Identification of Naturally Occurring Inhabitants of Vaginal Microbiota in Cows and Determination of Their Antibiotic Sensitivity
Simple Summary
Abstract
1. Introduction
- Identifying the naturally occurring inhabitants of vaginal bacterial flora of Holstein-Friesian cows;
- Determining the sensitivity of selected, potentially probiotic bacterial strains to ten commonly used antibiotics;
- Establishing an average vaginal mucosa surface pH value for healthy Holstein Friesian cows.
2. Materials and Methods
2.1. Animals and Samplings
2.2. Bacterial Culturing
2.3. Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) Analysis
2.4. Broth Microdilution Tests
2.5. Bacterial DNA Isolation and Polymerase Chain Reaction (PCR) Analysis
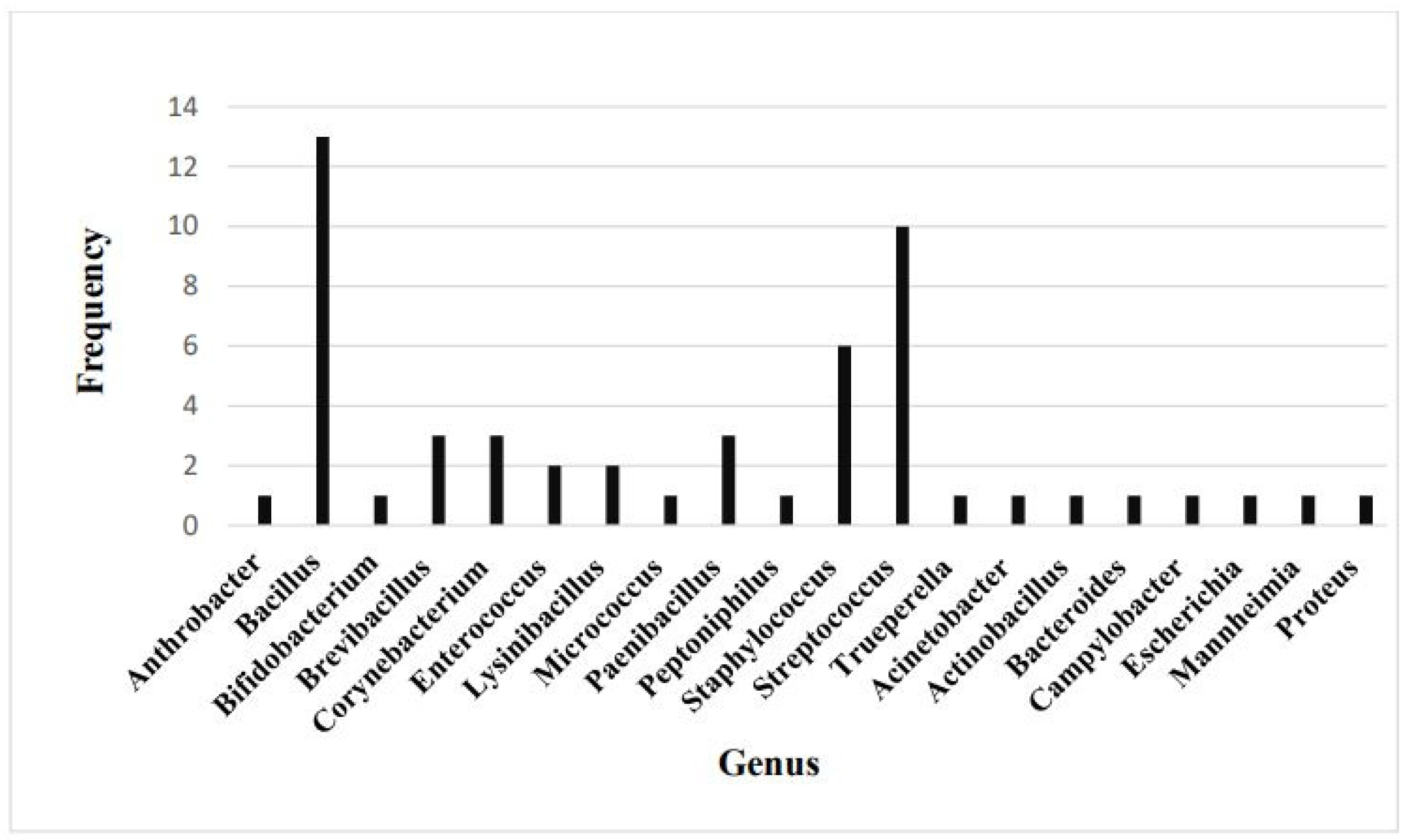
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tasara, T.; Meier, A.B.; Wambui, J.; Whiston, R.; Stevens, M.; Chapwanya, A.; Bleu, U. Interrogating the Diversity of Vaginal, Endometrial, and Fecal Microbiomes in Healthy and Metritis Dairy Cattle. Animals 2023, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Várhidi, Z.; Csikó, G.; Bajcsy, Á.C.; Jurkovich, V. Uterine disease in dairy cows: A comprehensive review highlighting new research areas. Vet. Sci. 2024, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.O.; Meijer, W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.; de Freitas, S.L.; da Costa, A.N.; Bicalho, R.C.; Lima, S.; et al. Uterine Microbiota Progression from Calving until Establishment of Metritis in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Zangirolamo, A.F.; Souza, A.K.; Yokomizo, D.N.; Miguel, A.K.A.; Costa, M.C.d.; Alfieri, A.A.; Seneda, M.M. Updates and current challenges in reproductive microbiome: A comparative analysis between cows and women. Animals 2024, 14, 2024. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenol. 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenol 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenol 2020, 150, 158–165. [Google Scholar] [CrossRef]
- Adnane, M.; Chapwanya, A. A Review of the Diversity of the Genital Tract Microbiome and Implications for Fertility of Cattle. Animals 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Chapwanya, A. Microbial gatekeepers of fertility in the female reproductive microbiome of cattle. Int. J. Mol. Sci. 2024, 25, 10923. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Uterine and vaginal bacterial community diversity prior to artificial insemination between pregnant and nonpregnant postpartum cows. J. Anim. Sci. 2019, 97, 4298–4304. [Google Scholar] [CrossRef]
- Quereda, J.J.; Barba, M.; Mocé, M.L.; Gomis, J.; Jiménez-Trigos, E.; García-Muñoz, Á.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal Microbiota Changes During Estrous Cycle in Dairy Heifers. Front. Vet. Sci. 2020, 7, 371. [Google Scholar]
- Rodrigues, N.F.; Kästle, J.; Coutinho, T.J.; Amorim, A.T.; Campos, G.B.; Santos, V.M.; Marques, L.M.; Timenetsky, J.; de Farias, S.T. Qualitative analysis of the vaginal microbiota of healthy cattle and cattle with genital-tract disease. Genet. Mol. Res. 2015, 14, 6518–6528. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Sun, C.; Liu, C.; Yang, Y.; Lu, W. Comparison of vaginal microbial community structure in healthy and endometritis dairy cows by PCR-DGGE and real-time PCR. Anaerobe 2016, 38, 1–6. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Santin, T.; Rodrigues, M.X.; Marques, C.E.; Lima, S.F.; Bicalho, R.C. Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: Associations with uterine diseases and reproductive outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; England, G.C.W.; Dobson, H.; Pfeiffer, D.U.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the inflammatory response to endometritis in cattle. Theriogenol 2005, 63, 102–117. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of Body Condition Score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenol 1997, 47, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Bazzazan, A.; Vallejo-Timaran, D.A.; Maldonado-Estrada, J.; Segura, M.; Lefebvre, R. Diagnosis of clinical cervicitis and vaginitis in dairy cows in relation to various postpartum uterine disorders. Clin. Theriogenol. 2024, 16, 1–6. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed.; CLSI Standard VET01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings, 2nd ed.; CLSI Report VET09; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Westermann, S.; Drillich, M.; Kaufmann, T.B.; Madoz, L.V.; Heuwieser, W. A clinical approach to determine false positive findings of clinical endometritis by vaginoscopy by the use of uterine bacteriology and cytology in dairy cows. Theriogenol 2010, 74, 1248–1255. [Google Scholar] [CrossRef]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral pH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef]
- Beckwith-Cohen, B.; Koren, O.; Blum, S.; Elad, D. Variations in Vaginal pH in Dairy Cattle Associated with Parity and the Periparturient Period. Israel J. Vet. Med. 2012, 67, 55–59. [Google Scholar]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Critic. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Molec. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Sanders, M.E.; Morelli, L.; Tompkins, T.A. Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compreh. Rev. Food Sci. Food Safety 2006, 2, 101–110. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Rodloff, A.; Bauer, T.; Ewig, S.; Kujath, P.; Müller, E. Susceptible, intermediate, and resistant—The intensity of antibiotic action. Deutsch. Arztebl. Int. 2008, 105, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Voigt, B.; Antelmann, H.; Albrecht, D.; Ehrenreich, A.; Maurer, K.-H.; Evers, S.; Gottschalk, G.; van Dijl, J.M.; Schweder, T.; Hecker, M. Cell Physiology and Protein Secretion of Bacillus licheniformis Compared to Bacillus subtilis. J. Molec. Microbiol. Biotechnol. 2009, 16, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Ahmed, I.; Ahmed, S.; Ahmed, Z.; Riaz, M.N.; Ghazanfar, S. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Ind. J. Anim. Res. 2018, 52, B-948. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.-S.; Ariton, A.-M.; Radu, C.; Porosnicu, I.; Sanduleanu, C.; Amariții, G. From Herd Health to Public Health: Digital Tools for Combating Antibiotic Resistance in Dairy Farms. Antibiotics 2024, 13, 634. [Google Scholar] [CrossRef]
- Panda, A.K.; Bisht, S.S.; DeMondal, S.; Kumar, N.S.; Gurusubramanian, G.; Panigrahi, A.K. Brevibacillus as a biological tool: A short review. Antonie Van Leeuwenhoek 2014, 105, 623–639. [Google Scholar] [CrossRef]
| Species | Gene Symbol | Gene | Accession Number | Primer | Product Size (Base Pair) | |
|---|---|---|---|---|---|---|
| Brevibacillus | penP | beta-lactamase class A | BA6348_08610 | F | CTGTGCAAAATGGCGCGTAT | 168 |
| agri | R | CCGATCCTCCCACCAAATCC | ||||
| ITS | internal transcribed spacer | AF478091.1 | F | CCCGAAGTCGGTGAGGTAAC | 214 | |
| R | ATGGACGCGAGTGCTCTTAG | |||||
| Bacillus | penP | beta-lactamase class A | BLi00280 | F | GCAATCACTCGAATGCCTCAC | 178 |
| licheniformis | R | ATCGTCGATGCAAAAGCGAAG | ||||
| ITS | internal transcribed spacer | AF478086.1 | F | ATGCCGCGGTGAATACGTTC | 161 | |
| R | CACCTTCCGATACGGCTACC | |||||
| B. pumilus | tetA_3 | major facilitator superfamily (MFS) | SAMEA4076707 | F | ATTGTCGGACCGAGCCTTG | 141 |
| transporter | _03279 | R | AGAAACTGTCGAAGGATGCTG | |||
| bpu-1 | beta-lactamase class D | BPUM_RS11670 | F | GAAGAGAAACACGCCACCCT | 124 | |
| R | TGCCGGTGCCTTTGATATTTG | |||||
| ITS | internal transcribed spacer | AF478070.1 | F | TATATGGAGCAGCGTGCGTT | 226 | |
| R | CATCGGCTCCTAGTGCCAAG | |||||
| B. subtilis | penP | beta-lactamase class A | BSU_18800 | F | TCTCACGACTGACAAACGCA | 122 |
| R | TTCCGGCTCCGGATTTATCG | |||||
| bsu-2 | beta-lactamase class D | BSU_02090 | F | AGTTTTGGCTGCAAAGCTCG | 168 | |
| R | TTCCGGTTTTCCCGTAGAGC | |||||
| ITS | internal transcribed spacer | AB050658.1 | F | ACAGAACGTTCCCTGTCTTGT | 124 | |
| R | TCACTACGTGATATCTTGCATTACT |
| Aerobic/Anaerobic Characteristics | Gram-Positive | Gram-Negative |
|---|---|---|
| Aerobe | Bacillus circulans, B. oceanisediminis, B. oleronius, B. pumilus, B. safensis B. sonorensis, Brevibacillus agri/parabrevis, Bb. borstelensis, Corynebacterium xerosis Lysinibacillus fusiformis, L. massiliensis, Bacillus amyloliquefaciens, B. clausii, B. megaterium, B. siralis, Arthrobacter gandavensis, Micrococcus luteus | Acinetobacter pittii |
| Facultative anaerobe | Bacillus cereus, B. licheniformis, B. subtilis, Corynebacterium camporealensis, C. renale, Enterococcus avium, E. hirae, Paenibacillus cookii, P. ihumii, P. lactis, Staphylococcus chromogenes, S. epidermidis, S. hominis, S. succinus, S. sciuri, S. xylosus, Streptococcus alactolyticus/lutetiensis, S. canis, S. dysgalactiae, S. equinus, S. pluranimalium/hyovaginalis, S. pneumoniae/pseudopneumoniae, S. suis, S. uberis, S. mitis/oralis/peroris, Trueperella pyogenes | Actinobacillus rossii, Escherichia coli, Mannheimia varigena/haemolytica/granulomatis, Proteus mirabilis |
| Obligate anaerobe | Bifidobacterium pseudolongum, Peptoniphilus indolicus | Bacteroides fragilis |
| Microaerophile | Campylobacter hyointestinalis |
| Bacterial Isolate | Resistance Gene | Present |
|---|---|---|
| Brevibacillus agri | Beta-lactamase class A | No |
| Bacillus licheniformis | Beta-lactamase class A | Yes |
| Bacillus licheniformis (W) | Beta-lactamase class A | No |
| Bacillus pumilus | Beta-lactamase class D | Yes |
| Tetracycline resistance MFS efflux pump | Yes | |
| Bacillus subtilis | Beta-lactamase class A | Yes |
| Beta-lactamase class D | Yes |
| AMX | CFQ | CTF | DOX | FFC | MBF | OTC | T-S | TUL | TYL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Brevibacillus agri | 0.5 | 0.06 | 0.016 | 0.05 | 2 | 0.5 | 1 | 500 | 0.5 | 0.5 |
| Bacillus licheniformis | >50 | 1 | 0.5 | 0.5 | 4 | 0.125 | 2 | 1 | 0.5 | 0.5 |
| B. licheniformis (W) | >5 | >8 | 8 | >0.05 | 4 | 0.25 | 0.5 | 2 | 2 | 0.25 |
| B. pumilus | >5 | >8 | >2 | 0.05 | 4 | 0.5 | 0.5 | >2 | 1 | 0.5 |
| B. subtilis | >50 | 0.25 | 0.25 | 0.5 | 1 | 0.25 | 8 | 1 | 2 | 0.5 |
| AMX | CFQ | CTF | DOX | FFC | MBF | OTC | T-S | TUL | TYL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Brevibacillus agri | S | S | S | S | S | S | S | R | S | S |
| Bacillus licheniformis | R | S | S | S | S | S | S | S | S | S |
| B. licheniformis (W) | I | R | R | S | S | S | S | S | S | S |
| B. pumilus | I | R | I | S | S | S | S | I | S | S |
| B. subtilis | R | S | S | S | S | S | R | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várhidi, Z.; Jurkovich, V.; Sátorhelyi, P.; Erdélyi, B.; Palócz, O.; Csikó, G. Identification of Naturally Occurring Inhabitants of Vaginal Microbiota in Cows and Determination of Their Antibiotic Sensitivity. Vet. Sci. 2025, 12, 423. https://doi.org/10.3390/vetsci12050423
Várhidi Z, Jurkovich V, Sátorhelyi P, Erdélyi B, Palócz O, Csikó G. Identification of Naturally Occurring Inhabitants of Vaginal Microbiota in Cows and Determination of Their Antibiotic Sensitivity. Veterinary Sciences. 2025; 12(5):423. https://doi.org/10.3390/vetsci12050423
Chicago/Turabian StyleVárhidi, Zsóka, Viktor Jurkovich, Péter Sátorhelyi, Balázs Erdélyi, Orsolya Palócz, and György Csikó. 2025. "Identification of Naturally Occurring Inhabitants of Vaginal Microbiota in Cows and Determination of Their Antibiotic Sensitivity" Veterinary Sciences 12, no. 5: 423. https://doi.org/10.3390/vetsci12050423
APA StyleVárhidi, Z., Jurkovich, V., Sátorhelyi, P., Erdélyi, B., Palócz, O., & Csikó, G. (2025). Identification of Naturally Occurring Inhabitants of Vaginal Microbiota in Cows and Determination of Their Antibiotic Sensitivity. Veterinary Sciences, 12(5), 423. https://doi.org/10.3390/vetsci12050423







