Antimicrobial Activity of Teat Antiseptic Formulations Based on Plant Extracts for Controlling Bovine Mastitis: In Vitro and In Vivo Evaluation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
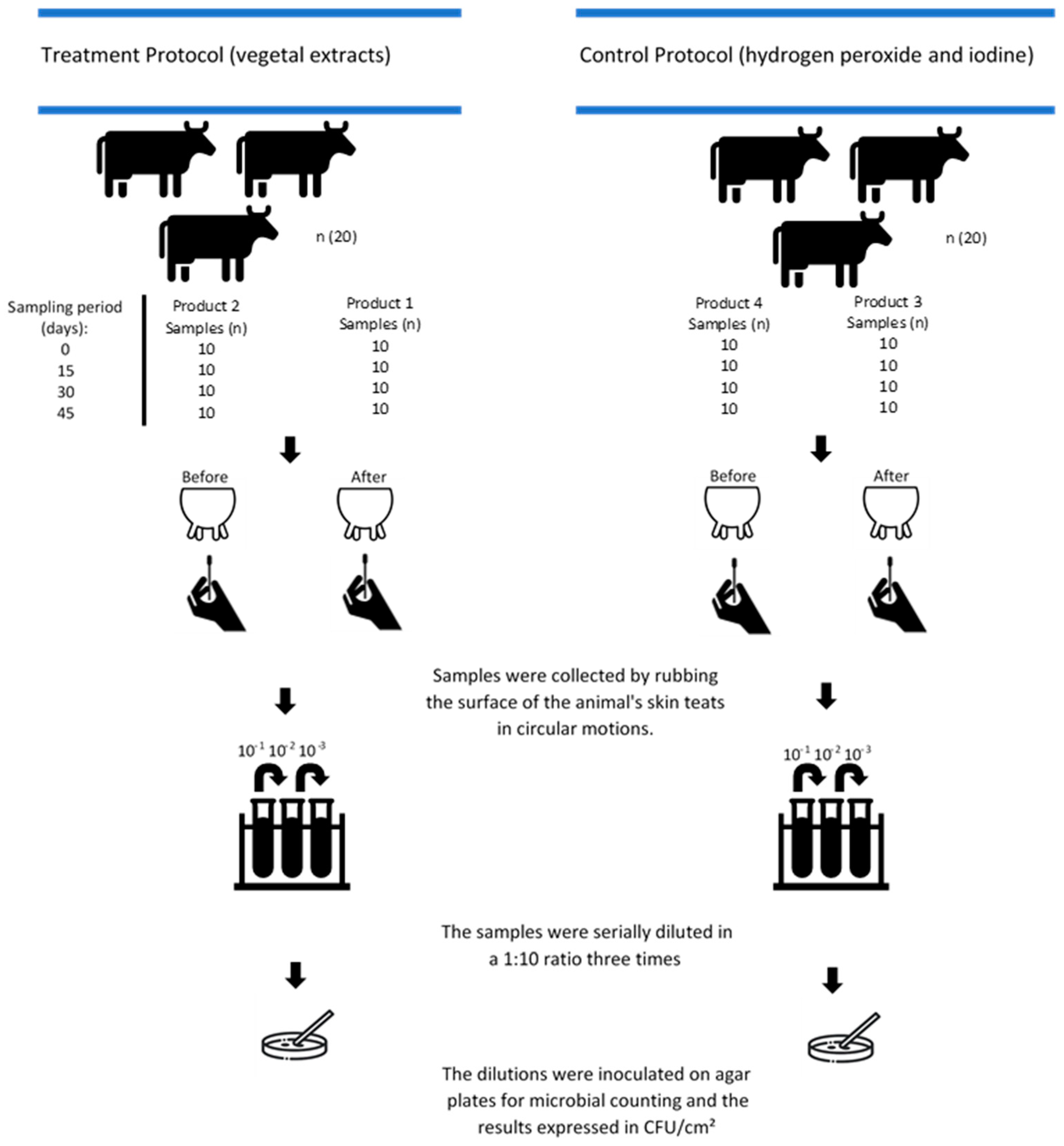
2.1. In Vitro Testing
2.2. In Vivo Testing
2.2.1. Microbial Quantification
2.2.2. Statistical Analysis
2.2.3. Evaluation of Post-Dipping Product Compliance
3. Results
3.1. In Vitro Testing
3.2. In Vivo Testing
3.2.1. Microbial Quantification
3.2.2. Statistical Analysis
3.2.3. Molecular Identification of Staphylococcus aureus
3.2.4. Evaluation of Post-Dipping Product Compliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.; Huang, X.; Xu, H.; Zhang, C.; Chen, S.; Liu, F.; Guan, S.; Zhang, S.; Zhu, K.; Wu, C. The Prevalence of Pathogens Causing Bovine Mastitis and Their Associated Risk Factors in 15 Large Dairy Farms in China: An Observational Study. Vet. Microbiol. 2020, 247, 108757. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, Types, Etiological Agents, Prevalence, Diagnosis, Treatment, Prevention, Effects on Human Health and Future Aspects of Bovine Mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Awandkar, S.P.; Kulkarni, M.B.; Khode, N.V. Bacteria from Bovine Clinical Mastitis Showed Multiple Drug Resistance. Vet. Res. Commun. 2022, 46, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Samardžija, M.; Kovačević, Z. Alternatives to Antimicrobial Treatment in Bovine Mastitis Therapy: A Review. Antibiotics 2023, 12, 683. [Google Scholar] [CrossRef]
- Enger, B.D.; Fox, L.K.; Gay, J.M.; Johnson, K.A. Reduction of Teat Skin Mastitis Pathogen Loads: Differences between Strains, Dips, and Contact Times. J. Dairy Sci. 2015, 98, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.Z.M.; Ronquillo, M.; Camacho, L.M.; Cerrillo, S.M.A.; Domínguez, I.A.; Bórquez, J.L. Beneficial Effects of Plant extracts in Ruminant Nutrition: A Review. Indian J. Anim. Sci. 2012, 82, 1117–1121. [Google Scholar] [CrossRef]
- French, E.A.; Mukai, M.; Zurakowski, M.; Rauch, B.; Gioia, G.; Hillebrandt, J.R.; Henderson, M.; Schukken, Y.H.; Hemling, T.C. Iodide Residues in Milk Vary between Iodine-Based Teat Disinfectants. J. Food Sci. 2016, 81, T1864–T1870. [Google Scholar] [CrossRef]
- Proestos, C. The Benefits of Plant extracts for Human Health. Foods 2020, 9, 10–12. [Google Scholar] [CrossRef]
- Li, P. Concise Review on Residual Chlorine Measurement: Interferences and Possible Solutions. J. Clean. Prod. 2021, 323, 129119. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Bovine Mastitis Prevention and Control in the Post-Antibiotic Era. Trop. Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef]
- van der Reijden, O.L.; Zimmermann, M.B.; Galetti, V. Iodine in dairy milk: Sources, concentrations and importance to human health. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Mureza, S.; Smit, C.J.; Muya, M.C.; Nherera-Chokuda, F.V. Effects of Plant extracts as Pre-Milking Dairy Cow Teat Sanitizer. J. Anim. Plant Sci. 2020, 31, 403–408. [Google Scholar] [CrossRef]
- Ayodipupo Babalola, B.; Ifeolu Akinwande, A.; Otunba, A.A.; Ebenezer Adebami, G.; Babalola, O.; Nwuofo, C. Therapeutic Benefits of Carica papaya: A Review on Its Pharmacological Activities and Characterization of Papain. Arab. J. Chem. 2024, 17, 105369. [Google Scholar] [CrossRef]
- Christopher Peterson, D. Potential Health Benefits of Aloe Vera in Livestock: A Review. J. Appl. Vet. Sci. 2024, 9, 94–104. [Google Scholar]
- de Souza de Aguiar, P.; Correa, Á.P.; Antunes, F.T.T.; de Barros Ferraz, A.F.; Vencato, S.B.; Amado, G.J.V.; Wiiland, E.; Corrêa, D.S.; Grivicich, I.; de Souza, A.H. Benefits of Stryphnodendron adstringens When Associated with Hydrogel on Wound Healing in Diabetic Rats. Clin. Phytoscience 2021, 7, 22. [Google Scholar] [CrossRef]
- Silva, B.A.; Scussel, V.M. Characteristics and Effects of the Amazonian Andiroba (Carapa Guianensis Aubl.) Oil Against Living Organisms—A Review. IOSR J. Biotechnol. Biochem. 2020, 6, 31–47. [Google Scholar] [CrossRef]
- de Faria, M.J.M.; Braga, C.A.D.S.B.; de Paula, J.R.; André, M.C.D.P.B.; Vaz, B.G.; de Carvalho, T.C.; Romão, W.; da Costa, H.B.; da Conceição, E.C. Antimicrobial Activity of Copaifera spp. Against Bacteria Isolated from Milk of Cows with Mastitis. Cienc. Anim. Bras. 2017, 18, 1–14. [Google Scholar] [CrossRef][Green Version]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Chatha, S.A.S.; Iqbal, Y.; Hussain, A.I.; Khan, I.; Xie, F. Recent trends in extraction, purification, and antioxidant activity evaluation of plant leaf-extract polysaccharides. Biofuels Bioprod. Biorefining. 2022, 16, 1820–1848. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100, Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2023. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- De Almeida, C.C.; Pizauro, L.J.L.; Soltes, G.A.; Slavic, D.; De Ávila, F.A.; Pizauro, J.M.; MacInnes, J.I. Some Coagulase Negative Staphylococcus spp. Isolated from Buffalo Can Be Misidentified as Staphylococcus aureus by Phenotypic and Sa442 PCR Methods. BMC Res. Notes 2018, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kybartas, M.; Virgailis, M.; Ruzauskas, M.; Klimienė, I.; Šiugždinienė, R.; Merkevičienė, L.; Štreimikytė-Mockeliūnė, Ž.; Mockeliūnas, R. A Study on the Stability and Antimicrobial Efficacy of a Newly Modeled Teat Dip Solution Containing Chlorhexidine. Vet. Sci. 2023, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.F.; Quevedo, B.V.; Asami, J.; Komatsu, D.; Hausen, M.d.A.; Duek, E.A.d.R. Electrospun Membrane Based on Poly(L-Co-D,L Lactic Acid) and Natural Rubber Containing Copaiba Oil Designed as a Dressing with Antimicrobial Properties. Antibiotics 2023, 12, 898. [Google Scholar] [CrossRef]
- Sampaio LT, R.; da Silva Santos, A.R.; de Sousa, A.P.; Fernandes HM, B.; de Oliveira Filho, A.A. Avaliação da atividade antimicrobiana e antiaderente do óleo essencial de Melaleuca alternifolia contra cepa de Staphylococcus saprophyticus. Rev. Colomb. De Cienc. Químico Farm. 2023, 52, 613–625. [Google Scholar]
- Suthovski, G.; Santa Catarina, A.; Perin, D.P.; Mainardes, R.M.; Starikoff, K.R.; Gallina, A.L.; Azevedo, M.G.B.; Dalmolin, F.; Cervo, L.V.; Benvegnú, D.M. Effect of Polycaprolactone Nanocapsules Loaded with Essential Oils on Biofilm Formation by Staphylococcus aureus Strains Isolated from Bovine Mastitis Cases. Brazilian J. Pharm. Sci. 2023, 59, e23068. [Google Scholar] [CrossRef]
- Puvača, N.; Čabarkapa, I.; Petrović, A.; Bursić, V.; Prodanović, R.; Soleša, D.; Lević, J. Tea Tree (Melaleuca alternifolia) and Its Essential Oil: Antimicrobial, Antioxidant and Acaricidal Effects in Poultry Production. Worlds. Poult. Sci. J. 2019, 75, 235–246. [Google Scholar] [CrossRef]
- Pasca, C.; Marghitas, L.A.; Dezmirean, D.S.; Matei, I.A.; Bonta, V.; Pasca, I.; Chirila, F.; Cîmpean, A.; Iosif Fit, N. Efficacy of Natural Formulations in Bovine Mastitis Pathology: Alternative Solution to Antibiotic Treatment. J. Vet. Res. 2020, 64, 523–529. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant Essential Oils as a Tool in the Control of Bovine Mastitis: An Update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Dewaele, P.; Hu, H.; Panda, S.K.; Luyten, W. Antimicrobial Efficacy of Select Medicinal Plant extracts from Bangladesh against Food-Borne Bacterial Pathogens. J. Food Saf. 2024, 44, e13147. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga, V.F.; Pinto, A.C.; Nakamura, C.V. Antimicrobial Activity of Brazilian Copaiba Oils Obtained from Different Species of the Copaifera Genus. Mem. Inst. Oswaldo Cruz 2008, 103, 277–281. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Weiwei, W.; Wei, X.; Ahmad, S.U.; Wu, L.; Zhang, J. Comparative Study of Antimicrobial Action of Aloe Vera and Antibiotics against Different Bacterial Isolates from Skin Infection. Vet. Med. Sci. 2021, 7, 2061–2067. [Google Scholar] [CrossRef]
- Yoruk, N.G.; Istanbullu Paksoy, Ö. GC/MS Evaluation of the Composition of the Aloe Vera Gel and Extract. Food Chem. X 2024, 23, 101536. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Corona-Gómez, L.; Hernández-Andrade, L.; Mendoza-Elvira, S.; Suazo, F.M.; Ricardo-González, D.I.; Quintanar-Guerrero, D. In Vitro Antimicrobial Effect of Essential Tea Tree Oil (Melaleuca alternifolia), Thymol, and Carvacrol on Microorganisms Isolated from Cases of Bovine Clinical Mastitis. Int. J. Vet. Sci. Med. 2022, 10, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.; do Rosário Estevam dos Santos, P.B.; Pereira, L.M.; do Rosário Estevam dos Santos, P.B.; Scorzoni, L.; Pereira, T.C.; Abu Hasna, A.; Carvalho, C.A.T.; de Oliveira, L.D. Antimicrobial Action of Four Herbal Plants over Mixed-Species Biofilms of Candida albicans with Four Different Microorganisms. Aust. Endod. J. 2023, 49, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Andrade, V.A.; Fonseca, F.S.A.; Macêdo, A.A.; Santos, R.L.; Colen, K.G.F.; Martins, E.R.; Marcelo, N.A. Acute and Chronic Toxicity and Antimicrobial Activity of the Extract of Stryphnodendron adstringens (Mart.) Coville. Pesqui. Vet. Bras. 2017, 37, 840–846. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Tuntun, M.; Jurusan Analis Kesehatan, P.K.T. Uji aktivitas antibakteri ekstrak daun pepaya (Carica papaya L.) terhadap bakteri Escherichia Coli DAN Staphylococcus aureus. J. Farm. J. Penelit. dan Pengabdi. Masy. 2016, 7, 497–502. [Google Scholar]
- Moirangthem, S.; Patra, G.; Biswas, S.; Das, A.; Nath, S.; Verma, A.K.; Pal, S.; Chatterjee, N.; Bandyopadhyay, S.; Nanda, P.K.; et al. Effect of Nutmeg (Myristica fragrans) and Tea Tree (Melaleuca alternifolia) Essential Oils on the Oxidative and Microbial Stability of Chicken Fillets During Refrigerated Storage. Foods 2024, 13, 4139. [Google Scholar] [CrossRef]
- Hudecová, P.; Koščová, J.; Hajdučková, V.; Király, J.; Horňak, P. Antibacterial and Antibiofilm Activity of Essential Oils Against Aeromonas spp. Isolated from Rainbow Trout. Animals 2024, 14, 3202. [Google Scholar] [CrossRef]
- de Souza Júnior, P.R.P.; Santos, G.S.; Prado, L.D.S.; Peters, L.P.; Carvalho, C.M. Antimicrobial Activity of Amazon Medicinal Plants. Acta Sci.—Biol. Sci. 2023, 45, e68565. [Google Scholar] [CrossRef]
- Buldain, D.; Buchamer, A.V.; Marchetti, M.L.; Aliverti, F.; Bandoni, A.; Mestorino, N. Combination of Cloxacillin and Essential Oil of Melaleuca armillaris as an Alternative against Staphylococcus aureus. Front. Vet. Sci. 2018, 5, 177. [Google Scholar] [CrossRef]
- Casanova, L.M.; Costa, S.S. Synergistic Interactions in Natural Products: Therapeutic Potential and Challenges. Rev. Virtual Quim. 2017, 9, 575–595. [Google Scholar] [CrossRef]
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef] [PubMed]
- Floriano, J.; Rodrigues, D.; Ohara, R.; Almeida, N.; Soares, V.; Sartorelli, P.; Graeff, C.; Grecco, S.; González, A.; Alpino, P.D. Bioactivity, Efficacy, and Safety of a Wound Healing Ointment with Medicinal Plant Bioactives: In Vitro and In Vivo Preclinical Evaluations. Sci. World J. 2025, 2024, 9466270. [Google Scholar] [CrossRef]
- Capurro, A.; Aspán, A.; Ericsson Unnerstad, H.; Persson Waller, K.; Artursson, K. Identification of Potential Sources of Staphylococcus aureus in Herds with Mastitis Problems. J. Dairy Sci. 2010, 93, 180–191. [Google Scholar] [CrossRef]
- Gleeson, D.; O’Brien, B.; Flynn, J.; O’Callaghan, E.; Galli, F. Effect of Pre-Milking Teat Preparation Pocedures on the Microbial Count on Teats Prior to Clusters Application. Ir. Vet. J. 2009, 62, 461–467. [Google Scholar] [CrossRef]
- Verdier-Metz, I.; Delbès, C.; Bouchon, M.; Pradel, P.; Theil, S.; Rifa, E.; Corbin, A.; Chassard, C. Influence of Post-Milking Treatment on Microbial Diversity on the Cow Teat Skin and in Milk. Dairy 2022, 3, 262–276. [Google Scholar] [CrossRef]
- Subramaniam, J.; Kovendan, K.; Mahesh Kumar, P.; Murugan, K.; Walton, W. Mosquito Larvicidal Activity of Aloe Vera (Family: Liliaceae) Leaf Extract and Bacillus sphaericus, against Chikungunya Vector, Aedes segypti. Saudi J. Biol. Sci. 2012, 19, 503–509. [Google Scholar] [CrossRef]
- Salim, R.H.; Salloum, A.M.; Alsalameh, S.A.; Khazem, M.R.; Hajeer, M.Y. Antimicrobial Properties of Aloe Vera Ethanol Extract as a Denture Disinfectant: An In Vitro Study. Cureus 2024, 16, e59916. [Google Scholar] [CrossRef]
- Mujawar, S.S.; Arbade, G.K.; Bisht, N.; Mane, M.; Tripathi, V.; Kumar, R.; Kashte, S.B. 3D Printed Aloe barbadensis Loaded Alginate-Gelatin Hydrogel for Wound Healing and Scar Reduction: In Vitro and in Vivo Study. Int. J. Biol. Macromol. 2025, 296, 139745. [Google Scholar]

| Product 1 * Pre-Dipping | Product 2 * Post-Dipping | Product 3 ** Pre-Dipping | Product 4 ** Post-Dipping |
|---|---|---|---|
| Aloe barbadensis leaf glycolic extract 2.5% | Aloe barbadensis leaf glycolic extract 2.5% | Hydrogen peroxide | Polyvynilpyrrolidone |
| E.D.T.A. 0.1% | Green dye 0.1% | Etidronic acid | Anionic surfactant |
| Carapa guianensis seed oil 2.5% | Carapa guianensis seed oil 2.5% | Decyl polyglucoside | Thickener |
| Carica papaya fruit extract 3.2% | Carica papaya fruit extract 3.2% | Benzalkonium chloride | Glycerin |
| Copaifera officinalis resin 0.2% | Copaifera officinalis resin 0.2% | Nanohydrate | Deonized water |
| Cosmoguard SL 0.5% | Glycerin 10% | Glycerin | |
| Melaleuca alternifolia leaf oil 0.2% | Melaleuca alternifolia leaf oil 0.4% | Hydroxyethylcellulose | |
| Propylene glycol 10% | Hyaluronic acid 0.3% | Formaldehyde | |
| Stryphnodendron barbatiman glycolic extract 2% | Stryphnodendron barbatiman glycolic extract 2% | Sodium hydroxixe | |
| Sodium lauryl ether sulfate 20% | Xantham gum 0.7% | Green HMC | |
| Deionized water 58.8% | Deionized water 77.8% | Deionized water |
| Serial Dilution (%) | Test Product | Staphylococcus aureus | Streptococcus agalactiae | Escherichia coli | |||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| 0 | 1 | - | - | - | - | - | - |
| 2 | - | - | - | - ** | - | - ** | |
| 50 | 1 | - | - | - | - | - | - |
| 2 | - | - | - * | + | - | + | |
| 25 | 1 | - | - | - | - | - | - |
| 2 | - | - | + | + | - | + | |
| 12.5 | 1 | - | - | - | - | - | - |
| 2 | - | - | + | + | - * | + | |
| 6.25 | 1 | - | - | - | - | - | - |
| 2 | - | - | + | + | + | + | |
| 3.125 | 1 | - | - | - | - | - | - |
| 2 | - | - | + | + | + | + | |
| 1.562 | 1 | - | - | - | - | - | - |
| 2 | - | - | + | + | + | + | |
| 0.781 | 1 | - | - | - | - | - | - |
| 2 | - * | - ** | + | + | + | + | |
| 0.390 | 1 | - | - | - | - | - | - ** |
| 2 | + | + | + | + | + | + | |
| 0.195 | 1 | - | - | - | - | - | + |
| 2 | + | + | + | + | + | + | |
| 0.097 | 1 | - * | - ** | - * | - | - * | + |
| 2 | + | + | + | + | + | + | |
| Treatment Group (CFU/cm2) | ||||||
| Time points (days) | Products | Staphylococcus spp. | Enterobacteriaceae | Streptococcus spp. | Total mesophiles | Total fungi and yeasts |
| 0 | Product 1 | 101 | 101 | 101 | 102 | 102 |
| Product 2 | 101 | 102 | 102 | 102 | 103 | |
| 15 | Product 1 | 101 | 101 | 101 | 102 | 102 |
| Product 2 | 102 | 101 | 101 | 103 | 103 | |
| 30 | Product 1 | 102 | 101 | 101 | 102 | 102 |
| Product 2 | 103 | 102 | 102 | 104 | 104 | |
| 45 | Product 1 | 101 | 101 | 101 | 103 | 102 |
| Product 2 | 102 | 101 | 103 | 103 | 103 | |
| Control Group (CFU/cm2) | ||||||
| Time points (days) | Products | Staphylococcus spp. | Enterobacteriaceae | Streptococcus spp. | Total mesophiles | Total fungi and yeasts |
| 0 | Product 3 | 101 | 101 | 101 | 102 | 102 |
| Product 4 | 102 | 102 | 101 | 102 | 103 | |
| 15 | Product 3 | 101 | 101 | 101 | 102 | 102 |
| Product 4 | 102 | 101 | 101 | 103 | 103 | |
| 30 | Product 3 | 102 | 102 | 102 | 103 | 102 |
| Product 4 | 104 | 104 | 102 | 104 | 104 | |
| 45 | Product 3 | 101 | 101 | 102 | 102 | 102 |
| Product 4 | 102 | 102 | 103 | 103 | 103 | |
| Reduction Capacity (%) | ||||||
|---|---|---|---|---|---|---|
| Group | Sampling Periods | Staphylococcus spp. | Enterobacteriaceae | Streptococcus spp. | Total Mesophiles | Total Fungi and Yeasts |
| Treatment (Product 1) | 0 | 92.0 | 91.5 | 92.2 | 81.2 | 90.8 |
| 15 | 96.3 | 89.8 | 97.6 | 93.2 | 88.5 | |
| 30 | 92.4 | 91.0 | 72.8 | 93.4 | 95.2 | |
| 45 | 91.6 | 95.1 | 93.7 | 87.9 | 88.1 | |
| Average | 93.0 | 91.8 | 89.1 | 88.9 | 90.6 | |
| Control (Product 3) | 0 | 81.7 | 53.1 | 96.1 | 80.7 | 92.1 |
| 15 | 85.9 | 91.7 | 95.7 | 93.5 | 89.5 | |
| 30 | 97.7 | 97.6 | 71.3 | 80.8 | 92.3 | |
| 45 | 90.8 | 89.1 | 91.8 | 75.3 | 88.1 | |
| Average | 89.0 | 82.9 | 88.7 | 82.6 | 90.5 | |
| Statistical Analysis | Comparison Between Averages in Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microbial Groups | Staphylococci | Enterobacteriaceae | Streptococci | Mesophilic Aerobes | Fungi and Yeast | ||||||
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | ||
| Tukey’s Test 1 | 1.71 a | 1.62 a | 0.93 a | 0.97 a | 1.24 a | 1.05 a | 2.95 a | 2.85 a | 2.78 a | 2.49 b | |
| Periods | Comparison Between Averages In Sampling Periods (days) | ||||||||||
| Farm 1 | 0 | 1.55 ab | 0.83 a | 0.79 b | 1.95 e | 1.95 c | |||||
| 15 | 1.86 ab | 0.97 a | 1.14 ab | 3.20 abc | 3.00 ab | ||||||
| 30 | 1.43 b | 0.81 a | 0.79 b | 2.51 d | 2.66 b | ||||||
| 45 | 1.38 b | 0.95 a | 1.04 ab | 2.90 bcd | 2.44 bc | ||||||
| Farm 2 | 0 | 1.81 ab | 0.87 a | 1.24 ab | 2.95 bcd | 2.56 b | |||||
| 15 | 1.54 ab | 1.25 a | 1.31 ab | 3.35 ab | 2.62 b | ||||||
| 30 | 1.66 ab | 0.88 a | 1.37 a | 2.75 cd | 2.56 b | ||||||
| 45 | 2.10 a | 1.07 a | 1.48 a | 3.60 a | 3.30 a | ||||||
| F test 2 | 0.94 NS | 0.72 NS | 0.42 NS | 0.63 NS | 0.82 NS | ||||||
| F-Test Results of the Total Average Reduction from All Microbial Groups Across All Sampling Time Periods | |||||
|---|---|---|---|---|---|
| Microbial Group | Group | Results | Group | Results | F-Test |
| Staphylococcus spp. | Control | 1.02 | Treatment | 0.94 | Non-significant |
| Enterobacteriaceae | Control | 0.26 | Treatment | 1.7 | Non-significant |
| Streptococcus spp. | Control | 3.94 | Treatment | 2.42 | Non-significant |
| Total mesophiles | Control | 1.34 | Treatment | 0.64 | Non-significant |
| Total fungi and yeasts | Control | 9.28 | Treatment | 7.96 | Non-significant |
| Parameters | Product 2 | Product 4 |
|---|---|---|
| Color | ||
| The color is bright and remains stable for 10 min. After 60 min, the color changed moderately. After 12 h, traces of the color persisted | The color is bright and remains stable for 10 min. After 60 min, the color changed moderately. After 12 h, traces of the color are slightly visible | |
| Points (1 to 5) | 4 points | 3 points |
| Dripping immediately after dipping | ||
| No dripping after immersion (no more than one drop in the first minute) | No dripping after immersion (no more than one drop in the first minute) | |
| Points (1 to 5) | 5 points | 5 points |
| Formation of a drop on the teat end | ||
| After a few minutes, a stable suspended drop is formed. After 60 min, the drop is no longer visible | After a few minutes, a stable suspended drop is formed. After 60 min, the drop is no longer visible | |
| Points (1 to 5) | 4 points | 4 points |
| Teat covered with a film | ||
| It covers the teat skin with a uniform film in a single layer | It covers the teat skin with a uniform film, though slightly inconspicuous | |
| Points (1 to 5) | 5 points | 4 points |
| Evenness of teat coverage | ||
| Covers uniformly. | Covers uniformly. | |
| Points (1 to 5) | 5 points | 5 points |
| Total Points | 23 points | 21 points |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, G.M.; Rodrigues, R.A.; Brugnera, H.C.; Barbosa, J.C.; Favaron, F.R., Jr.; Rossi, G.A.M.; de Bragança, C.R.S.; Schocken-Iturrino, R.P.; de Ávila, F.A.; Cardozo, M.V. Antimicrobial Activity of Teat Antiseptic Formulations Based on Plant Extracts for Controlling Bovine Mastitis: In Vitro and In Vivo Evaluation. Vet. Sci. 2025, 12, 293. https://doi.org/10.3390/vetsci12040293
do Nascimento GM, Rodrigues RA, Brugnera HC, Barbosa JC, Favaron FR Jr., Rossi GAM, de Bragança CRS, Schocken-Iturrino RP, de Ávila FA, Cardozo MV. Antimicrobial Activity of Teat Antiseptic Formulations Based on Plant Extracts for Controlling Bovine Mastitis: In Vitro and In Vivo Evaluation. Veterinary Sciences. 2025; 12(4):293. https://doi.org/10.3390/vetsci12040293
Chicago/Turabian Styledo Nascimento, Gabriel Michelutti, Romário Alves Rodrigues, Heloisa Cristina Brugnera, José Carlos Barbosa, Flavio Rubens Favaron, Jr., Gabriel Augusto Marques Rossi, Caio Roberto Soares de Bragança, Ruben Pablo Schocken-Iturrino, Fernando Antônio de Ávila, and Marita Vedovelli Cardozo. 2025. "Antimicrobial Activity of Teat Antiseptic Formulations Based on Plant Extracts for Controlling Bovine Mastitis: In Vitro and In Vivo Evaluation" Veterinary Sciences 12, no. 4: 293. https://doi.org/10.3390/vetsci12040293
APA Styledo Nascimento, G. M., Rodrigues, R. A., Brugnera, H. C., Barbosa, J. C., Favaron, F. R., Jr., Rossi, G. A. M., de Bragança, C. R. S., Schocken-Iturrino, R. P., de Ávila, F. A., & Cardozo, M. V. (2025). Antimicrobial Activity of Teat Antiseptic Formulations Based on Plant Extracts for Controlling Bovine Mastitis: In Vitro and In Vivo Evaluation. Veterinary Sciences, 12(4), 293. https://doi.org/10.3390/vetsci12040293








