Identification and Characterization of Viral and Bacterial Pathogens in Free-Living Bats of Kopaonik National Park, Serbia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Bat Pathogen Analyses
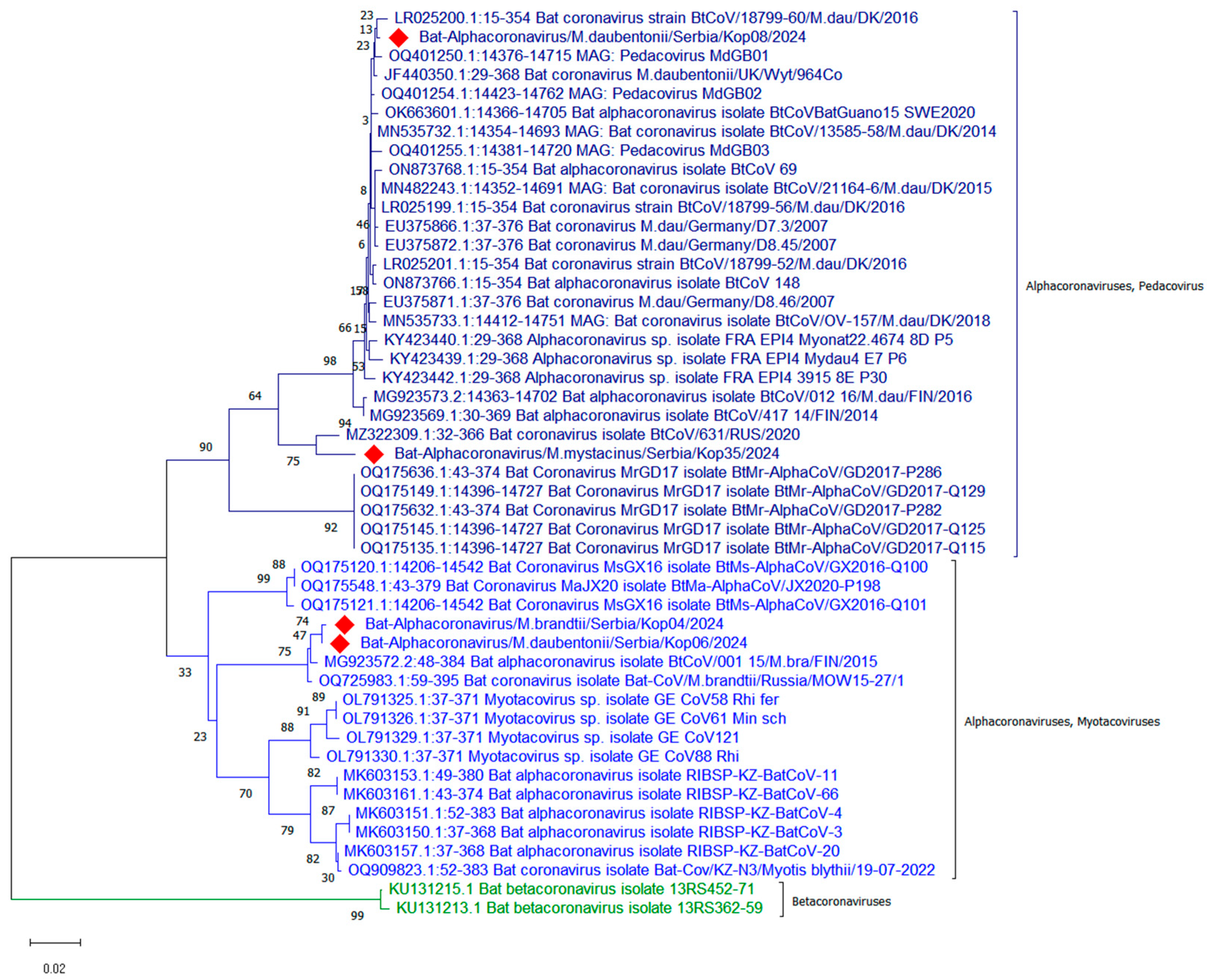
3. Results
4. Discussion
- Coronaviruses
- Lyssaviruses, Filoviruses
- Mycoplasma spp.
- Rickettsia spp.
- Other potentially pathogenic bacteria in bats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, R.; Debnath, C.; Samanta, I.; Barua, R.; Singh, A.D. Ecology of Bats and Their Role in Emerging Zoonotic Diseases: A Review. Rev. Sci. Tech. OIE 2020, 39, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.B.; Cirranello, A.L. Bat Species of the World: A Taxonomic and Geographic Database. Version 1.6. Available online: https://batnames.org/home.html (accessed on 8 December 2024).
- Fenton, M.B.; Simmons, N.B. Bats: A World of Science and Mystery; University of Chicago Press: Chicago, IL, USA, 2015; ISBN 978-0-226-06512-0. [Google Scholar]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important Reservoir Hosts of Emerging Viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Wang, L.-F.; Walker, P.J.; Poon, L.L.M. Mass Extinctions, Biodiversity and Mitochondrial Function: Are Bats ‘Special’ as Reservoirs for Emerging Viruses? Curr. Opin. Virol. 2011, 1, 649–657. [Google Scholar] [CrossRef]
- Young, C.C.W.; Olival, K.J. Optimizing Viral Discovery in Bats. PLoS ONE 2016, 11, e0149237. [Google Scholar] [CrossRef] [PubMed]
- Carini, A. Sur une grande epizootie de rage. Ann. L’inst. Pasteur 1911, 25, 843–846. [Google Scholar]
- Farina, L.L.; Lankton, J.S. Chiroptera. In Pathology of Wildlife and Zoo Animals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 607–633. ISBN 978-0-12-805306-5. [Google Scholar]
- Kohl, C.; Nitsche, A.; Kurth, A. Update on Potentially Zoonotic Viruses of European Bats. Vaccines 2021, 9, 690. [Google Scholar] [CrossRef]
- Chomel, B.B.; Boulouis, H.-J.; Chang, C.; Setién, A.A.; Stuckey, M.J. Bat-Related Zoonoses. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–36. ISBN 978-3-030-85877-3. [Google Scholar]
- Hayman, D.T.S.; Bowen, R.A.; Cryan, P.M.; McCracken, G.F.; O’Shea, T.J.; Peel, A.J.; Gilbert, A.; Webb, C.T.; Wood, J.L.N. Ecology of Zoonotic Infectious Diseases in Bats: Current Knowledge and Future Directions. Zoonoses Public Health 2013, 60, 2–21. [Google Scholar] [CrossRef]
- Paunović, M.; Karapandža, B.; Budinski, I.; Stamenković, S. Bats (Mammalia, Chiroptera) of Serbia; Serbian Academy of Sciences and Arts: Beograd, Serbia, 2020; Volume 13, ISBN 978-86-7025-887-7. [Google Scholar]
- Dietz, C.; Kiefer, A. Bats of Britain and Europe; Bloomsbury Publishing: London, UK, 2016; ISBN 978-1-4729-2202-1. [Google Scholar]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration, and Serotyping of Salmonella 2017. International Organization for Standardization: Geneva, Switzerland, 2017.
- Marston, D.A.; Jennings, D.L.; MacLaren, N.C.; Dorey-Robinson, D.; Fooks, A.R.; Banyard, A.C.; McElhinney, L.M. Pan-Lyssavirus Real Time RT-PCR for Rabies Diagnosis. JoVE 2019, 149, 59709. [Google Scholar] [CrossRef]
- He, B.; Feng, Y.; Zhang, H.; Xu, L.; Yang, W.; Zhang, Y.; Li, X.; Tu, C. Filovirus RNA in Fruit Bats, China. Emerg. Infect. Dis. 2015, 21, 1675–1677. [Google Scholar] [CrossRef]
- Coertse, J.; Mortlock, M.; Grobbelaar, A.; Moolla, N.; Markotter, W.; Weyer, J. Development of a Pan-Filoviridae SYBR Green qPCR Assay for Biosurveillance Studies in Bats. Viruses 2023, 15, 987. [Google Scholar] [CrossRef]
- WOAH. Chapter 3.1.16 Nipah and Hendra Virus Diseases (Version Adopted in May 2022). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; WOAH: Paris, France, 2024. [Google Scholar]
- Holbrook, M.G.; Anthony, S.J.; Navarrete-Macias, I.; Bestebroer, T.; Munster, V.J.; Van Doremalen, N. Updated and Validated Pan-Coronavirus PCR Assay to Detect All Coronavirus Genera. Viruses 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Masangkay, J.S.; Nagata, N.; Morikawa, S.; Mizutani, T.; Fukushi, S.; Alviola, P.; Omatsu, T.; Ueda, N.; Iha, K.; et al. Bat Coronaviruses and Experimental Infection of Bats, the Philippines. Emerg. Infect. Dis. 2010, 16, 1217–1223. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Cultrera, R.; Seraceni, S.; Germani, R.; Contini, C. Molecular Evidence of Ureaplasma urealyticum and Ureaplasma parvum Colonization in Preterm Infants during Respiratory Distress Syndrome. BMC Infect. Dis. 2006, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Ehricht, R.; Slickers, P.; Goellner, S.; Hotzel, H.; Sachse, K. Optimized DNA Microarray Assay Allows Detection and Genotyping of Single PCR-Amplifiable Target Copies. Mol. Cell. Probes 2006, 20, 60–63. [Google Scholar] [CrossRef]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly Sensitive Real-Time PCR for Specific Detection and Quantification of Coxiella burnetii. BMC Microbiol. 2006, 6, 2. [Google Scholar] [CrossRef]
- Kato, C.Y.; Chung, I.H.; Robinson, L.K.; Austin, A.L.; Dasch, G.A.; Massung, R.F. Assessment of Real-Time PCR Assay for Detection of Rickettsia spp. and Rickettsia rickettsii in Banked Clinical Samples. J. Clin. Microbiol. 2013, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of Pathogenic Leptospira Spp. through TaqMan Polymerase Chain Reaction Targeting the LipL32 Gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, Evolution and Spillover of Coronaviruses from Bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- Hemnani, M.; Da Silva, P.G.; Thompson, G.; Poeta, P.; Rebelo, H.; Mesquita, J.R. Detection and Prevalence of Coronaviruses in European Bats: A Systematic Review. EcoHealth 2024, 21, 125–140. [Google Scholar] [CrossRef]
- Petrović, T.; Lupulović, D.; Paunović, M.; Vidanović, D.; Lazić, G.; Samojlović, M.; Lazić, S. Detection and Typing of Coronaviruses in Bats in Serbia. In Proceedings of the 12th International Congress for Veterinary Virology, Ghent, Belgium, 20–23 September 2022. [Google Scholar]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Lorusso, A. Novel Human Coronavirus (SARS-CoV-2): A Lesson from Animal Coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Korneenko, E.V.; Samoilov, A.E.; Chudinov, I.K.; Butenko, I.O.; Sonets, I.V.; Artyushin, I.V.; Yusefovich, A.P.; Kruskop, S.V.; Sinitsyn, S.O.; Klyuchnikova, E.O.; et al. Alphacoronaviruses from Bats Captured in European Russia in 2015 and 2021 Are Closely Related to Those of Northern Europe. Front. Ecol. Evol. 2024, 12, 1324605. [Google Scholar] [CrossRef]
- Ohlopkova, O.V.; Popov, I.V.; Popov, I.V.; Stolbunova, K.A.; Stepanyuk, M.A.; Moshkin, A.D.; Maslov, A.A.; Sobolev, I.A.; Malinovkin, A.V.; Tkacheva, E.V.; et al. Detection and Phylogenetic Analysis of Alphacoronaviruses in Bat Populations of Rostov and Novosibirsk Regions of Russia, 2021–2023. Microbiol. Res. 2024, 16, 3. [Google Scholar] [CrossRef]
- Corduneanu, A.; Zając, Z.; Kulisz, J.; Wozniak, A.; Foucault-Simonin, A.; Moutailler, S.; Wu-Chuang, A.; Peter, Á.; Sándor, A.D.; Cabezas-Cruz, A. Detection of Bacterial and Protozoan Pathogens in Individual Bats and Their Ectoparasites Using High-Throughput Microfluidic Real-Time PCR. Microbiol. Spectr. 2023, 11, e01531-23. [Google Scholar] [CrossRef]
- Nikolić, M. Pasteur’s Institutes in Serbia. Bull. Inst. Hyg. 1954, III, 53–72. [Google Scholar]
- Stankov, S.; Lalošević, D.; Fooks, A.R. History of Rabies Incidence and Rabies Control in Serbia in Support of the Zero by 2030 Campaign to Eliminate Dog-Mediated Human Rabies. Viruses 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Goletic, S.; Goletic, T.; Omeragic, J.; Supic, J.; Kapo, N.; Nicevic, M.; Skapur, V.; Rukavina, D.; Maksimovic, Z.; Softic, A.; et al. Metagenomic Sequencing of Lloviu Virus from Dead Schreiber’s Bats in Bosnia and Herzegovina. Microorganisms 2023, 11, 2892. [Google Scholar] [CrossRef]
- Kemenesi, G.; Tóth, G.E.; Mayora-Neto, M.; Scott, S.; Temperton, N.; Wright, E.; Mühlberger, E.; Hume, A.J.; Suder, E.L.; Zana, B.; et al. Isolation of Infectious Lloviu Virus from Schreiber’s Bats in Hungary. Nat. Commun. 2022, 13, 1706. [Google Scholar] [CrossRef]
- Szentivanyi, T.; McKee, C.; Jones, G.; Foster, J.T. Trends in Bacterial Pathogens of Bats: Global Distribution and Knowledge Gaps. Transbound. Emerg. Dis. 2023, 2023, 9285855. [Google Scholar] [CrossRef]
- Fritschi, J.; Marti, H.; Seth-Smith, H.M.B.; Aeby, S.; Greub, G.; Meli, M.L.; Hofmann-Lehmann, R.; Mühldorfer, K.; Stokar-Regenscheit, N.; Wiederkehr, D.; et al. Prevalence and Phylogeny of Chlamydiae and Hemotropic Mycoplasma Species in Captive and Free-Living Bats. BMC Microbiol. 2020, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, P.E.; Keel, M.K.; Yabsley, M.; Last, L.A.; Breitschwerdt, E.B.; Maggi, R.G. Hemotropic Mycoplasmas in Little Brown Bats (Myotis lucifugus). Parasites Vectors 2014, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Vengust, M.; Knapic, T.; Weese, J.S. The Fecal Bacterial Microbiota of Bats; Slovenia. PLoS ONE 2018, 13, e0196728. [Google Scholar] [CrossRef]
- Matei, I.A.; Corduneanu, A.; Sándor, A.D.; Ionică, A.M.; Panait, L.; Kalmár, Z.; Ivan, T.; Papuc, I.; Bouari, C.; Fit, N.; et al. Rickettsia Spp. in Bats of Romania: High Prevalence of Rickettsia Monacensis in Two Insectivorous Bat Species. Parasites Vectors 2021, 14, 107. [Google Scholar] [CrossRef]
- Mühldorfer, K. Bats and Bacterial Pathogens: A Review. Zoonoses Public Health 2013, 60, 93–103. [Google Scholar] [CrossRef]
- Allocati, N.; Petrucci, A.G.; Di Giovanni, P.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Bat-Man Disease Transmission: Zoonotic Pathogens from Wildlife Reservoirs to Human Populations. Cell Death Discov. 2016, 2, 16048. [Google Scholar] [CrossRef]
- Di Bella, S.; Giacchino, I.; Blanda, V.; Gucciardi, F.; Scibetta, S.; La Russa, F.; Lastra, A.; Purpari, G.; Grasso, R.; Spena, M.T.; et al. Zoonotic Bacteria and Vector-Borne Protozoa in Troglophilus Bat Colonies in Sicily (Southern Italy): A Biomolecular Survey. Animals 2025, 15, 488. [Google Scholar] [CrossRef]
- Verde, R.S.; Di Azevedo, M.I.N.; Dias, D.; Tavares de Freitas, T.P.; Carvalho-Costa, F.A.; Bonvicino, C.; Lilenbaum, W.; D’Andrea, P.S.; Medeiros, L.S. Bat-Associated Pathogenic Leptospira spp. from Forest Fragments in Southwestern Brazilian Amazonia. Transbound. Emerg. Dis. 2024, 2024, 6633866. [Google Scholar] [CrossRef]
| Assay | Primer/Probename | Primer/Probe Sequences |
|---|---|---|
| Lyssavirus [15] | JW12 | ATG TAA CAC CYC TAC AAT G |
| N165 | GCA GGG TAY TTR TAC TCA TA | |
| Filoviruses [16] | Filo-F | TGATATATGATCATCTTCCAGG |
| Filo-in-F | GCATTTCACCAATTAACACAGG | |
| Filo-R | TTTATATGAATCAGTGGAGGTG | |
| FV-F1 | GCMTTYCCIAGYAAYATGATGG | |
| FV-R1 | GTDATRCAYTGRTTRTCHCCCAT | |
| FV-F2 | TDCAYCARGCITCDTGGCAYC | |
| FV-R2 | GIGCACADGADATRCWIGTCC | |
| Filoviruses [17] | Filo-SYBR-F | GRGARTAYGCICCITTYGC |
| Filo-SYBR-R | AGYTGYTGRTAYTGYTCICC | |
| Hendra [18] | HeV M 5755F | CTTCGACAAAGACGGAACCAA |
| HeV M 5823R | CCAGCTCGTCGGACAAAATT | |
| Nipah [18] | NiV_N_1198F | TCAGCAGGAAGGCAAGAGAGTAA |
| NiV_N_1297R | CCCCTTCATCGATATCTTGATCA | |
| Pan-corona [19] | Pan_CoV_F1 | GGTTGGGAYTAYCCHAARTGYGA |
| Pan_CoV_R1 | CCRTCATCAGAHARWATCAT | |
| Pan_CoV_R2 | CCRTCATCACTHARWATCAT | |
| Pan_CoV_F2 | GAYTAYCCHAARTGTGAYAGA | |
| Pan_CoV_F3 | GAYTAYCCHAARTGTGAYMGH | |
| Coronavirus [20] | Watanabe conventional_F | GGTTGGGACTATCCTAAGTGTGA |
| Watanabe conventional_R | CCATCATCAGATAGAATCATCATA | |
| SARS-CoV2 [21] | SARS2-IP4-14059F | GGT AAC TGG TAT GAT TTC G |
| SARS2-IP4-14146R | CTG GTC AAG GTT AAT ATA GG | |
| SARS2-IP4-14084FAM | FAM-TCA TAC AAA CCA CGC CAG G-BHQ1 | |
| Mycoplasma [22] | GPO-1 | ACTCCTACGGGAGGCAGCAGTA |
| MGSO | TGCACCATCTGTCACTCTGTTAACCTC | |
| Chlamydiae [23] | Ch23S-F | CTGAAACCAGTAGCTTATAAGCGGT |
| Ch23S-R | ACCTCGCCGTTTAACTTAACTCC | |
| Ch23S-Pro | FAM-CTCATCATGCAAAAGGCACGCCG-BHQ1 | |
| Coxiella burnetii [24] | Cox-F | GTCTTAAGGTGGGCTGCGTG |
| Cox-R | CCCCGAATCTCATTGATCAGC | |
| Cox-TM | FAM-AGCGAACCATTGGTATCGGACGTT-TAMRA-TATGG | |
| Rickettsia [25] | PanR8_F | AGCTTGCTTTTGGATCATTTGG |
| PanR8_R | TTCCTTGCCTTTTCATACATCTAGT | |
| PanR8_P | FAM-CCTGCTTCTATTTGTCTTGCAGTAACACGCCA-BHQ1 | |
| Pathogenic Leptospira spp. [26] | LipL32-45F | AAGCATTACCGCTTGTGGTG |
| LipL32-286R | GAACTCCCATTTCAGCGATT | |
| LipL32-189P | FAM-AAAGCCAGGACAAGCGCCG-BHQ1 |
| Bat No. | Bat Species | Coronaviruses | Mycoplasma sp. | Rickettsia sp. |
|---|---|---|---|---|
| 1 | Rhinolophus ferrumequinum | − | + | − |
| 2 | Rhinolophus ferrumequinum | − | + | − |
| 3 | Rhinolophus hipposideros | − | − | − |
| 4 | Myotis brandtii | + | + | + |
| 5 | Myotis brandtii | − | − | − |
| 6 | Myotis daubentonii | + | + | − |
| 7 | Myotis cf. mystacinus | − | − | + |
| 8 | Myotis daubentonii | + | − | − |
| 9 | Barbastella barbastellus | − | − | + |
| 10 | Myotis blythii | − | + | − |
| 11 | Myotis myotis | − | + | − |
| 12 | Rhinolophus ferrumequinum | − | + | − |
| 13 | Myotis daubentonii | − | − | − |
| 14 | Myotis nattereri | − | − | − |
| 15 | Myotis alcathoe | − | − | − |
| 16 | Myotis cf. mystacinus | − | − | − |
| 17 | Myotis brandtii | − | − | + |
| 18 | Myotis daubentonii | − | − | − |
| 19 | Rhinolophus ferrumequinum | − | + | − |
| 20 | Myotis myotis | − | + | − |
| 21 | Plecotus auritus | − | − | + |
| 22 | Plecotus auritus | − | − | − |
| 23 | Plecotus auritus | − | − | − |
| 24 | Myotis emarginatus | − | + | − |
| 25 | Myotis emarginatus | − | + | − |
| 26 | Myotis daubentonii | − | − | − |
| 27 | Myotis cf. mystacinus | − | + | − |
| 28 | Myotis daubentonii | − | − | − |
| 29 | Plecotus auritus | − | + | − |
| 30 | Myotis myotis | − | − | − |
| 31 | Rhinolophus ferrumequinum | − | + | − |
| 32 | Plecotus auritus | − | + | − |
| 33 | Plecotus auritus | − | + | − |
| 34 | Rhinolophus ferrumequinum | − | + | − |
| 35 | Myotis cf. mystacinus | + | − | − |
| 36 | Myotis myotis | − | + | − |
| 37 | Plecotus auritus | − | − | − |
| 38 | Barbastella barbastellus | − | − | − |
| 39 | Myotis daubentonii | − | − | − |
| 40 | Rhinolophus hipposideros | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidanović, D.; Vasković, N.; Dmitrić, M.; Tešović, B.; Debeljak, M.; Stojanović, M.; Budinski, I. Identification and Characterization of Viral and Bacterial Pathogens in Free-Living Bats of Kopaonik National Park, Serbia. Vet. Sci. 2025, 12, 401. https://doi.org/10.3390/vetsci12050401
Vidanović D, Vasković N, Dmitrić M, Tešović B, Debeljak M, Stojanović M, Budinski I. Identification and Characterization of Viral and Bacterial Pathogens in Free-Living Bats of Kopaonik National Park, Serbia. Veterinary Sciences. 2025; 12(5):401. https://doi.org/10.3390/vetsci12050401
Chicago/Turabian StyleVidanović, Dejan, Nikola Vasković, Marko Dmitrić, Bojana Tešović, Mihailo Debeljak, Milovan Stojanović, and Ivana Budinski. 2025. "Identification and Characterization of Viral and Bacterial Pathogens in Free-Living Bats of Kopaonik National Park, Serbia" Veterinary Sciences 12, no. 5: 401. https://doi.org/10.3390/vetsci12050401
APA StyleVidanović, D., Vasković, N., Dmitrić, M., Tešović, B., Debeljak, M., Stojanović, M., & Budinski, I. (2025). Identification and Characterization of Viral and Bacterial Pathogens in Free-Living Bats of Kopaonik National Park, Serbia. Veterinary Sciences, 12(5), 401. https://doi.org/10.3390/vetsci12050401







