Effects of Oligomeric Proanthocyanidins on Cadmium-Induced Extracellular Matrix Damage via Inhibiting the ERK1/2 Signaling Pathway in Chicken Chondrocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. SPF Grade Eggs
2.3. Isolation of Chondrocytes in Chicken Embryo Joints
2.4. Identification of Chondrocytes
2.5. Measurement of Cell Proliferation of Chondrocytes
2.6. ELISA Analysis
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
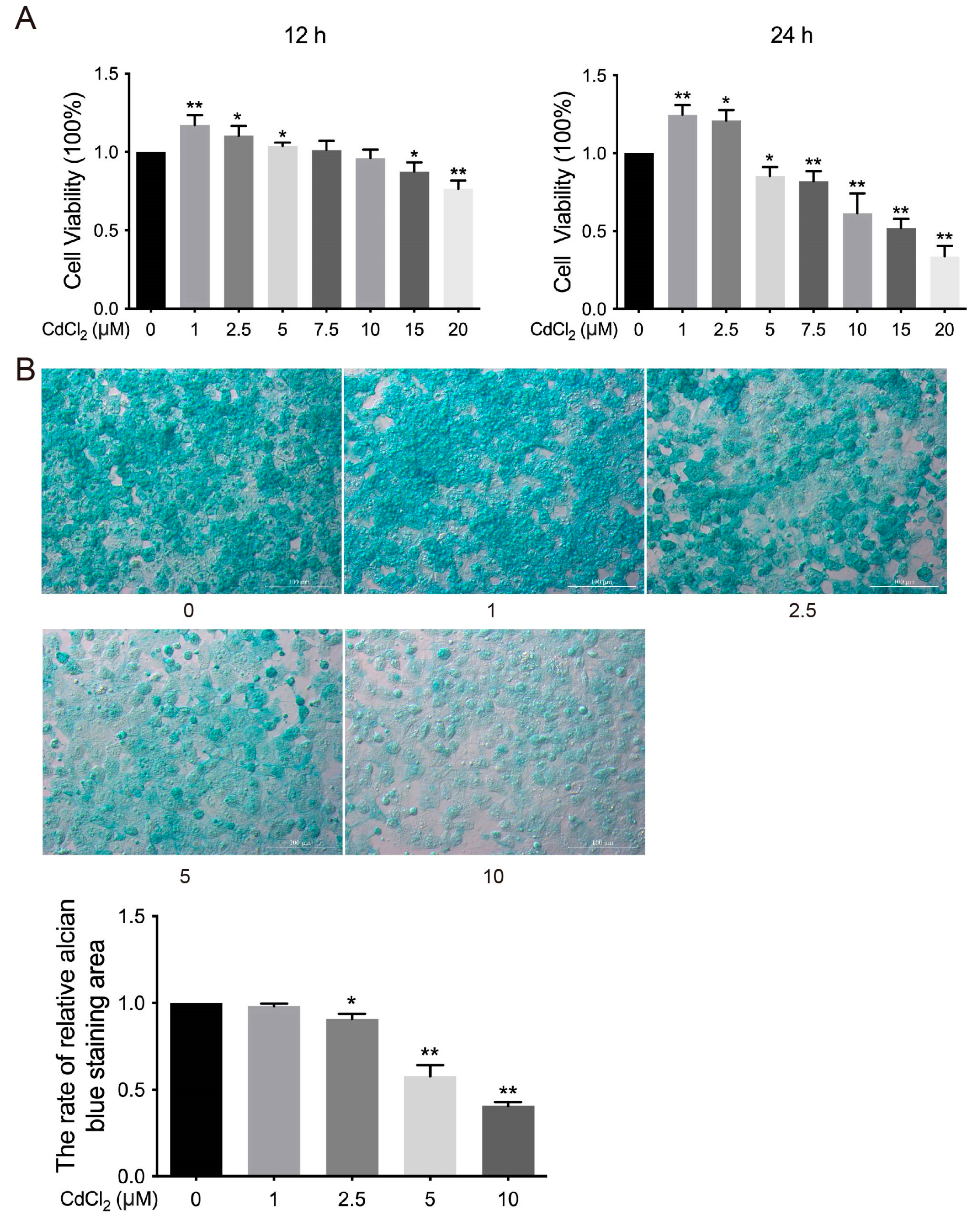
3.1. Effects of Cd on the Viability of Chondrocytes in Chickens In Vitro
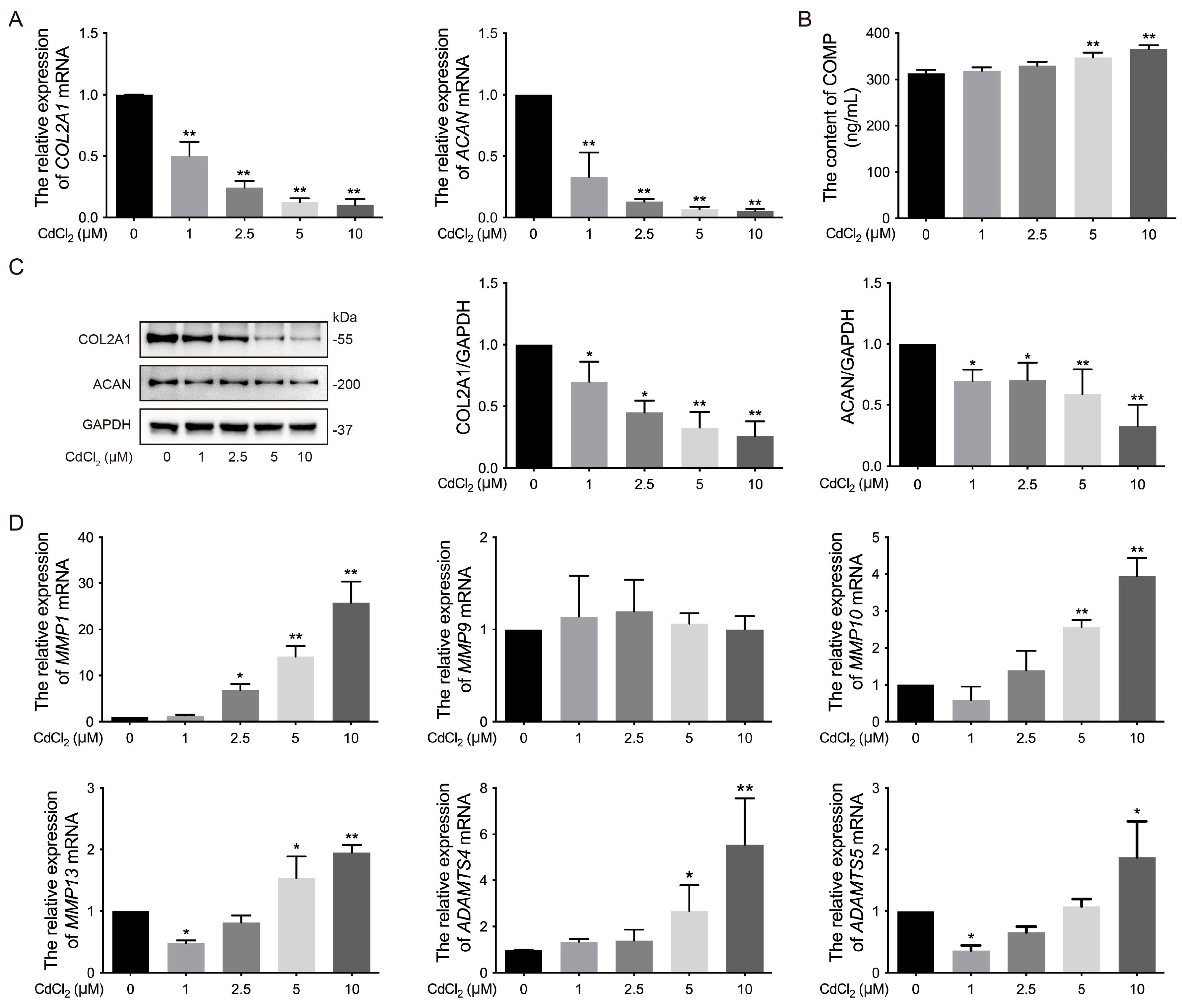
3.2. Effects of Cd on the ECM of Chondrocytes in Chickens In Vitro
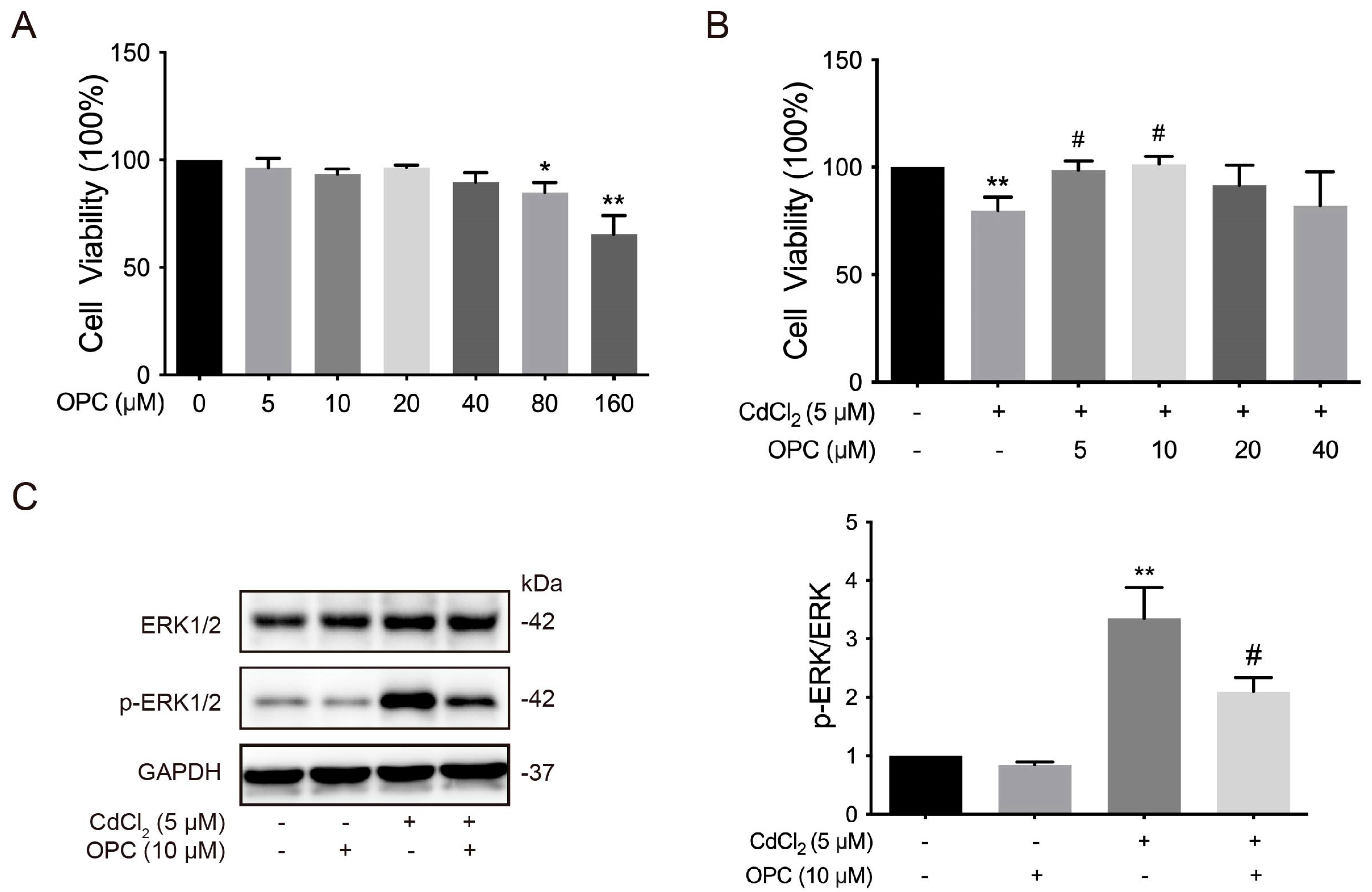
3.3. Effects of the ERK Signaling Pathway on Cd-Induced Phosphorylation of ERK1/2 of Chondrocytes in Chickens In Vitro
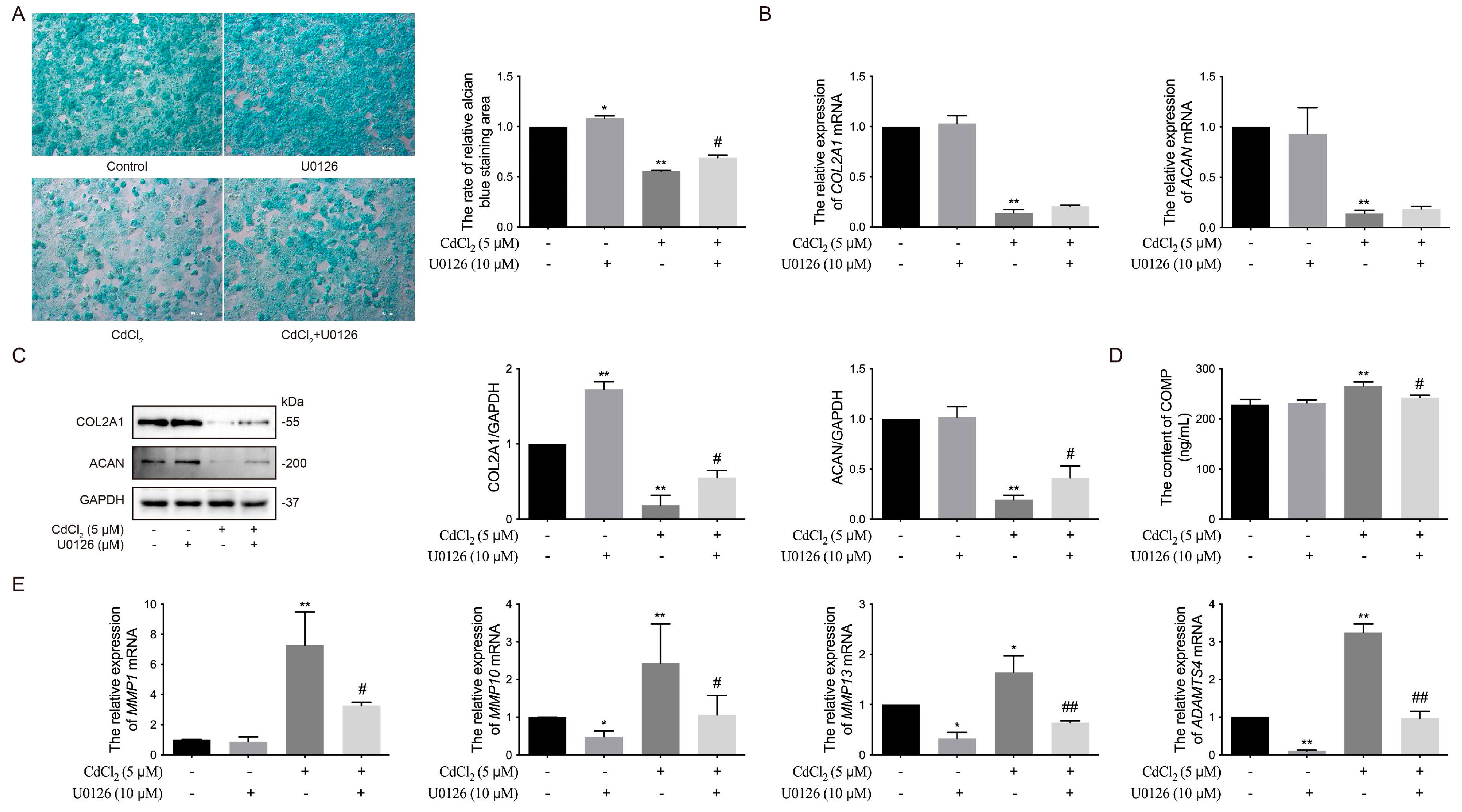
3.4. Effects of the ERK Signaling Pathway on Cd-Induced ECM Damage in Chicken Chondrocytes In Vitro
3.5. Effects of OPCs on the Cd-Induced ERK Signaling Pathway of Chondrocytes in Chickens In Vitro
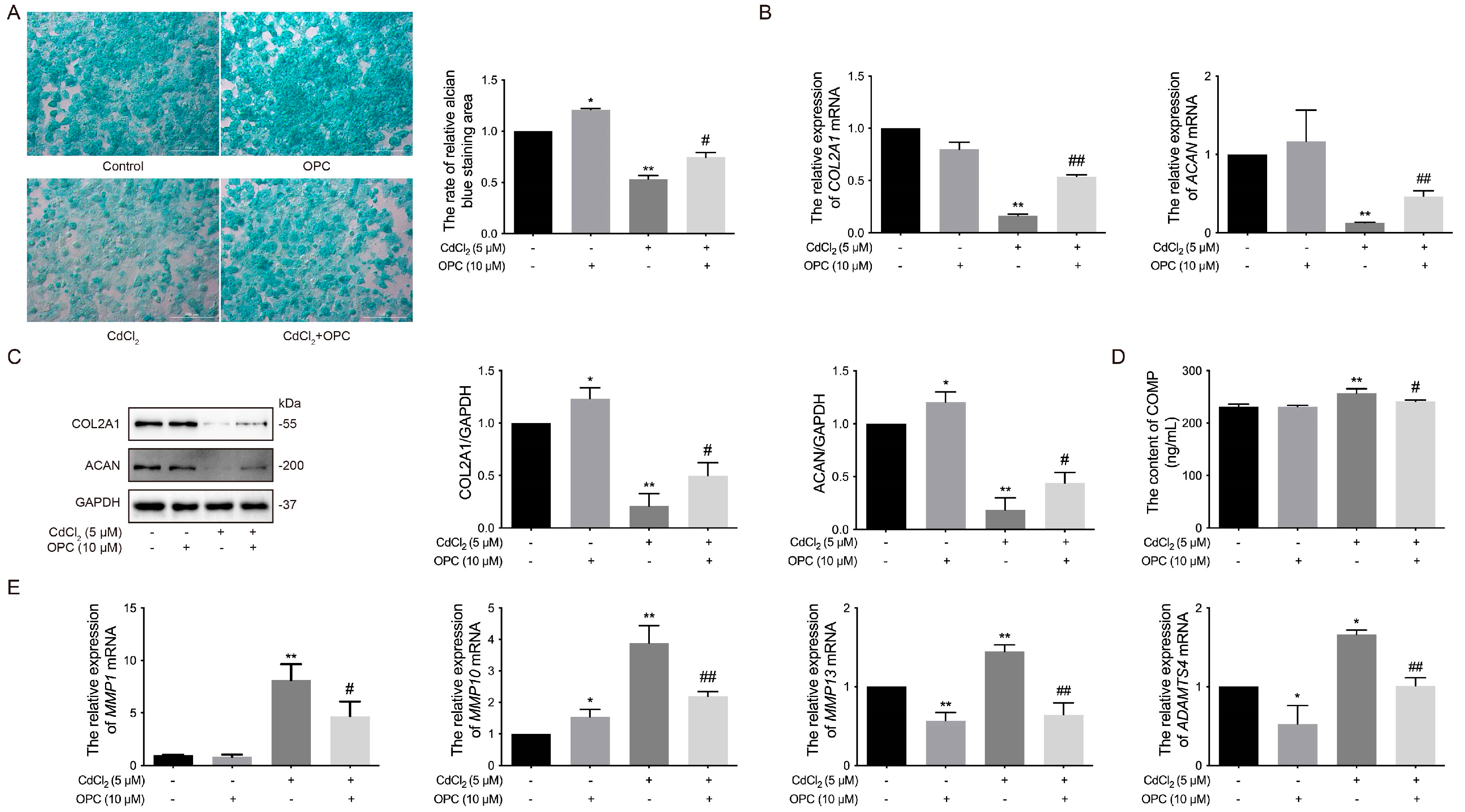
3.6. Effects of OPCs on Cd-Induced ECM Damage to Chondrocytes in Chickens In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| ACAN | aggrecan |
| ACLT | anterior cruciate ligament transection |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AFB1 | Aflatoxin B1 |
| ALP | alkaline phosphatase |
| BMD | bone mineral density |
| Bax | Bcl-2 associated X-protein |
| Bcl-2 | B-cell lymphoma 2 |
| BMSCs | bone marrow mesenchymal stem cells |
| Ca | calcium |
| CCK-8 | cell counting kit-8 |
| Cd | cadmium |
| CdCl2 | cadmium chloride |
| COL2A1 | type II collagen alpha 1 |
| COMP | cartilage oligomeric matrix protein |
| DMEM | dulbecco modified Eagle’s medium/nutrient mixture |
| ECL | electrochemiluminescence |
| ECM | extracellular matrix |
| ERK1/2 | extracellular signal-regulated kinases 1/2 |
| FBS | fetal bovine serum |
| GAGs | glycosaminoglycans |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GSH-Px | glutathione peroxidase |
| GST | glutathione S transferase |
| HA | hyaluronic acid |
| HRP | horseradish peroxidase |
| IGD | interglobular domain |
| IGF1 | insulin-like growth factor 1 |
| IPFP | infrapatellar fat pad |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MIA | monosodium iodoacetate |
| MMPs | matrix metalloproteinases |
| MMP1 | matrix metalloproteinases 1 |
| MTs | metallothioneins |
| NOAEL | no observed adverse effect level |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | nuclear factor-erythroid-2-related factor |
| OA | osteoarthritis |
| OP | osteoporosis |
| OPC | oligomeric proanthocyanidins |
| P | phosphorus |
| PA | procyanidins |
| PBS | phosphate buffered saline |
| PCO | protein carbonyl |
| PGs | polysaccharides |
| PI3K/AKT | phosphoinositide-3 kinase/protein kinase B |
| PVDF | polyvinylidene fluoride |
| RA | rheumatoid arthritis |
| RIPA | radioimmunoprecipitation assay |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SOD | superoxide dismutase |
| TBST | tris-buffered saline solution with 0.1% Tween-20 |
| TIMP1 | tissue inhibitor of metalloproteinases1 |
| TRPV5 | transient receptor potential vanilloid 5 |
| TSP | thrombospondin |
| Zn | zinc |
References
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium sources, toxicity, resistance and removal by microorganisms-A potential strategy for cadmium eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [PubMed]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Zamudio Cuevas, Y.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, C.; Gutiérrez-Ruiz, M.C.; Rojas Del Castillo, E.; et al. Toxicity of cadmium in musculoskeletal diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar]
- Rinaldi, M.; Micali, A.; Marini, H.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484–1494. [Google Scholar] [CrossRef]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects-A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar]
- Chen, X.; Gan, C.; Zhu, G.; Jin, T. Benchmark dose for estimation of cadmium reference level for osteoporosis in a Chinese female population. Food Chem. Toxicol. 2013, 55, 592–595. [Google Scholar]
- Chen, X.; Wang, G.; Li, X.; Gan, C.; Zhu, G.; Jin, T.; Wang, Z. Environmental level of cadmium exposure stimulates osteoclasts formation in male rats. Food Chem. Toxicol. 2013, 60, 530–535. [Google Scholar] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. Effect of cadmium on bone tissue in growing animals. Exp. Toxicol. Pathol. 2016, 68, 391–397. [Google Scholar] [PubMed]
- Wang, M.; Liu, J.; Zhu, G.; Chen, X. Low levels of cadmium exposure affect bone by inhibiting Lgr4 expression in osteoblasts and osteoclasts. J. Trace Elem. Med. Biol. 2022, 73, 127025. [Google Scholar]
- Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I.; Prokopowicz, A.; Kalisinska, E.; Sokolowski, S.; Karaczun, M.; Zietek, P.; Podlasińska, J.; Pilarczyk, B.; Tomza-Marciniak, A.; et al. The Effect of Risk Factors on the Levels of Chemical Elements in the Tibial Plateau of Patients with Osteoarthritis following Knee Surgery. BioMed Res. Int. 2015, 2015, 650282. [Google Scholar]
- Attalla, S. A study of the toxic effects of some environmental pollutants and cigarette smoking in the development of osteoarthritis. In Proceedings of the the International Conference of Environmental and Occupational Health (ICEOH 2014), Putrajaya, Malaysia, 7–9 April 2014. [Google Scholar]
- Yessica Eduviges, Z.C.; Martínez-Nava, G.; Reyes-Hinojosa, D.; Mendoza-Soto, L.; Fernández-Torres, J.; López-Reyes, A.; Olivos-Meza, A.; Armienta-Hernández, M.A.; Ruíz-Huerta, E.A.; de Jesús González-Guadarrama, M.; et al. Impact of cadmium toxicity on cartilage loss in a 3D in vitro model. Environ. Toxicol. Pharmacol. 2020, 74, 103307. [Google Scholar]
- Hung, C.T.; Racine-Avila, J.; Pellicore, M.J.; Aaron, R. Biophysical Modulation of Mesenchymal Stem Cell Differentiation in the Context of Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 3919. [Google Scholar] [CrossRef]
- Mow, V.C.; Wang, C.C.; Hung, C.T. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthr. Cartil. 1999, 7, 41–58. [Google Scholar]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar]
- Wang, X.; Xu, H.; Huang, Y.; Gu, S.; Jiang, J.X. Coupling Effect of Water and Proteoglycans on the In Situ Toughness of Bone. J. Bone Miner. Res. 2016, 31, 1026–1029. [Google Scholar]
- Ntenti, C.; Papakonstantinou, E.; Fidani, L.; Stolz, D.; Goulas, A. The Genetics behind Sulfation: Impact on Airway Remodeling. J. Pers. Med. 2024, 14, 248. [Google Scholar] [CrossRef]
- Michelacci, Y.M.; Baccarin, R.Y.A.; Rodrigues, N.N.P. Chondrocyte Homeostasis and Differentiation: Transcriptional Control and Signaling in Healthy and Osteoarthritic Conditions. Life 2023, 13, 1460. [Google Scholar] [CrossRef]
- Empere, M.; Wang, X.; Prein, C.; Aspberg, A.; Moser, M.; Oohashi, T.; Clausen-Schaumann, H.; Aszodi, A.; Alberton, P. Aggrecan governs intervertebral discs development by providing critical mechanical cues of the extracellular matrix. Front. Bioeng. Biotechnol. 2023, 11, 1128587. [Google Scholar]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [PubMed]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Arner, E.C. Aggrecanase-mediated cartilage degradation. Curr. Opin. Pharmacol. 2002, 2, 322–329. [Google Scholar]
- Lin, W.; Kang, H.; Niu, Y.; Niu, J.; Fan, C.; Feng, X.; Wang, F. Cartilage degeneration is associated with activation of the PI3K/AKT signaling pathway in a growing rat experimental model of developmental trochlear dysplasia. J. Adv. Res. 2022, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, G.; Zhang, F.; Yang, X.; Chen, Y.; Duan, Y.; Yu, M.; Zhang, S.; Han, J. Procyanidin B2 Reduces Vascular Calcification through Inactivation of ERK1/2-RUNX2 Pathway. Antioxidants 2021, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Steitz, M.; Schlicht, C.; Kurth, H.; Gaedcke, F. Anthocyanin- and proanthocyanidin-rich extracts of berries in food supplements—Analysis with problems. Pharmazie 2007, 62, 803–812. [Google Scholar]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB1-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef]
- Barbe, A.; Mellouk, N.; Ramé, C.; Grandhaye, J.; Anger, K.; Chahnamian, M.; Ganier, P.; Brionne, A.; Riva, A.; Froment, P.; et al. A grape seed extract maternal dietary supplementation improves egg quality and reduces ovarian steroidogenesis without affecting fertility parameters in reproductive hens. PLoS ONE 2020, 15, e0233169. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, R.; Li, W.M.; Niu, Y.J.; Guo, H.C.; Liu, X.H.; Hou, Y.C.; Zhao, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environ. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, P.; Pi, S.; Guo, Y.; Pei, S.; Yang, W.; Chang, X.; Wang, L.; Chen, F. Proanthocyanidins Protect Against Cadmium-Induced Diabetic Nephropathy Through p38 MAPK and Keap1/Nrf2 Signaling Pathways. Front. Pharmacol. 2021, 12, 801048. [Google Scholar] [CrossRef]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019, 39, BSR20180515. [Google Scholar] [CrossRef]
- He, L.; Li, P.; Yu, L.H.; Li, L.; Zhang, Y.; Guo, Y.; Long, M.; He, J.B.; Yang, S.H. Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ. Toxicol. Pharmacol. 2018, 57, 1–8. [Google Scholar] [PubMed]
- Gu, J.H.; Li, S.H.; Wang, G.S.; Zhang, X.Q.; Yuan, Y.; Liu, X.Z.; Bian, J.C.; Tong, X.S.; Liu, Z.P. Cadmium Toxicity on Chondrocytes and the Palliative Effects of 1α, 25-Dihydroxy Vitamin D3 in White Leghorns Chicken’s Embryo. Front. Vet. Sci. 2021, 8, 637369. [Google Scholar]
- Gu, J.; Zhang, X.; Zhang, C.; Li, Y.; Bian, J.; Liu, X.; Yuan, Y.; Zou, H.; Tong, X.; Liu, Z. Galectin-3 Contributes to the Inhibitory Effect of lα,25-(OH)2D3 on Osteoclastogenesis. Int. J. Mol. Sci. 2021, 22, 13334. [Google Scholar] [CrossRef]
- Yu, G.; Fu, X.; Gong, A.; Gu, J.; Zou, H.; Yuan, Y.; Song, R.; Ma, Y.; Bian, J.; Liu, Z.; et al. Oligomeric proanthocyanidins ameliorates osteoclastogenesis through reducing OPG/RANKL ratio in chicken’s embryos. Poult. Sci. 2024, 103, 103706. [Google Scholar]
- Tong, X.; Yu, G.; Liu, Q.; Zhang, X.; Bian, J.; Liu, Z.; Gu, J. Puerarin alleviates cadmium-induced oxidative damage to bone by reducing autophagy in rats. Environ. Toxicol. 2022, 37, 720–729. [Google Scholar] [CrossRef]
- Vincent, T.L.; McClurg, O.; Troeberg, L. The Extracellular Matrix of Articular Cartilage Controls the Bioavailability of Pericellular Matrix-Bound Growth Factors to Drive Tissue Homeostasis and Repair. Int. J. Mol. Sci. 2022, 23, 6003. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [PubMed]
- Ariosa-Morejon, Y.; Santos, A.; Fischer, R.; Davis, S.; Charles, P.; Thakker, R.; Wann, A.K.; Vincent, T.L. Age-dependent changes in protein incorporation into collagen-rich tissues of mice by in vivo pulsed SILAC labelling. eLife 2021, 10, e66635. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Chen, S.; Tang, X.X. Role of ADAM and ADAMTS proteases in pathological tissue remodeling. Cell Death Discov. 2023, 9, 447. [Google Scholar]
- Ou, L.; Wang, H.; Wu, Z.; Wang, P.; Yang, L.; Li, X.; Sun, K.; Zhu, X.; Zhang, R. Effects of cadmium on osteoblast cell line: Exportin 1 accumulation, p-JNK activation, DNA damage and cell apoptosis. Ecotoxicol. Environ. Saf. 2021, 208, 111668. [Google Scholar]
- Wang, B.; Xiao, J.L.; Ling, Y.H.; Meng, X.J.; Wu, B.; Yang, X.Y.; Zou, F. BNIP3 upregulation by ERK and JNK mediates cadmium-induced necrosis in neuronal cells. Toxicol. Sci. 2014, 140, 393–402. [Google Scholar]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. BioMed Pharmacother. 2019, 116, 108999. [Google Scholar]
- Chen, H.; Li, J.; Li, S.; Wang, X.; Xu, G.; Li, M.; Li, G. Research progress of procyanidins in repairing cartilage injury after anterior cruciate ligament tear. Heliyon 2024, 10, e26070. [Google Scholar] [PubMed]
- Chen, L.; You, Q.; Hu, L.; Gao, J.; Meng, Q.; Liu, W.; Wu, X.; Xu, Q. The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice. Front. Immunol. 2017, 8, 1910. [Google Scholar]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar]
- Fernández-Torres, J.; Plata-Rodríguez, R.; Zamudio-Cuevas, Y.; Martínez-Nava, G.A.; Landa-Solís, C.; Mendoza Soto, L.; Olivos-Meza, A.; Suárez-Ahedo, C.; Barbier, O.C.; Narváez-Morales, J.; et al. Effect of cadmium on the viability on monolayer cultures of synoviocytes, chondrocytes, and Hoffa: A preliminary study. Toxicol. Ind. Health 2020, 36, 940–945. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013, 2013, 284873. [Google Scholar]
- Verma, P.; Dalal, K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013, 31, 999–1006. [Google Scholar]
- Ishikawa, T.; Nishigaki, F.; Miyata, S.; Hirayama, Y.; Minoura, K.; Imanishi, J.; Neya, M.; Mizutani, T.; Imamura, Y.; Naritomi, Y.; et al. Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br. J. Pharmacol. 2005, 144, 133–143. [Google Scholar]
- Chen, H.; Qin, Z.; Zhao, J.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [PubMed]
- Séguin, C.A.; Bernier, S.M. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J. Cell Physiol. 2003, 197, 356–369. [Google Scholar]
- Luo, P.; Yuan, Q.L.; Yang, M.; Wan, X.; Xu, P. The role of cells and signal pathways in subchondral bone in osteoarthritis. Bone Jt. Res. 2023, 12, 536–545. [Google Scholar]
- Oh, C.D.; Chang, S.H.; Yoon, Y.M.; Lee, S.J.; Lee, Y.S.; Kang, S.S.; Chun, J.S. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J. Biol. Chem. 2000, 275, 5613–5619. [Google Scholar]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar]
- Lu, N.; Malemud, C.J. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int. J. Mol. Sci. 2019, 20, 3792. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar]
- Zhou, Z.; Tang, S.; Nie, X.; Zhang, Y.; Li, D.; Zhao, Y.; Cao, Y.; Yin, J.; Chen, T.; Ruan, G.; et al. Osteoarthritic infrapatellar fat pad aggravates cartilage degradation via activation of p38MAPK and ERK1/2 pathways. Inflamm. Res. 2021, 70, 1129–1139. [Google Scholar]
- Kim, J.H.; Huh, J.E.; Baek, Y.H.; Lee, J.D.; Choi, D.Y.; Park, D.S. Effect of Phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J. Ethnopharmacol. 2011, 134, 234–242. [Google Scholar]
- Zhou, F.; Mei, J.; Han, X.; Li, H.; Yang, S.; Wang, M.; Chu, L.; Qiao, H.; Tang, T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 2019, 9, 973–985. [Google Scholar] [PubMed]
- Sondergaard, B.C.; Schultz, N.; Madsen, S.H.; Bay-Jensen, A.C.; Kassem, M.; Karsdal, M.A. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation—Divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthr. Cartilage 2010, 18, 279–288. [Google Scholar]
- Prasadam, I.; Crawford, R.; Xiao, Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—Possible pathogenic role in osteoarthritis. J. Rheumatol. 2012, 39, 621–634. [Google Scholar] [PubMed]
- Willcockson, H.; Ozkan, H.; Chubinskaya, S.; Loeser, R.F.; Longobardi, L. CCL2 induces articular chondrocyte MMP expression through ERK and p38 signaling pathways. Osteoarthr. Cartil. Open 2021, 3, 100136. [Google Scholar]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016, 7, 483–490. [Google Scholar]
- Miller, M.J.; Bobrowski, P.; Shukla, M.; Gupta, K.; Haqqi, T.M. Chondroprotective effects of a proanthocyanidin rich Amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes. J. Inflamm. 2007, 4, 16. [Google Scholar]
- Bai, Z.; Hu, K.; Shou, Z.; Yu, J.; Meng, H.; Zhou, H.; Chen, L.; Yu, T.; Lu, R.; Li, N.; et al. Layer-by-layer assembly of procyanidin and collagen promotes mesenchymal stem cell proliferation and osteogenic differentiation in vitro and in vivo. Regen. Biomater. 2023, 10, rbac107. [Google Scholar] [CrossRef]
- Miyake, M.; Ide, K.; Sasaki, K.; Matsukura, Y.; Shijima, K.; Fujiwara, D. Oral administration of highly oligomeric procyanidins of Jatoba reduces the severity of collagen-induced arthritis. Biosci. Biotechnol. Biochem. 2008, 72, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Primer Sequence (5′–3′) | Gene Number | Length (bp) |
|---|---|---|---|
| COL2A1 | F: GGAGTTTGGCGTGGATATTGG | NM_204426.2 | 164 |
| R: GGGTGGGACTGGTTTGCTTT | |||
| ACAN | F: TGGGCGTGCGGACCGTTTA | NM_001396161.1 | 255 |
| R: TGGGCTCCAGGGTAGCGATG | |||
| MMP1 | F: ATTTGATGCCATTACCACTT | XM_040658536.2 | 172 |
| R: ACTTCATCCCTTTCAATGTTCT | |||
| MMP9 | F: GTGCCGTGATAGATGATGCCTTCC | NM_204667.2 | 95 |
| R: GTCTGCCTCGCCGCTGTAAATC | |||
| MMP10 | F: ATCAGGCTCTACAGTGGTG | NM_001278089.2 | 275 |
| R: ATGGGATACATCAAGGCAC | |||
| MMP13 | F: CCCAACCCAAAACATCCCAAAACG | NM_001293090.2 | 94 |
| R: TGAAGACCAGCATTTCTCCACGAAG | |||
| ADAMTS4 | F: GACGGCGTGGGAGAAACAGAAAG | XM_040690827.2 | 113 |
| R: GGAGGGGCTGAGGTAGACACAG | |||
| ADAMTS5 | F: AGAGCAGTGTGAAGCAAGGAATGG | XM_040658789.2 | 96 |
| R: CCAGGAAGCACTCCAGCATACTTG | |||
| GAPDH | F: ATGGCATCCAAGGAGTGA | NM_204305.2 | 141 |
| R: GGGAGACAGAAGGGAACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Liu, D.; Gong, A.; Zhao, X.; Zhou, J.; Wang, P.; Xia, H.; Song, R.; Ma, Y.; Zou, H.; et al. Effects of Oligomeric Proanthocyanidins on Cadmium-Induced Extracellular Matrix Damage via Inhibiting the ERK1/2 Signaling Pathway in Chicken Chondrocytes. Vet. Sci. 2025, 12, 317. https://doi.org/10.3390/vetsci12040317
Gu J, Liu D, Gong A, Zhao X, Zhou J, Wang P, Xia H, Song R, Ma Y, Zou H, et al. Effects of Oligomeric Proanthocyanidins on Cadmium-Induced Extracellular Matrix Damage via Inhibiting the ERK1/2 Signaling Pathway in Chicken Chondrocytes. Veterinary Sciences. 2025; 12(4):317. https://doi.org/10.3390/vetsci12040317
Chicago/Turabian StyleGu, Jianhong, Dan Liu, Anqing Gong, Xinrui Zhao, Jiatao Zhou, Panting Wang, Han Xia, Ruilong Song, Yonggang Ma, Hui Zou, and et al. 2025. "Effects of Oligomeric Proanthocyanidins on Cadmium-Induced Extracellular Matrix Damage via Inhibiting the ERK1/2 Signaling Pathway in Chicken Chondrocytes" Veterinary Sciences 12, no. 4: 317. https://doi.org/10.3390/vetsci12040317
APA StyleGu, J., Liu, D., Gong, A., Zhao, X., Zhou, J., Wang, P., Xia, H., Song, R., Ma, Y., Zou, H., Memon, M. A., Yuan, Y., Liu, X., Bian, J., Liu, Z., & Tong, X. (2025). Effects of Oligomeric Proanthocyanidins on Cadmium-Induced Extracellular Matrix Damage via Inhibiting the ERK1/2 Signaling Pathway in Chicken Chondrocytes. Veterinary Sciences, 12(4), 317. https://doi.org/10.3390/vetsci12040317









