Mechanism of GBE Combined with TP on the Effect of AMPK/SREBP-1C/ACC Pathway on Lipid Metabolism in Heat-Stressed Broiler Liver
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Basic Rations
2.3. Sample Collection
2.4. Measurement Methods
2.4.1. Measurement of Growth Performance
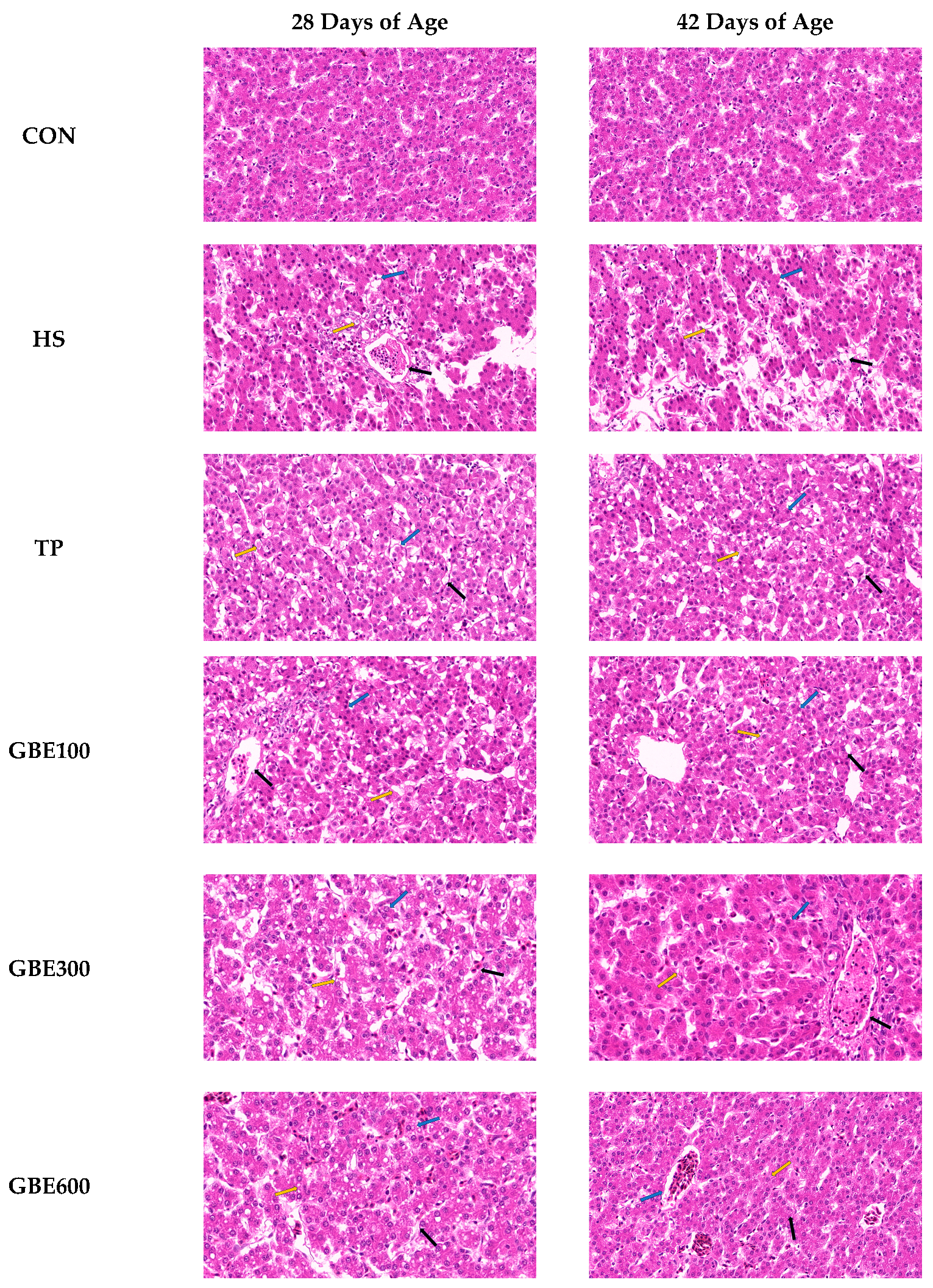
2.4.2. Histopathological Changes in the Liver
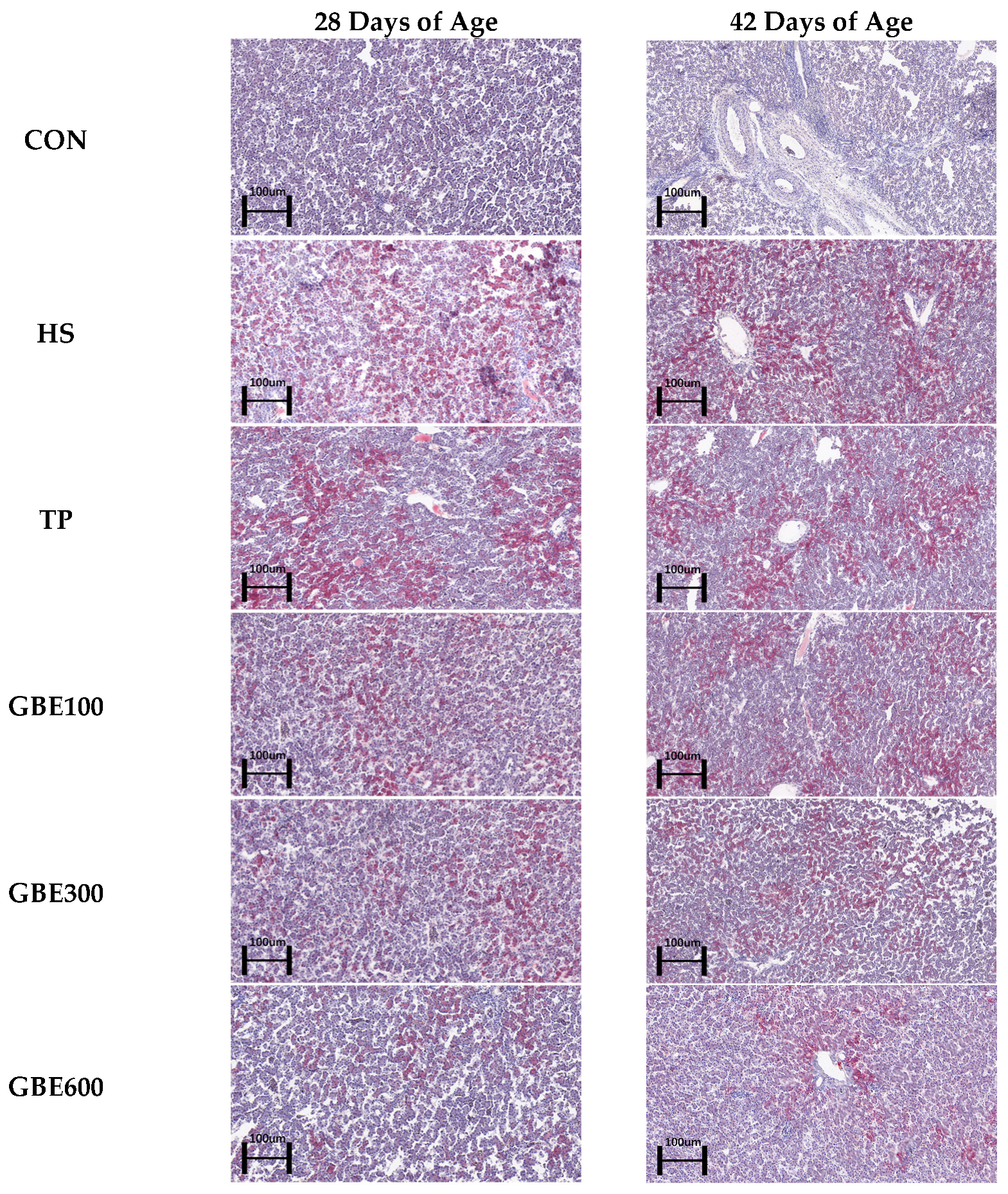
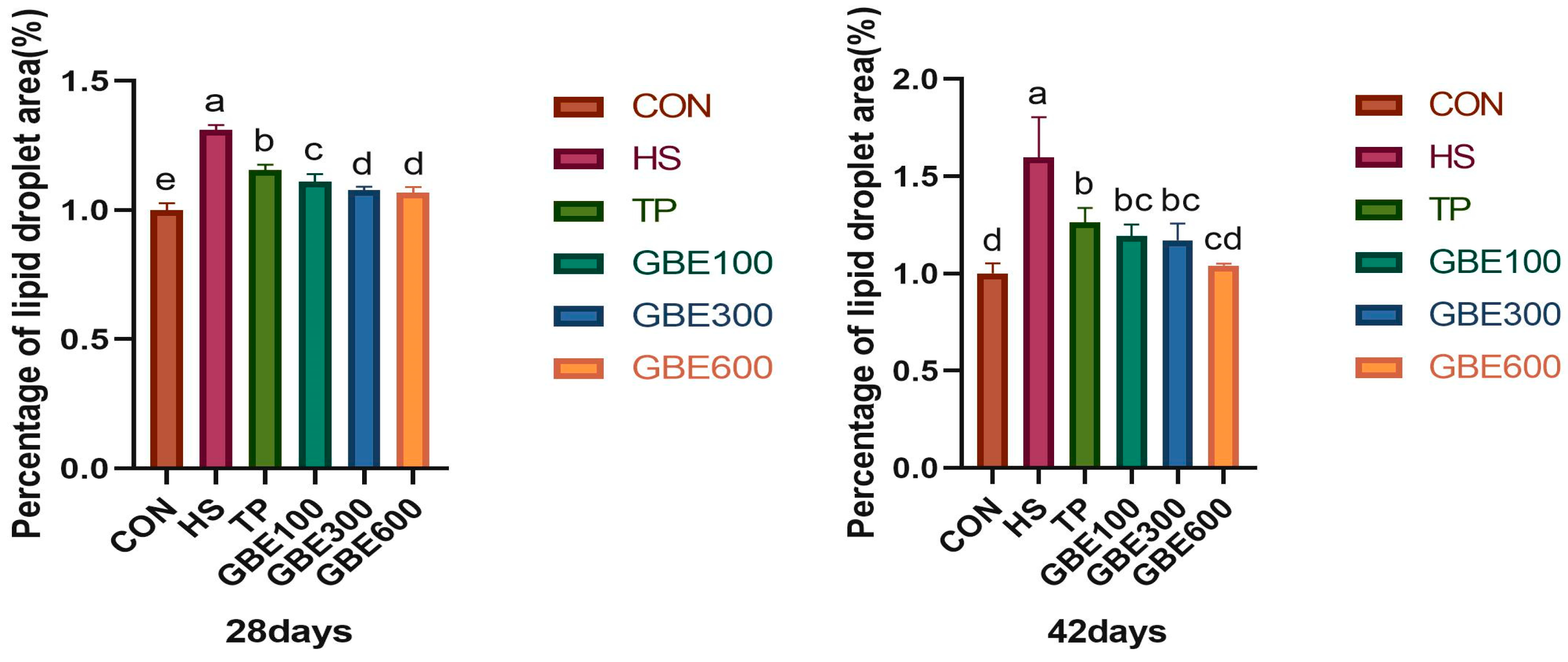
2.4.3. Hepatic Lipid Area Analysis
2.4.4. Measurement of Serum Lipid Content
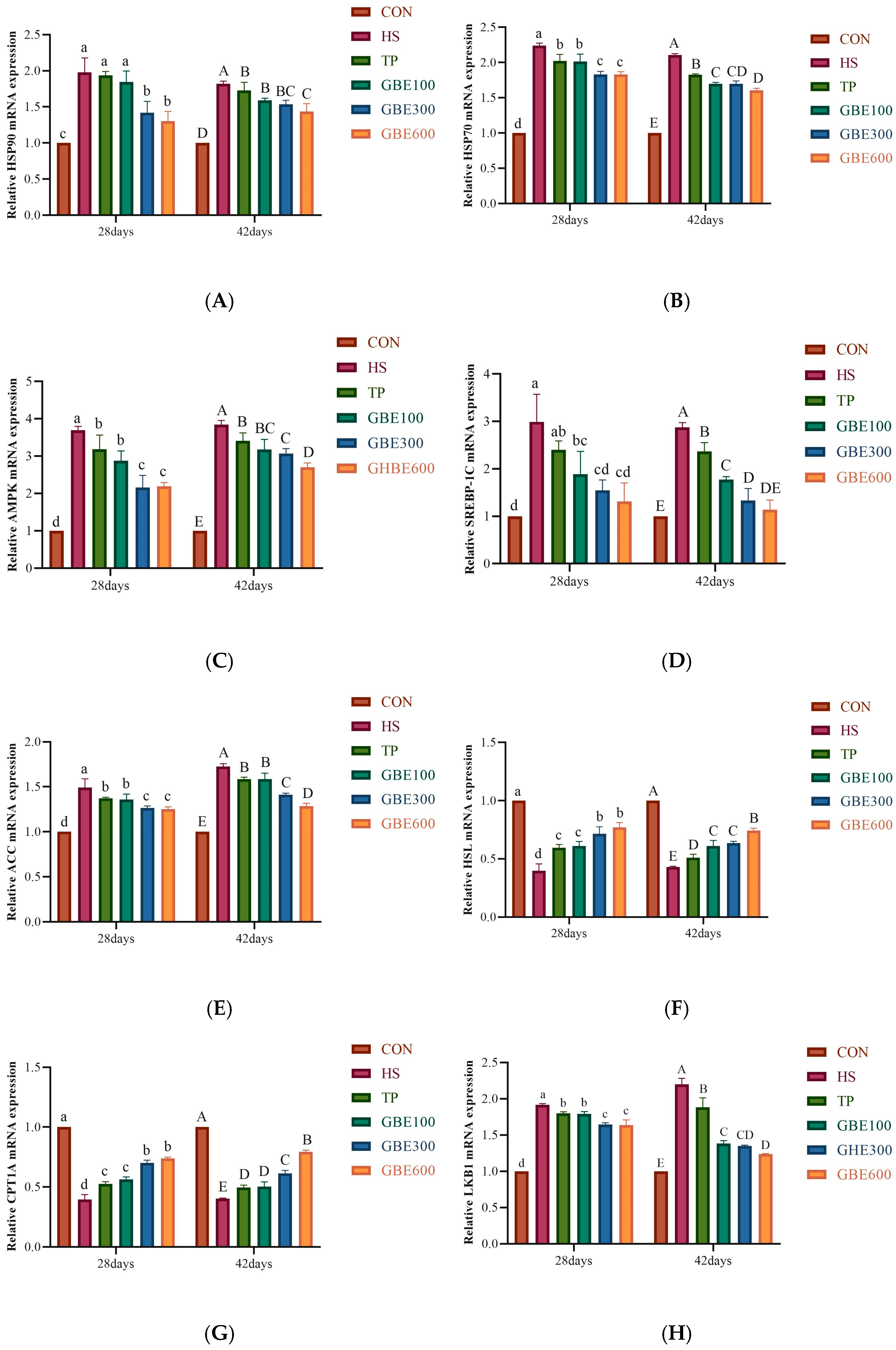
2.4.5. Expression of Genes Related to Hepatic Lipid Metabolism
2.5. Statistics and Analysis of Data
3. Results
3.1. Production Performance Measurement
3.2. HE Pathologic Observation of Liver Tissue
3.3. Liver ORO Lipid Observations
3.4. Serum Lipid Profile Test
3.5. Determination of mRNA for Lipid Metabolism-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shokryazdan, P.; Jahromi, M.F.; Saadand, S.M.; Ebrahimi, M.; Idrus, Z.; Zhou, H.; Diao, X.P.; Liang, J.B. Chinese Herbal Medicines as Potential Agents for Alleviation of Heat Stress in Poultry. Scientifica 2017, 2017, 8208261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-M.; Ju, Y.-X.; Yang, M.-M.; Wu, X.-J. Physiological hazards of heat stress on broiler chickens and mitigation measures. Feed Res. 2024, 47, 175–179. [Google Scholar] [CrossRef]
- Ma, B.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Chronic heat stress causes liver damage via endoplasmic reticulum stress-induced apoptosis in broilers. Poult. Sci. 2022, 101, 102063. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Li, Z.; Yin, Y.; Zhao, Y. Adipogenesis and its regulatory mechanisms in chickens. Anim. Nutr. 2022, 34, 6860–6872. [Google Scholar]
- Hao, J.; Lu, Q.; Zhang, H.; Zhang, X. Effects of sustained high temperature on fat metabolism and blood adipocytokine levels in Beijing oil chickens of different sexes. Anim. Nutr. 2012, 24, 1038–1043. [Google Scholar]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2021, 22, 429–443. [Google Scholar] [CrossRef]
- Liu, M. Effects of acute heat stress on growth performance and fat metabolism of broiler chicks. Anim. Nutr. 2011, 23, 862–868. [Google Scholar]
- Ge, Y. Effects of Chronic Heat Stress on Lipid Metabolism and Mitigating Effect of Vitamin C in Breeding Laying Hens. Doctoral Dissertation, Jiangxi Agricultural University, Nanchang, China, 2023. [Google Scholar]
- Yanli, L.; Jing, S.; Xin, Y.; Qingzhu, S.; Xiaojun, Y. Folic Acid Reduced Triglycerides Deposition in Primary Chicken Hepatocytes. J. Agric. Food Chem. 2018, 66, 13162–13172. [Google Scholar]
- Wang, W.W.; Chen, L.; Zhang, J.Y.; Wang, W.; Jiang, H.Y. Characterization of tea polyphenols and their application in food. Chin. Tea 2020, 42, 1–7. [Google Scholar]
- Long, J.; Wang, M.H.; Li, D.; Sun, Y.; Wang, J. Progress of Tea Polyphenol Extraction Process. Food Saf. Guide 2024, 187–189. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, S.; Xia, R.; Fan, X.; Han, W. Chemical Composition of Tea Polyphenols, Determination of Their Content and Structural Identification. Mechatron. Inf. 2018, 1–9. [Google Scholar] [CrossRef]
- Jinbao, H.; Yong, Z.; Yibin, Z.; Zhengzhu, Z.; Zhongwen, X.; Jinsong, Z.; Xiaochun, W. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013, 61, 8565–8572. [Google Scholar]
- Quan, M.; Su, Z.; Fang, D.; Xie, X.; Xie, J. Progress in the extraction and functional study of ginkgo biloba flavonoids. Pharmacol. Today 2020, 30, 789–792. [Google Scholar]
- Liu, X.-G.; Wu, S.-Q.; Li, P.; Yang, H. Advancement in the chemical analysis and quality control of flavonoid in Ginkgo biloba. J. Pharm. Biomed. Anal. 2015, 113, 212–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Quan, M. Research progress on antioxidant efficacy of Ginkgo biloba extract. Heilongjiang Anim. Husb. Vet. Med. 2016, 69–71. [Google Scholar] [CrossRef]
- Zhang, X.h. Ginkgo biloba extract EGB761 improved anti-heat stress responses in chickens in vivo via regulation of heat-shock protein expression and distribution. Turk. J. Vet. Anim. Sci. 2020, 44, 191–200. [Google Scholar] [CrossRef]
- Luo, P. Study on the Role of AMPK on Mitochondrial Autophagy and Steroid Synthesis in Duck Granulosa Cells Under Heat Treatment Conditions. Doctoral Dissertation, Zhongkai University of Agriculture and Engineering, Guangzhou, China, 2023. [Google Scholar]
- Pompili, S.; Vetuschi, A.; Gaudio, E.; Tessitore, A.; Capelli, R.; Alesse, E.; Latella, G.; Sferra, R.; Onori, P. Long-term abuse of a high-carbohydrate diet is as harmful as a high-fat diet for development and progression of liver injury in a mouse model of NAFLD/NASH. Nutrition 2020, 75–76, 110782. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Ren, H.; Huang, X.; Shen, T.; Tang, W.; Dou, L.; Li, J. miR-23a/b-3p promotes hepatic lipid accumulation by regulating Srebp-1c and Fas. J. Mol. Endocrinol. 2021, 68, 35–49. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Vivian, S.; Prisma, A.; Miskiyah, I.; Christensen, M.C.; Alward, H.J.; Kevin, K.I.; Imaduddin, S.M.N.; Melva, L.; Ari, E. Alpha-Mangosteen lessens high-fat/high-glucose diet and low-dose streptozotocin induced-hepatic manifestations in the insulin resistance rat model. Pharm. Biol. 2023, 61, 241–248. [Google Scholar]
- Li, H.-W.; Guo, S.; Wang, Q.-X.; Qian, D.-W.; Duan, J.-A. Analysis of the current status of the development of the feed additive industry of plant extracts from Chinese medicine resources and its outlook. Chin. Herbal. Med. 2023, 54, 3745–3758. [Google Scholar]
- Lu, Y. Study on the Effect of Tea Polyphenols in Lowering Egg Cholesterol and Its Mechanism. Doctoral Dissertation, Southwest University, Chongqing, China, 2019. [Google Scholar]
- Huang, Y.; Lan, B.; Hou, X.; Zhou, Z.; Xie, S.; Wu, J. Effects of tea polyphenols and flax oil on the meat quality and antioxidant properties of three-yellow chickens. Feed Ind. 2021, 42, 48–52. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wan, X.L.; Zhang, X.H.; Zhao, L.G.; He, J.T.; Zhang, J.F.; Zhang, L.L.; Wang, T. Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult. Sci. 2017, 96, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zeng, Q.; Yin, C.; Yang, X.; Wang, Y.; Ru, M.; Liu, J.; Xie, X.; Liang, H.; Wei, Q.; et al. Progress in the study of fatty liver hemorrhagic syndrome and its molecular mechanism in laying hens. Chin. J. Vet. Med. 2021, 41, 1658–1665. [Google Scholar] [CrossRef]
- Junichi, F.; Takujiro, H.; Sho, K.; Geuk, S.H. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J. Biol. Chem. 2018, 9, 1–15. [Google Scholar]
- Qi, Q. Genistein Alleviates Heat Stress-Induced Hepatic Lipid Deposition in Broiler Chickens by Modulating Mfn2-PLIN1 Interactions. Doctoral Dissertation, Jiangxi Agricultural University, Nanchang, China, 2024. [Google Scholar]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef]
- Wang, Y. Determination and analysis of main physiological and biochemical indexes of Bode generation sheep. Gansu Anim. Husb. Vet. Med. 2003, 2–4. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.Y.; Wei, X.C.; Wang, Q.; Yang, J.Y.; Hou, H.; Du, Z.W.; Wu, X.A. Effects of dibutyl phthalate on lipid metabolism in liver and hepatocytes based on PPARα/SREBP-1c/FAS/GPAT/AMPK signal pathway. Food Chem. Toxicol. 2021, 149, 112029. [Google Scholar] [CrossRef]
- Wang, Z.; Mou, X.; Yang, G.; Li, J.; Liu, D. Effects of dietary nutrient levels on growth performance and blood biochemical indexes of northeastern meat geese (1–28 days of age). Nucl. Agron. Lett. 2009, 23, 891–897. [Google Scholar]
- Gu, M.; Zhang, J.; Li, G.; Liu, X. Changes in blood lipid levels in patients with liver disease. Exam. Med. 2008, 447–449. [Google Scholar]
- Peng, Y. Treatment of hyperlipoproteinemia with ginkgo lipid-lowering drink in 40 cases. Jiangxi J. Tradit. Chin. Med. 2014, 45, 35–37. [Google Scholar]
- Wang, G.; Liu, Z.; Li, M.; Li, Y.; Alvi, S.S.; Ansari, I.A.; Khan, M.S. Ginkgolide B Mediated Alleviation of Inflammatory Cascades and Altered Lipid Metabolism in HUVECs via Targeting PCSK-9 Expression and Functionality. BioMed Res. Int. 2019, 2019, 7284767. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.-M.; Wang, X.-J. Effects of Ginkgo biloba extract on growth performance and lipid metabolism of broiler chicks. Feed. Expo. 2006, 6–9. [Google Scholar]
- Sun, P.-M. Heat Stress Injury and Heat Shock Protein 70 Expression in Broiler Chickens. Doctoral Dissertation, Nanjing Agricultural University, Nanjing, China, 2006. [Google Scholar]
- Yang, C.; Xiao, X.; Luo, P.; Deng, Z.; Su, M.; Chen, S.; Fu, X.; Cao, L.; Liu, W. Effects of acute heat stress on the expression of NLRP3 inflammatory vesicle gene in goose tissues. Zhongkai Agric. Eng. Coll. J. 2021, 34, 1–5+11. [Google Scholar]
- Yang, H.; Gao, R. Progress in the study of medicinal components and pharmacological effects of Ginkgo biloba. Anim. Med. 2017, 38, 96–99. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Hu, X.-Y.; Yang, J.-C.; Ding, X.W.; Wang, Y.; Song, G. Effect of heat stress on the gene expression of hepatic AMPKα1 and fat metabolism-related molecules in broiler chickens. Anim. Husb. Vet. Sci. Lett. 2018, 49, 102–110. [Google Scholar]
- Richards, M.P.; Proszkowiec-Weglarz, M. Mechanisms Regulating Feed Intake, Energy Expenditure, and Body Weight in Poultry12. Poult. Sci. 2007, 86, 1478–1490. [Google Scholar] [CrossRef]
- Liang, K.; Lin, Y.; Yu, Y.; Wang, Y.; Zhu, J. Clonal expression of CPT1A gene and correlation analysis of intramuscular fat content in goats. North China Agric. 2019, 34, 231–238. [Google Scholar]
- Tobita, H.; Sato, S.; Yazaki, T.; Mishiro, T.; Ishimura, N.; Ishihara, S.; Kinoshita, Y. Alogliptin alleviates hepatic steatosis in a mouse model of nonalcoholic fatty liver disease by promoting CPT1a expression via Thr172 phosphorylation of AMPKα in the liver. Mol. Med. Rep. 2018, 17, 6840–6846. [Google Scholar] [CrossRef]
- Schindler, M.; Pendzialek, M.; Grybel, K.J.; Seeling, T.; Gürke, J.; Fischer, B.; Navarrete Santos, A. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum. Reprod. 2017, 32, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Kellner, T.A.; Baumgard, L.H.; Prusa, K.J.; Gabler, N.K.; Patience, J.F. Does heat stress alter the pig’s response to dietary fat? J. Anim. Sci. 2016, 94, 4688–4703. [Google Scholar] [CrossRef] [PubMed]

| Raw Material | Content (%) |
|---|---|
| Soybean Meal | 15–30 |
| Maize | 60–65 |
| Stone Powder | 1–5 |
| Talc | 0.8–1 |
| Calcium Bicarbonate | 0.5–1 |
| Composite Enzyme | 0.03–0.05 |
| Vitamin Complex Premix | 1.5–2 |
| Amino Acids | 1.5–2 |
| Crude Protein ≥ | 21.0 |
| Total Phosphorus ≥ | 0.5 |
| Methionine ≥ | 0.3–0.8 |
| Crude Fiber ≤ | 5.0 |
| Crude Ash ≤ | 8.0 |
| Moisture ≤ | 14.0 |
| Edible Salt | 0.3–0.8 |
| Soybean Meal | 15–30 |
| Maize | 60–65 |
| Genetics | Sequences (5′-3′) | Number |
|---|---|---|
| β-actin F | CCGCTCTATGAAGGCTACGC | 20 |
| β-actin R | CTCTCGGCTGTGGTGGTGAA | 20 |
| AMPK F | ATCTGTCTCGCCCTCATCCT | 20 |
| AMPK R | CCACTTCGCTCTTCTTACACCTT | 23 |
| SREBP1-c F | GAGACCATCTACAGCTCCGC | 20 |
| SREBP1-c R | CATCCGAAAAGCACCCCTCT | 20 |
| ACC F | TCCAGCAGAACCGCATT | 17 |
| ACC R | GTATGAGCAGGCAGGACTT | 19 |
| LKB1 F | AGACTCTGGTGCCCATACCT | 20 |
| LKB1 R | CTCAGGCACCTGTCCTGGTA | 20 |
| HMGCR F | GCGGCAGATTTGCTGACTG | 19 |
| HMGCR R | TGGGCACTCATAGTTCCAGC | 20 |
| CPT1A F | GCATTGACCGCCATCTGTTC | 20 |
| CPT1A R | CAGGTCCAAATCCACCACCA | 20 |
| HSP70 F | ACAGTGCCCGCTTACTTCAA | 20 |
| HSP70 R | ACACATCAAAAGTGCCCCCT | 20 |
| HSP90 F | GGACCAACCAATGGAGGAGG | 20 |
| GROUPS | ||||||
|---|---|---|---|---|---|---|
| CON | HS | TP | GBE100 | GBE300 | GBE600 | |
| 21 Weight (G/Only) | 859.4 ± 20.12 | 869.6 ± 29.71 | 871 ± 25.3 | 865 ± 34.69 | 872.8 ± 23.44 | 887.2 ± 27.69 |
| 42 Weight (G/Only) | 2556.4 ± 20.12 a | 1936 ± 29.71 d | 2187.4 ± 25.3 c | 2210.2 ± 34.69 c | 2421.8 ± 23.44 b | 2508.4 ± 27.69 a |
| ADG (G/D) | 80.81 ± 3.38 a | 50.78 ± 3.02 c | 62.69 ± 4.5 b | 64.01 ± 4.26 b | 68.24 ± 4.81 b | 78.57 ± 2.47 a |
| ADFI (G/D) | 86.66 ± 1.13 a | 67.78 ± 1.5 e | 72.16 ± 0.69 d | 73.21 ± 1.42 d | 76.71 ± 1.4 c | 82.68 ± 0.95 ab |
| (F/G) | 1.07 ± 0.14 d | 1.33 ± 0.03 a | 1.15 ± 0.11 b | 1.14 ± 0.02 bc | 1.12 ± 0.02 c | 1.05 ± 0.12 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Chen, H.; Wu, X.; Dong, H.; Feng, S.; Tie, Y.; Zhao, Z.; Si, L. Mechanism of GBE Combined with TP on the Effect of AMPK/SREBP-1C/ACC Pathway on Lipid Metabolism in Heat-Stressed Broiler Liver. Vet. Sci. 2025, 12, 424. https://doi.org/10.3390/vetsci12050424
Zhou C, Chen H, Wu X, Dong H, Feng S, Tie Y, Zhao Z, Si L. Mechanism of GBE Combined with TP on the Effect of AMPK/SREBP-1C/ACC Pathway on Lipid Metabolism in Heat-Stressed Broiler Liver. Veterinary Sciences. 2025; 12(5):424. https://doi.org/10.3390/vetsci12050424
Chicago/Turabian StyleZhou, Chenyang, Haoxiang Chen, Xingyue Wu, Huili Dong, Siliang Feng, Yajin Tie, Zhanqin Zhao, and Lifang Si. 2025. "Mechanism of GBE Combined with TP on the Effect of AMPK/SREBP-1C/ACC Pathway on Lipid Metabolism in Heat-Stressed Broiler Liver" Veterinary Sciences 12, no. 5: 424. https://doi.org/10.3390/vetsci12050424
APA StyleZhou, C., Chen, H., Wu, X., Dong, H., Feng, S., Tie, Y., Zhao, Z., & Si, L. (2025). Mechanism of GBE Combined with TP on the Effect of AMPK/SREBP-1C/ACC Pathway on Lipid Metabolism in Heat-Stressed Broiler Liver. Veterinary Sciences, 12(5), 424. https://doi.org/10.3390/vetsci12050424






