Comparative Assessment of Morphometry, Morphology, and Maturation Capacity of Vitrified Cattle Oocytes in Different Media
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Experiment I: Morphometric Evaluation of Immature and Mature Cattle Oocytes Pre and Post Cryopreservation
- 1.
- Control group (non-vitrified immature oocytes);
- 2.
- Vitrification of immature oocytes with ART Lab Solutions vitrification medium;
- 3.
- Vitrification of mature oocytes with ART Lab Solutions vitrification medium.
2.1.2. Experiment II: Three Different Vitrification Protocols, Together with Their Respective Warming and Maturation Media, Were Evaluated
- 1.
- Control group (non-vitrified oocytes);
- 2.
- Immature oocytes were vitrified using TCM199-based vitrification medium (Gibco-Invitrogen Life Technologies, Grand Island, NY, USA) and subsequently subjected to IVM in TCM199 medium;
- 3.
- Immature oocytes were vitrified using ART Lab Solutions vitrification medium and subsequently subjected to IVM in VitroMat-Protect™ (ART Lab Solutions, Adelaide, Australia) medium;
- 4.
- Immature oocytes were vitrified using BO-VitriCool™ (Bioscience, Guangzhou, China) medium and subsequently subjected to IVM in BO-IVM™ (Bioscience, Guangzhou, China) medium.
2.2. Cattle Ovary Collection
2.3. Aspiration Method for Retrieving Cattle Oocytes
2.4. Experiment 1: Morphometric Evaluation of Immature and Mature Cattle Oocytes Pre and Post Cryopreservation
2.4.1. Vitrification and Warming of Immature and Mature Cattle Oocytes
2.4.2. Thawing of the Immature and Mature Cattle Oocytes
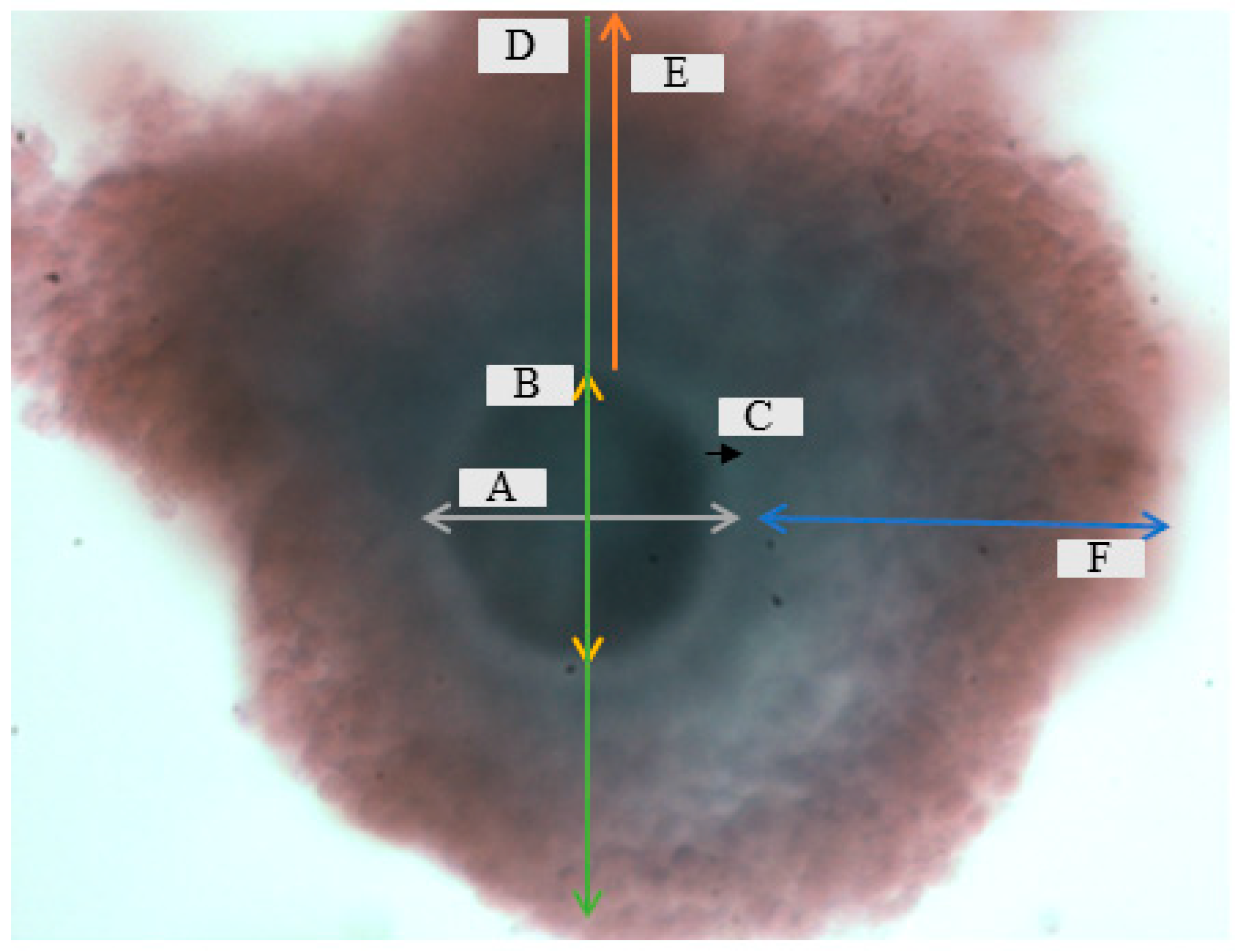
2.4.3. Morphometric Evaluation of Immature and Mature Cattle Oocytes
2.5. Experiment 2: To Compare the Maturation Rate and Morphological Characteristics of Oocytes Following Cryopreservation in Different Media
2.5.1. In Vitro Maturation of Cattle Oocytes
2.5.2. Oocyte Cumulus Cells’ Expansion
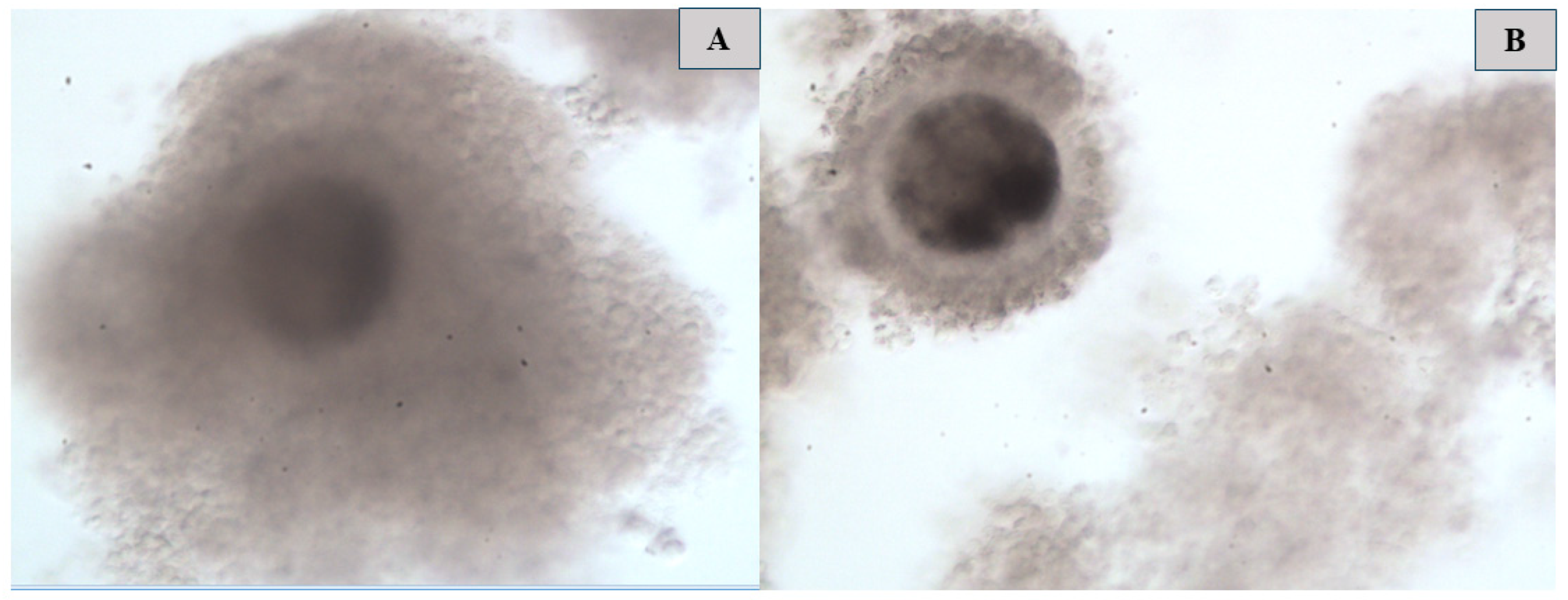
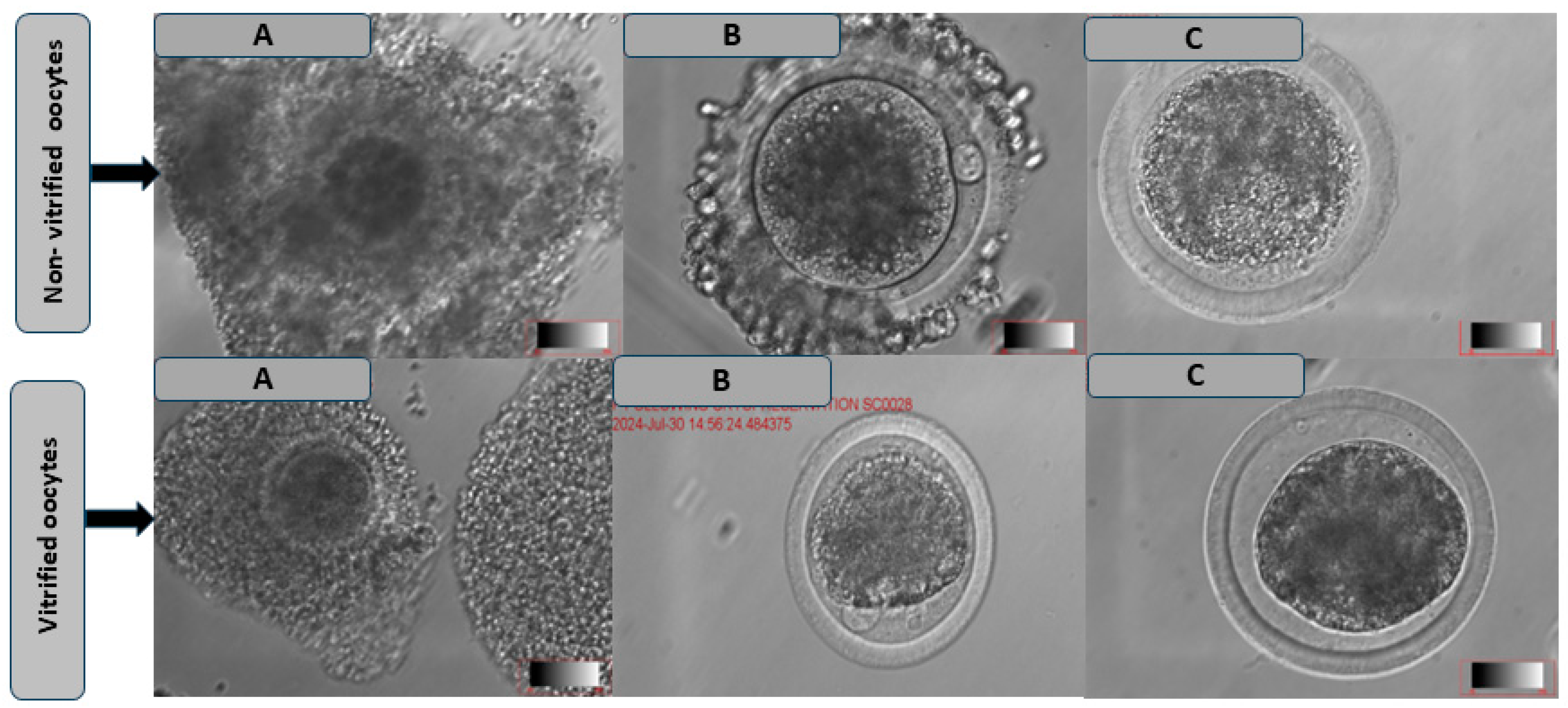
2.5.3. Oocytes’ Polar Body Extrusion and Morphological Characteristics’ Evaluation
2.6. Statistical Analysis
3. Results
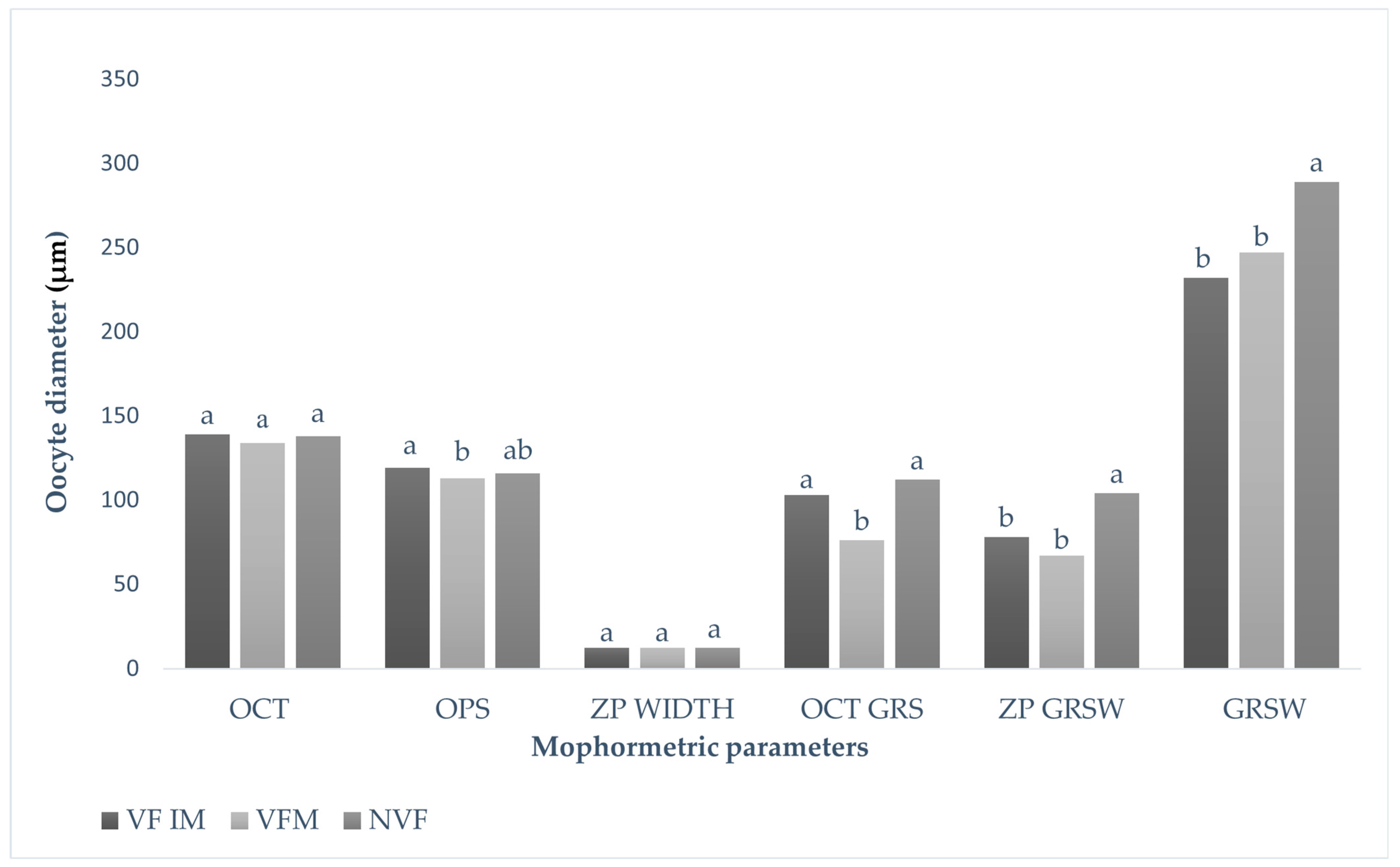
3.1. Morphometric Evaluation of Immature and Mature Cattle Oocytes Pre and Post Cryopreservation
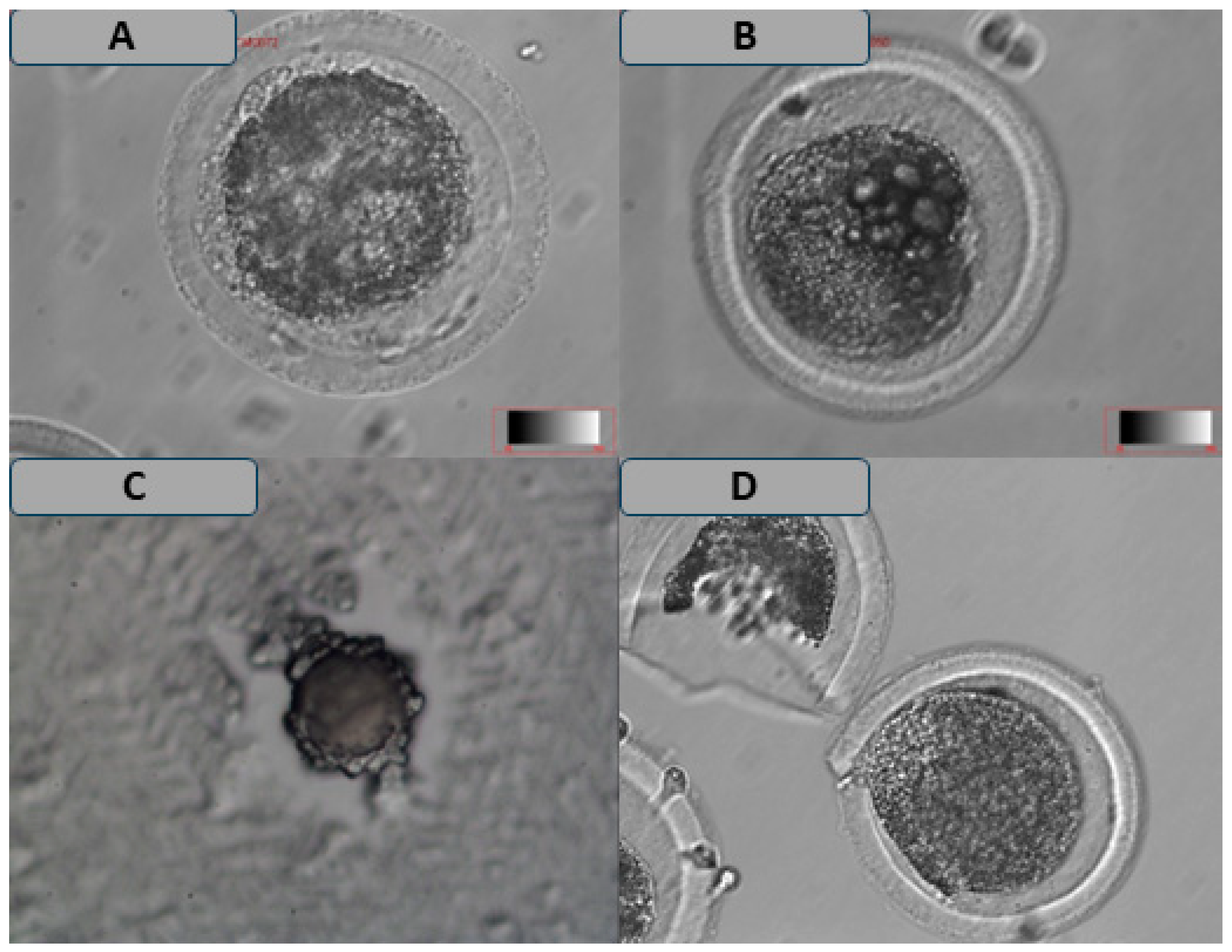
3.2. Comparison of the Maturation Rate and Morphological Characteristics of Oocytes Following Cryopreservation in Different Media
4. Discussion
5. Conclusions
6. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angel-Velez, D.; De Coster, T.; Azari-Dolatabad, N. Embryo morphokinetics derived from non-vitrified and vitrified bovine oocytes predict blastocyst development and nuclear abnormalities. Sci. Rep. 2023, 13, 4765. [Google Scholar] [CrossRef] [PubMed]
- Dujíčková, L.; Makarevich, A.; Olexiková, L.; Kubovičová, E.; Strejček, F. Methodological approaches for vitrification of bovine oocytes. Zygote 2021, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Harasanit, T.; Thuwanut, P. Oocyte cryopreservation in domestic animals and humans: Principles, techniques and updated outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Vajta, G.; Rindom, N.; Peura, T.T.; Holm, P.; Greve, T.; Callesen, H. The effect of media, serum and temperature on in vitro survival of bovine blastocysts after Open Pulled Straw (OPS) vitrification. Theriogenology 1999, 52, 939–948. [Google Scholar] [CrossRef]
- Paynter, S.J. A rational approach to oocyte cryopreservation. Reprod. Biomed. Online 2005, 10, 578–586. [Google Scholar] [CrossRef]
- Maside, C.; Sánchez-Ajofrín, I.; Medina-Chávez, D.; Alves, B.; Garde, J.J.; Soler, A.J. Oocyte morphometric assessment and gene expression profiling of oocytes and cumulus cells as biomarkers of oocyte competence in sheep. Animals 2021, 11, 2818. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Elias, M.H.; Mat Jin, N.; Abu, M.A.; Syafruddin, S.E.; Zainuddin, A.A.; Suzuki, N.; Karim, A.K. Oocytes Quality Assessment-The Current Insight: A Systematic Review. Biology 2024, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Arav, A.; Natan, Y.; Kalo, D.; Komsky-Elbaz, A.; Roth, Z.; Levi-Setti, P.E.; Leong, M.; Patrizio, P. A new, simple, automatic vitrification device: Preliminary results with murine and bovine oocytes and embryos. J. Assist. Reprod. Genet. 2018, 35, 1161–1168. [Google Scholar] [CrossRef]
- Asa, E.; Tabatabaee, R.; Farrokhi, A.; Nejatbakhsh, R. Relationship between meiotic spindles visualization and intracytoplasmic sperm injection outcomes in human oocytes. Anat. Cell Biol. 2017, 50, 26–32. [Google Scholar] [CrossRef]
- Hyttel, P.F.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Raghu, H.M.; Nandi, S.; Reddy, S.M. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod. Fertil. Dev. 2002, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fair, T.; Hyttel, P.; Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol. Reprod. Dev. 1995, 42, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, J.; Maside, C.; Ferreira, A.C.A.; Vieira, L.A.; Leiva-Revilla, J.; Paes, V.M.; Alves, B.G.; Brandão, F.Z.; Rodrigues, A.P.R.; Wheeler, M.B.; et al. Relationship between follicular dynamics and oocyte maturation during in vitro culture as a non-invasive sign of caprine oocyte meiotic competence. Theriogenology 2018, 107, 95–103. [Google Scholar] [CrossRef]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte Selection for In Vitro Embryo Production in Bovine Species: Noninvasive Approaches for New Challenges of Oocyte Competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef]
- Grifo, J.; Adler, A.; Lee, H.L.; Morin, S.J.; Smith, M.; Lu, L.; Hodes-Wertz, B.; McCaffrey, C.; Berkeley, A.; Munné, S. Deliveries from trophectoderm biopsied, fresh and vitrified blastocysts derived from polar body biopsied, vitrified oocytes. Reprod. Biomed. Online 2015, 31, 210–216. [Google Scholar] [CrossRef][Green Version]
- Chronopoulou, E.; Harper, J.C. IVF culture media: Past, present and future. Hum. Reprod. Update 2015, 21, 39–55. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Rapani, A.; Giannelou, P.; Anifandis, G.; Bolaris, S.; Pantou, A.; Lambropoulou, M.; Pappas, A.; Deligeoroglou, E.; et al. Considerations Regarding Embryo Culture Conditions: From Media to Epigenetics. In Vivo 2018, 32, 451–460. [Google Scholar] [CrossRef]
- Martinez, C.A.; Nohalez, A.; Parrilla, I. The overlaying oil type influences vitro embryo production: Differences in composition and compound transfer into incubation medium between oils. Sci. Rep. 2017, 7, 10505. [Google Scholar] [CrossRef]
- Martinez-Rodero, I.; Gallardo, M.; Pisaturo, M.; Scarica, C.; Conaghan, J.; Liebermann, J.; Cuevas-Saiz, I. The development of shorter protocols for vitrification and post-warming dilution of human oocytes and embryos: A narrative review. Reprod. Biomed. Online 2025, 104857, 1472–6483. [Google Scholar] [CrossRef]
- Sithole, S.M.; Mphaphathi, M.L.; Sebopela, M.D.; Nedambale, T.L. Comparison of in vitro Maturation Media on Cattle Oocytes after in vitro Embryo Production. Am. J. Anim. Vet. Sci. 2023, 18, 27–39. [Google Scholar] [CrossRef]
- Lekola, K.P.M. Improvement of Cattle Oocyte Retrieval Techniques and Hormonal Influence on In Vitro Embryonic Development. Ph.D. Dissertation, University of Limpopo, Polokwane, South Africa, 2015. Available online: https://www.mobt3ath.com/uplode/book/book-33325.pdf?ver=accessable (accessed on 12 May 2016).
- Daly, J.; Smith, H.; Mcgrice, H.A.; Kind, K.L.; Van Wettere, W.H. Towards Improving the Outcomes of Assisted Reproductive Technologies of Cattle and Sheep, with Particular Focus on Recipient Management. Animals 2020, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Maksura, H.; Akon, N.; Islam, M.N.; Akter, I.; Modak, A.K.; Khatun, A.; Alam, M.H.; Hashem, M.A.; Amin, M.R.; Moniruzzaman, M. Effects of estradiol on in vitro maturation of buffalo and goat oocytes. Reprod. Med. Biol. 2020, 20, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Maruska, D.V.; Leibfried, M.L.; First, N.L. Role of calcium and the calcium-calmodulin complex in resumption of meiosis, cumulus expansion, viability and hyaluronidase sensitivity of bovine cumulus-oocyte complexes. Biol. Reprod. 1984, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Otoi, T.; Yamamoto, K.; Koyama, N.; Tachikawa, S.; Suzuki, T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997, 48, 769–774. [Google Scholar] [CrossRef]
- Lucas, X.; Martínez, E.A.; Roca, J.; Vazquez, J.M.; Gil, M.A.; Pastor, L.M.; Alabart, J.L. Relationship between antral follicle size, oocyte diameters and nuclear maturation of immature oocytes in pigs. Theriogenology 2002, 58, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.R.; Chohan, K.R. Nuclear morphology, diameter and meiotic competence of buffalo oocytes relative to follicle size. Reprod. Fertil. Dev. 2003, 15, 223–229. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.; Yaqoob, S.H.; Alowaimer, A.N. Morphometric assessment of in vitro matured dromedary camel oocytes determines the developmental competence after parthenogenetic activation. Theriogenology 2017, 95, 141–148. [Google Scholar] [CrossRef]
- Lasiene, K.; Lasys, V.; Glinskyte, S.; Valanciute, A.; Vitkus, A. Relevance and methodology for the morphological analysis of oocyte quality in IVF and ICSI. J. Reprod. Stem Cell Biotechnol. 2011, 2, 1–3. [Google Scholar] [CrossRef][Green Version]
- De Wit, A.A.; Wurth, Y.A.; Kruip, T.A. Effect of ovarian phase and follicle quality on morphology and developmental capacity of the bovine cumulus-oocyte complex. J. Anim. Sci. 2000, 78, 1277–1283. [Google Scholar] [CrossRef]
- Arlotto, T.; Schwartz, J.L.; First, N.L.; Leibfried-Rutledge, M.L. Aspects of follicle and oocyte stage that affect in vitro maturation and development of bovine oocytes. Theriogenology 1996, 45, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Brambillasca, F.; Guglielmo, M.C.; Coticchio, G.; Mignini Renzini, M.; Dal Canto, M.; Fadini, R. The current challenges to efficient immature oocyte cryopreservation. J. Assist. Reprod. Genet. 2013, 30, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Mphaphathi, M.L. Factors Affecting Cryopreservation of Immature and Matured Cattle Oocytes. Ph.D. Thesis, Department of Animal, Wildlife and Grassland Sciences, Faculty of Natural and Agricultural Sciences, University of the Free State, Bloemfontein, South Africa, 2022. [Google Scholar]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.M.; Chian, R.C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bartolacci, A.; Intra, G.; Coticchio, G.; dell’Aquila, M.; Patria, G.; Borini, A. Does morphological assessment predict oocyte developmental competence? A systematic review and proposed score. Assist. Reprod. Genet. 2022, 1, 1–5. [Google Scholar] [CrossRef]
- Fasano, G.; Demeestere, I.; Englert, Y. In-vitro maturation of human oocytes: Before or after vitrification? J. Assist. Reprod. Genet. 2012, 29, 507–512. [Google Scholar] [CrossRef]
- Rose, B.I.; Laky, D. Polar body fragmentation in IVM oocytes is associated with impaired fertilization and embryo development. J. Assist. Reprod. Genet. 2013, 30, 679–682. [Google Scholar] [CrossRef]
- Navarro, P.A.; de Araújo, M.M.; de Araújo, C.M.; Rocha, M.; dos Reis, R.; Martins, W. Relationship between first polar body morphology before intracytoplasmic sperm injection and fertilization rate, cleavage rate, and embryo quality. Int. J. Gynaecol. Obstet. 2009, 104, 226–229. [Google Scholar] [CrossRef]
- Fancsovits, P.; Tóthné, Z.G.; Murber, A.; Takács, F.Z.; Papp, Z.; Urbancsek, J. Correlation between first polar body morphology and further embryo development. Acta Biol. Hung. 2006, 57, 331–338. [Google Scholar] [CrossRef]
- Lasiene, K.; Vitkuse, A.; Valanciute, A.; Lasys, V. morphological criteria of oocyte quality. Medicina 2009, 45, 509–514. [Google Scholar] [CrossRef] [PubMed]
| Solution | ART Lab Solutions™-Vitrification and Warming | |
|---|---|---|
| Composition | Time | |
| Handling media | Buffered medium + bovine serum albumin | 5–10 min |
| Vitrification solution 1 | Buffered medium + bovine serum albumin + ethylene glycol dimethylsulfoxide | 3 min |
| Vitrification solution 2 | Sucrose solution + ethylene glycol + dimethylsulfoxide | 30 s |
| Warm 1 | Handling media + 1 M sucrose solution | <1 min |
| Warm 2 | Handling media + 1 M sucrose solution | 5 min |
| Warm 3 | Handling media + 1 M sucrose solution | 5 min |
| Warm 4 | Handling media | 1–5 min |
| Solution | TCM 199-Vitrification and Warming | |
|---|---|---|
| Composition | Time | |
| Base (BS) | TCM199 + fetal bovine serum | 1 min |
| Rinsing | Base + fetal bovine serum | 3 min |
| Holding medium | Base + fetal bovine serum + dimethylsulfoxide + ethylene glycol | 3 min |
| Vitrification solution | TCM199 + fetal bovine serum + ethylene glycol + dimethylsulfoxide + sucrose | 30 s |
| Warming | Dulbecco’s phosphate-buffered saline + fetal bovine serum + sucrose | 30 s |
| Rehydration | Dulbecco’s phosphate-buffered saline + fetal bovine serum + sucrose | 1 min |
| Solution | BO-VitriCoolTM and BO-VitriWarmTM | |
|---|---|---|
| Composition | Time | |
| Pre-incubation | Ethylene glycol + dimethylsulfoxide-based formula (serum-free) | 2 min |
| Cooling 1 | Ethylene glycol + dimethylsulfoxide-based formula | 2 min |
| Cooling 2 | Ethylene glycol + dimethylsulfoxide-based formula | 30 s |
| Warming 1 | Sucrose + albumin (serum free) | 3 min |
| Warming 2 | Sucrose + albumin | 2 min |
| Warming 3 | Sucrose + albumin | 2 min |
| Warming 4 | Sucrose + albumin | 1 min |
| Treatment | Type of the Oocyte (n = 900) | COC Expansion | PB% |
|---|---|---|---|
| Vitromat-ProtectTM | Non-vitrified | 93.30 ± 6.96 a | 67.30 ± 5.67 a |
| Vitrified | 34.00 ± 11.02 c | 24.60 ± 11.93 bc | |
| BO-IVMTM | Non-vitrified | 94.75 ± 6.70 a | 71.50 ± 12.76 a |
| Vitrified | 52.28 ± 7.06 b | 35.14 ± 5.01 b | |
| TCM199 | Non-vitrified | 87.33 ± 13.08 a | 61.90 ± 7.79 a |
| Vitrified | 28.70 ± 10.03 c | 18.44 ± 8.00 c |
| Treatment | Type of the Oocyte | FPB | LV | DG | CC |
|---|---|---|---|---|---|
| Vitromat-ProtectTM | Non-vitrified | 2.00 ± 4.47 c | 15.30 ± 8.44 abc | 2.00 ± 4.47 cd | 0.00 ± 0.00 d |
| Vitrified | 19.30 ± 3.97 a | 22.60 ± 6.51 a | 15.80 ± 8.24 a | 24.50 ± 10.53 a | |
| BO-IVMTM | Non-vitrified | 1.75 ± 3.52 c | 10.00 ± 3.46 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| Vitrified | 16.14 ± 5.20 ab | 24.00 ± 5.50 a | 15.14 ± 9.38 ab | 31.42 ± 7.32 a | |
| TCM199 | Non-vitrified | 0.70 ± 2.21 c | 12.00 ± 7.95 bc | 1.40 ± 2.95 d | 0.00 ± 0.00 d |
| Vitrified | 13.90 ± 4.54 b | 20.70 ± 8.01 ab | 16.00 ± 6.44 a | 18.70 ± 7.04 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebopela, M.D.; Mkhize, N.R.; Thema, M.A.; Mphaphathi, M.L. Comparative Assessment of Morphometry, Morphology, and Maturation Capacity of Vitrified Cattle Oocytes in Different Media. Vet. Sci. 2025, 12, 461. https://doi.org/10.3390/vetsci12050461
Sebopela MD, Mkhize NR, Thema MA, Mphaphathi ML. Comparative Assessment of Morphometry, Morphology, and Maturation Capacity of Vitrified Cattle Oocytes in Different Media. Veterinary Sciences. 2025; 12(5):461. https://doi.org/10.3390/vetsci12050461
Chicago/Turabian StyleSebopela, Maleke Dimpho, Ntuthuko Raphael Mkhize, Mamonene Angelinah Thema, and Masindi Lottus Mphaphathi. 2025. "Comparative Assessment of Morphometry, Morphology, and Maturation Capacity of Vitrified Cattle Oocytes in Different Media" Veterinary Sciences 12, no. 5: 461. https://doi.org/10.3390/vetsci12050461
APA StyleSebopela, M. D., Mkhize, N. R., Thema, M. A., & Mphaphathi, M. L. (2025). Comparative Assessment of Morphometry, Morphology, and Maturation Capacity of Vitrified Cattle Oocytes in Different Media. Veterinary Sciences, 12(5), 461. https://doi.org/10.3390/vetsci12050461








