2002–2022 Quinolone Resistance in Escherichia coli of Swine in Mainland China: A Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Selection Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
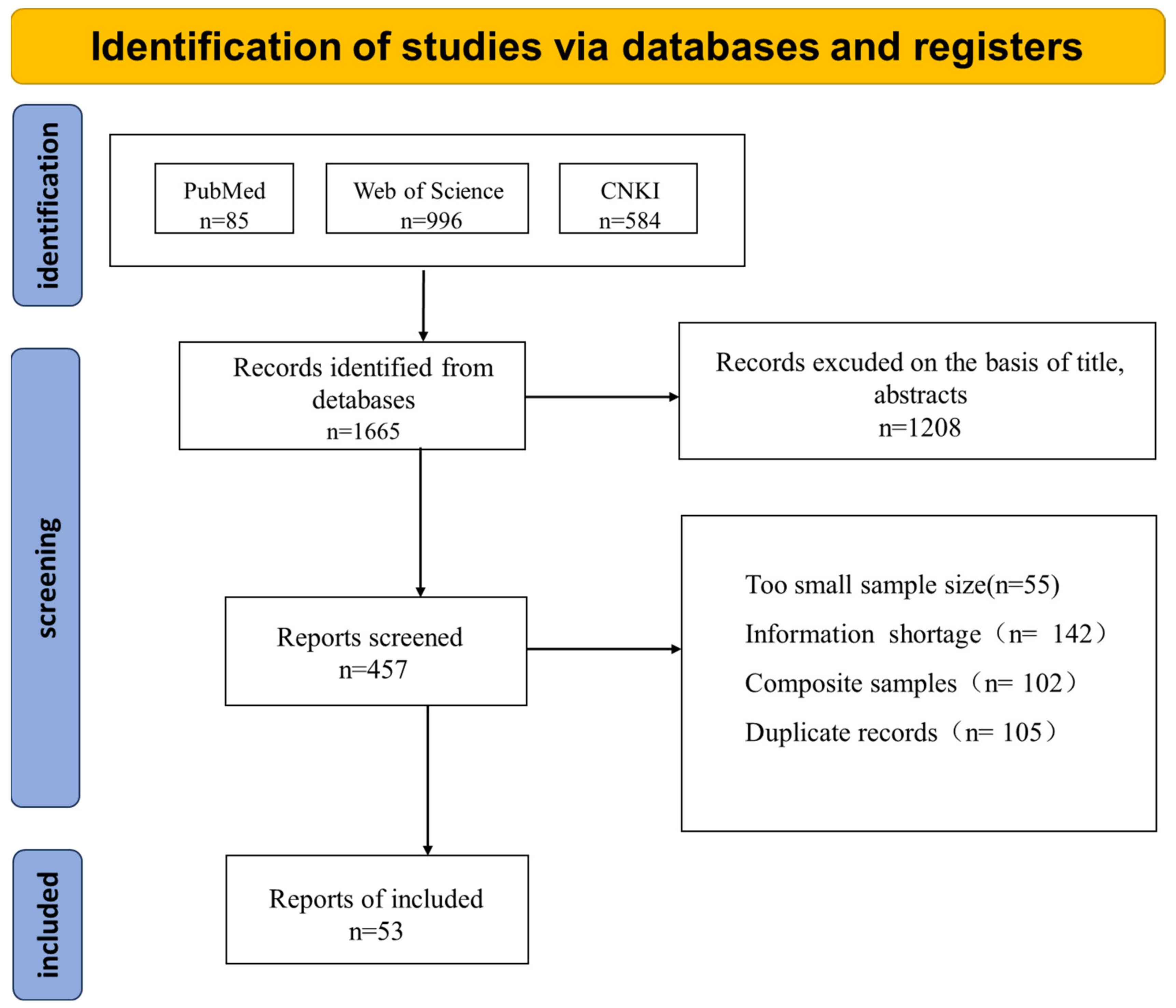
3.1. Study Screening
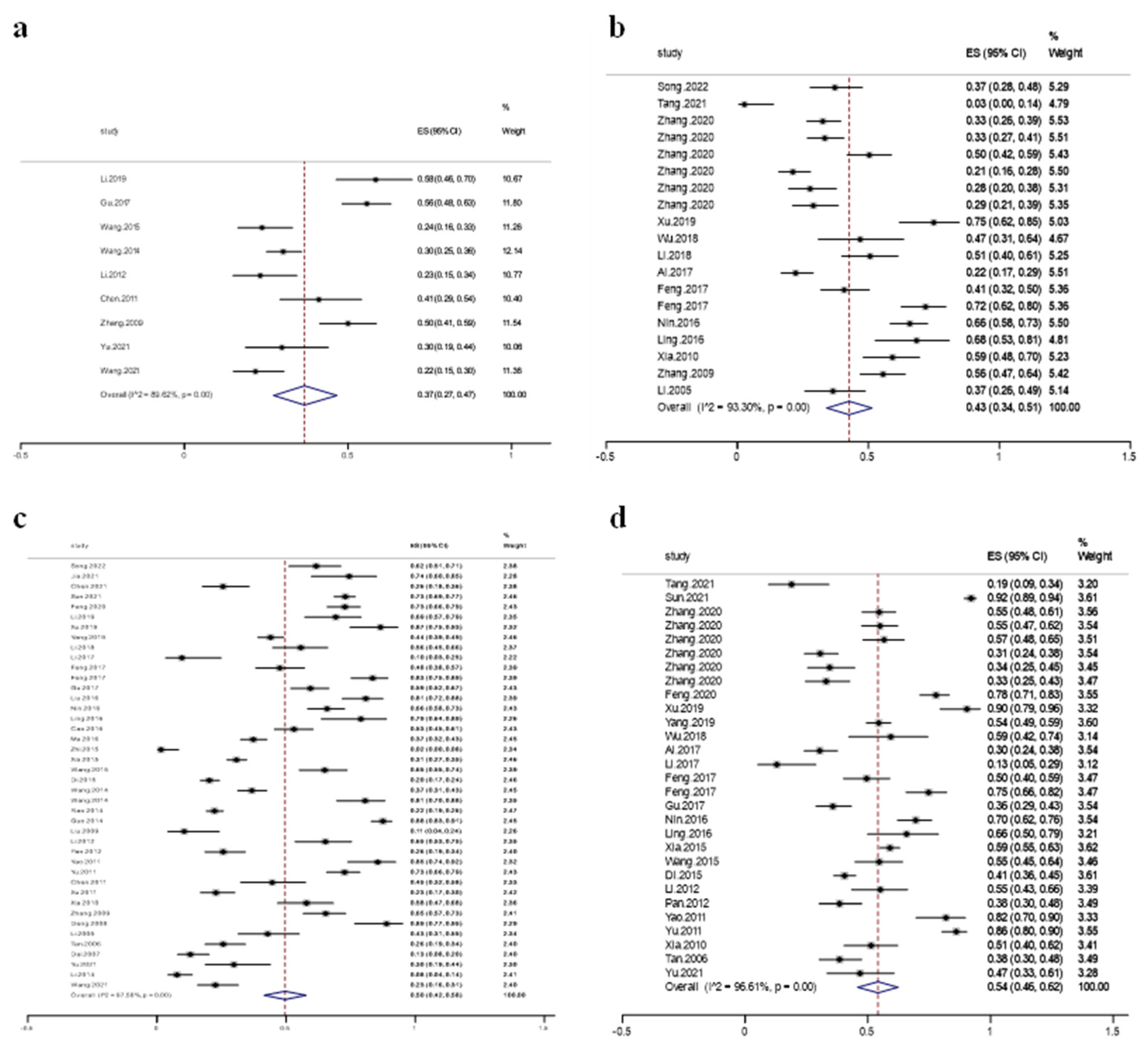
3.2. Quinolone Resistance in Swine Escherichia coli
3.3. Publication Bias
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Baum, H.; Marre, R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int. J. Med. Microbiol. 2005, 295, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.D.; Shome, R.; Bandopadhyay, S.; Geddam, S.; Kumar, A.M.P.; Murugesan, D.; Shome, A.; Shome, B.R. Genetic insights of antibiotic resistance, pathogenicity (virulence) and phylogenetic relationship of Escherichia coli strains isolated from livestock, poultry and their handlers—A one health snapshot. Mol. Biol. Rep. 2024, 51, 404. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Alonso, M.P.; Blanco, J.E.; Blanco, J.; Mora, A. Clones of enterotoxigenic and Shiga toxin-producing Escherichia coli implicated in swine enteric colibacillosis in Spain and rates of antibiotic resistance. Vet. Microbiol. 2021, 252, 108924. [Google Scholar] [CrossRef]
- Abubakar, R.H.; Madoroba, E.; Adebowale, O.; Fasanmi, O.G.; Fasina, F.O. Antimicrobial usage in pig production: Effects on Escherichia coli virulence profiles and antimicrobial resistance. Onderstepoort J. Vet. Res. 2019, 86, e1–e11. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y.; Li, F.; Li, X.; Liu, H.; Fazilani, S.A.; Guo, W.; Xu, G.; Zhang, X. The prevalence and mechanism of fluoroquinolone resistance in Escherichia coli isolated from swine farms in China. BMC Vet. Res. 2020, 16, 258. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, Z.; Zhang, C.; Song, L.; Wang, B.; Shang, J.; Yue, X.; Qu, Z.; Li, X.; Wu, L.; et al. Surveillance of antimicrobial resistance among Escherichia coli from chicken and swine, China, 2008-2015. Vet. Microbiol. 2017, 203, 49–55. [Google Scholar] [CrossRef]
- Belotindos, L.; Villanueva, M.; Miguel, J., Jr.; Bwalya, P.; Harada, T.; Kawahara, R.; Nakajima, C.; Mingala, C.; Suzuki, Y. Prevalence and Characterization of Quinolone-Resistance Determinants in Escherichia coli Isolated from Food-Producing Animals and Animal-Derived Food in the Philippines. Antibiotics 2021, 10, 413. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Frimodt-Møller, N.; Hasman, H.; Guardabassi, L.; Nielsen, L.; Aarestrup, F.M. Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb. Drug Resist. 2008, 14, 163–169. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, J.; Cheng, P.; Xiao, T.; Muhammad, I.; Yu, H.; Liu, R.; Zhang, X. Susceptibility breakpoint for Danofloxacin against swine Escherichia coli. BMC Vet. Res. 2019, 15, 51. [Google Scholar] [CrossRef]
- Seo, K.; Do, K.H.; Lee, W.K. Molecular characteristics of fluoroquinolone-resistant Escherichia coli isolated from suckling piglets with colibacillosis. BMC Microbiol. 2022, 22, 216. [Google Scholar] [CrossRef]
- Lei, T.; Tian, W.; He, L.; Huang, X.H.; Sun, Y.X.; Deng, Y.T.; Sun, Y.; Lv, D.H.; Wu, C.M.; Huang, L.Z.; et al. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. Vet. Microbiol. 2010, 146, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Shao, B.; Shen, J.; Wang, S.; Wu, Y. Plasmid-mediated quinolone resistance genes and antibiotic residues in wastewater and soil adjacent to swine feedlots: Potential transfer to agricultural lands. Environ. Health Perspect. 2012, 120, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Pierini, E.; Famiglini, G.; Mangani, F.; Cappiello, A. Fate of enrofloxacin in swine sewage. J. Agric. Food Chem. 2004, 52, 3473–3477. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Zhou, H. Occurrence of (fluoro)quinolones and (fluoro)quinolone resistance in soil receiving swine manure for 11 years. Sci. Total Environ. 2015, 530–531, 191–197. [Google Scholar] [CrossRef]
- Wu, B.; Qi, Q.; Zhang, X.; Cai, Y.; Yu, G.; Lv, J.; Gao, L.; Wei, L.; Chai, T. Dissemination of Escherichia coli carrying plasmid-mediated quinolone resistance (PMQR) genes from swine farms to surroundings. Sci. Total Environ. 2019, 665, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.E.; Fox, J.T.; Reinstein, S.L.; Nagaraja, T.G. Antimicrobial susceptibility of foodborne pathogens in organic or natural production systems: An overview. Foodborne Pathog. Dis. 2008, 5, 721–730. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Ma, L.; Liu, J.; An, Q.; Zhang, C.; Yin, G.; Cao, Z.; Pan, H. Comparative Analyses of Antibiotic Resistance Genes in Jejunum Microbiota of Pigs in Different Areas. Front. Cell. Infect. Microbiol. 2022, 12, 887428. [Google Scholar] [CrossRef]
- Lugsomya, K.; Yindee, J.; Niyomtham, W.; Tribuddharat, C.; Tummaruk, P.; Hampson, D.J.; Prapasarakul, N. Antimicrobial Resistance in Commensal Escherichia coli Isolated from Pigs and Pork Derived from Farms Either Routinely Using or Not Using In-Feed Antimicrobials. Microb. Drug Resist. 2018, 24, 1054–1066. [Google Scholar] [CrossRef]
- Niu, K.; Zhong, J.; Hu, X. Impacts of climate change-induced heat stress on pig productivity in China. Sci. Total Environ. 2024, 908, 168215. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Wang, Y.; Han, Y. Using Freeman-Tukey Double Arcsine Transformation in Meta-analysis of Single Proportions. Aesthetic Plast. Surg. 2023, 47, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, H.; Zhang, X.; Hong, X.; Zhang, T. Prevalence and Risk Factors Associated with Feline Infectious Peritonitis (FIP) in Mainland China between 2008 and 2023: A Systematic Review and Meta-Analysis. Animals 2024, 14, 1220. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef]
- Thorlund, K.; Imberger, G.; Johnston, B.C.; Walsh, M.; Awad, T.; Thabane, L.; Gluud, C.; Devereaux, P.J.; Wetterslev, J. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 2012, 7, e39471. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Comparison of four heterogeneity measures for meta-analysis. J. Eval. Clin. Pract. 2020, 26, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.R. When should meta-analysis avoid making hidden normality assumptions? Biom. J. 2018, 60, 1040–1058. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development of the People’s Republic of China. Ministry of Agriculture of the People’s Republic of China Announcement No. 2292. Available online: https://www.moa.gov.cn/nybgb/2015/jiuqi/201712/t20171219_6103873.htm (accessed on 10 February 2023).
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef]
- Spineli, L.M.; Pandis, N. Exploring heterogeneity in meta-analysis: Subgroup analysis. Part 1. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 302–304.e301. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gong, J.; Butaye, P.; Lu, G.; Huang, K.; Zhu, G.; Zhang, J.; Hathcock, T.; Cheng, D.; Wang, C. CRISPR/Cas9/sgRNA-mediated targeted gene modification confirms the cause-effect relationship between gyrA mutation and quinolone resistance in Escherichia coli. FEMS Microbiol. Lett. 2018, 365, fny127. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, Y.; Jiang, J.; Wang, X.; Shao, D.; Lam, M.M.C.; Holt, K.E.; Shao, B.; Wu, C.; Shen, J.; et al. Distinct increase in antimicrobial resistance genes among Escherichia coli during 50 years of antimicrobial use in livestock production in China. Nat. Food 2022, 3, 197–205. [Google Scholar] [CrossRef]
- Amsler, M.; Zurfluh, K.; Hartnack, S.; Sidler, X.; Stephan, R.; Kümmerlen, D. Occurrence of Escherichia coli non-susceptible to quinolones in faecal samples from fluoroquinolone-treated, contact and control pigs of different ages from 24 Swiss pig farms. Porc. Health Manag. 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z.; Chen, S.; Deng, Y.; Liu, Y.; Tian, W.; Huang, X.; Wu, C.; Sun, Y.; Sun, Y.; et al. Prevalence and dissemination of oqxAB in Escherichia coli isolates from animals, farmworkers, and the environment. Antimicrob. Agents Chemother. 2010, 54, 4219–4224. [Google Scholar] [CrossRef]
- Xia, L.N.; Li, L.; Wu, C.M.; Liu, Y.Q.; Tao, X.Q.; Dai, L.; Qi, Y.H.; Lu, L.M.; Shen, J.Z. A survey of plasmid-mediated fluoroquinolone resistance genes from Escherichia coli isolates and their dissemination in Shandong, China. Foodborne Pathog. Dis. 2010, 7, 207–215. [Google Scholar] [CrossRef]
- Tang, X.; Tan, C.; Zhang, X.; Zhao, Z.; Xia, X.; Wu, B.; Guo, A.; Zhou, R.; Chen, H. Antimicrobial resistances of extraintestinal pathogenic Escherichia coli isolates from swine in China. Microb. Pathog. 2011, 50, 207–212. [Google Scholar] [CrossRef]
- Mitra, S.; Mukherjee, S.; Naha, S.; Chattopadhyay, P.; Dutta, S.; Basu, S. Evaluation of co-transfer of plasmid-mediated fluoroquinolone resistance genes and bla(NDM) gene in Enterobacteriaceae causing neonatal septicaemia. Antimicrob. Resist. Infect. Control 2019, 8, 46. [Google Scholar] [CrossRef]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 2005, 56, 463–469. [Google Scholar] [CrossRef]
- Jiang, H.X.; Lü, D.H.; Chen, Z.L.; Wang, X.M.; Chen, J.R.; Liu, Y.H.; Liao, X.P.; Liu, J.H.; Zeng, Z.L. High prevalence and widespread distribution of multi-resistant Escherichia coli isolates in pigs and poultry in China. Vet. J. 2011, 187, 99–103. [Google Scholar] [CrossRef]
- Kindle, P.; Zurfluh, K.; Nüesch-Inderbinen, M.; von Ah, S.; Sidler, X.; Stephan, R.; Kümmerlen, D. Phenotypic and genotypic characteristics of Escherichia coli with non-susceptibility to quinolones isolated from environmental samples on pig farms. Porc. Health Manag. 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Boothe, D.M.; Thungrat, K.; Aly, S. Mechanisms accounting for fluoroquinolone multidrug resistance Escherichia coli isolated from companion animals. Vet. Microbiol. 2012, 161, 159–168. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Tiseo, K.; Pires, J.; Criscuolo, N.G.; Van Boeckel, T.P. Geographically targeted surveillance of livestock could help prioritize intervention against antimicrobial resistance in China. Nat. Food 2021, 2, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hu, Z.; Li, Z.; Zhang, X.; Jia, C.; Li, T.; Dai, M.; Tan, C.; Xu, Z.; Wu, B.; et al. Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat. Commun. 2022, 13, 1116. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development of the People's Republic of China. Report on the Use of Veterinary Antimicrobials in China in 2020. Available online: https://www.moa.gov.cn/gk/sygb/202111/P020211104353940540082.pdf (accessed on 10 February 2023).
- Chen, Y.H.; Du, L.; Geng, X.Y.; Liu, G.J. Implement meta-analysis with non-comparative binary data in RevMan software. Chin. J. Evid.-Based Med. 2014, 14, 889–896. [Google Scholar] [CrossRef]
- Österberg, J.; Wingstrand, A.; Nygaard Jensen, A.; Kerouanton, A.; Cibin, V.; Barco, L.; Denis, M.; Aabo, S.; Bengtsson, B. Antibiotic Resistance in Escherichia coli from Pigs in Organic and Conventional Farming in Four European Countries. PLoS ONE 2016, 11, e0157049. [Google Scholar] [CrossRef]
- Mathew, A.G.; Saxton, A.M.; Upchurch, W.G.; Chattin, S.E. Multiple antibiotic resistance patterns of Escherichia coli isolates from swine farms. Appl. Environ. Microbiol. 1999, 65, 2770–2772. [Google Scholar] [CrossRef]
- Mathew, A.G.; Upchurch, W.G.; Chattin, S.E. Incidence of antibiotic resistance in fecal Escherichia coli isolated from commercial swine farms. J. Anim. Sci. 1998, 76, 429–434. [Google Scholar] [CrossRef]

| Subgroups | No. of Study | Resistance Rate (95% CI) | Heterogeneity Test | ||
|---|---|---|---|---|---|
| p | I2 | ||||
| Area | North China | 2 | 0.55 (0.47, 0.62) | NA | NA |
| East China | 4 | 0.54 (0.42, 0.66) | 0.00 | 89.28% | |
| South China | 4 | 0.68 (0.53, 0.80) | 0.00 | 88.08% | |
| Central China | 13 | 0.47 (0.34, 0.59) | 0.00 | 95.75% | |
| Northeast China | 1 | 0.82 (0.70, 0.90) | NA | NA | |
| Northwest China | 3 | 0.66 (0.33, 0.92) | NA | NA | |
| Southwestern China | 2 | 0.38 (0.32, 0.45) | NA | NA | |
| method | dilution method | 15 | 0.49 (0.37, 0.61) | 0.00 | 97.66% |
| disk diffusion method | 14 | 0.60 (0.48, 0.70) | 0.00 | 94.18% | |
| time | after 2018 | 26 | 0.54 (0.48, 0.61) | 0.00 | 94.27% |
| before 2018 | 3 | 0.50 (0.05, 0.96) | NA | NA | |
| Subgroups | No. of Study | Resistance Rate (95% CI) | Heterogeneity Test | ||
|---|---|---|---|---|---|
| p | I2 | ||||
| Area | North China | 3 | 0.46 (0.16, 0.78) | NA | NA |
| East China | 7 | 0.51 (0.36, 0.66) | 0.00 | 95.53% | |
| South China | 10 | 0.58 (0.35, 0.79) | 0.00 | 98.57% | |
| Central China | 10 | 0.51 (0.33, 0.69) | 0.00 | 96.58% | |
| Northeast China | 2 | 0.45 (0.40, 0.51) | NA | NA | |
| Northwest China | 5 | 0.34 (0.16, 0.55) | 0.00 | 98.86% | |
| Southwestern China | 5 | 0.42 (0.20, 0.66) | 0.00 | 97.28% | |
| method | dilution method | 30 | 0.48 (0.31, 0.64) | 0.00 | 98.79% |
| disk diffusion method | 12 | 0.50 (0.42, 0.59) | 0.00 | 96.25% | |
| time | after 2018 | 5 | 0.51 (0.27, 0.75) | 0.00 | 97.22% |
| before 2018 | 37 | 0.50 (0.42, 0.58) | 0.00 | 97.57% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Liu, S.; Kang, R.; Sun, Y.; Tian, M.; Zhao, L.; Lei, C.; Wang, H.; Yang, X. 2002–2022 Quinolone Resistance in Escherichia coli of Swine in Mainland China: A Meta-Analysis. Vet. Sci. 2025, 12, 345. https://doi.org/10.3390/vetsci12040345
Long X, Liu S, Kang R, Sun Y, Tian M, Zhao L, Lei C, Wang H, Yang X. 2002–2022 Quinolone Resistance in Escherichia coli of Swine in Mainland China: A Meta-Analysis. Veterinary Sciences. 2025; 12(4):345. https://doi.org/10.3390/vetsci12040345
Chicago/Turabian StyleLong, Xuelin, Shujun Liu, Runmin Kang, Yue Sun, Mingyue Tian, Lijun Zhao, Changwei Lei, Hongning Wang, and Xin Yang. 2025. "2002–2022 Quinolone Resistance in Escherichia coli of Swine in Mainland China: A Meta-Analysis" Veterinary Sciences 12, no. 4: 345. https://doi.org/10.3390/vetsci12040345
APA StyleLong, X., Liu, S., Kang, R., Sun, Y., Tian, M., Zhao, L., Lei, C., Wang, H., & Yang, X. (2025). 2002–2022 Quinolone Resistance in Escherichia coli of Swine in Mainland China: A Meta-Analysis. Veterinary Sciences, 12(4), 345. https://doi.org/10.3390/vetsci12040345









