Effects of Botanical Blend of Turmeric, Capsicum, and Pepper Extracts on Colostrum and Milk Yield and Quality, Passive Transfer of Immunity, and Performance of Beef Cow–Calf Pairs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Treatments
2.2. Blood Collections, Calving Procedures, and Colostrum Sampling
2.3. Milk Sampling
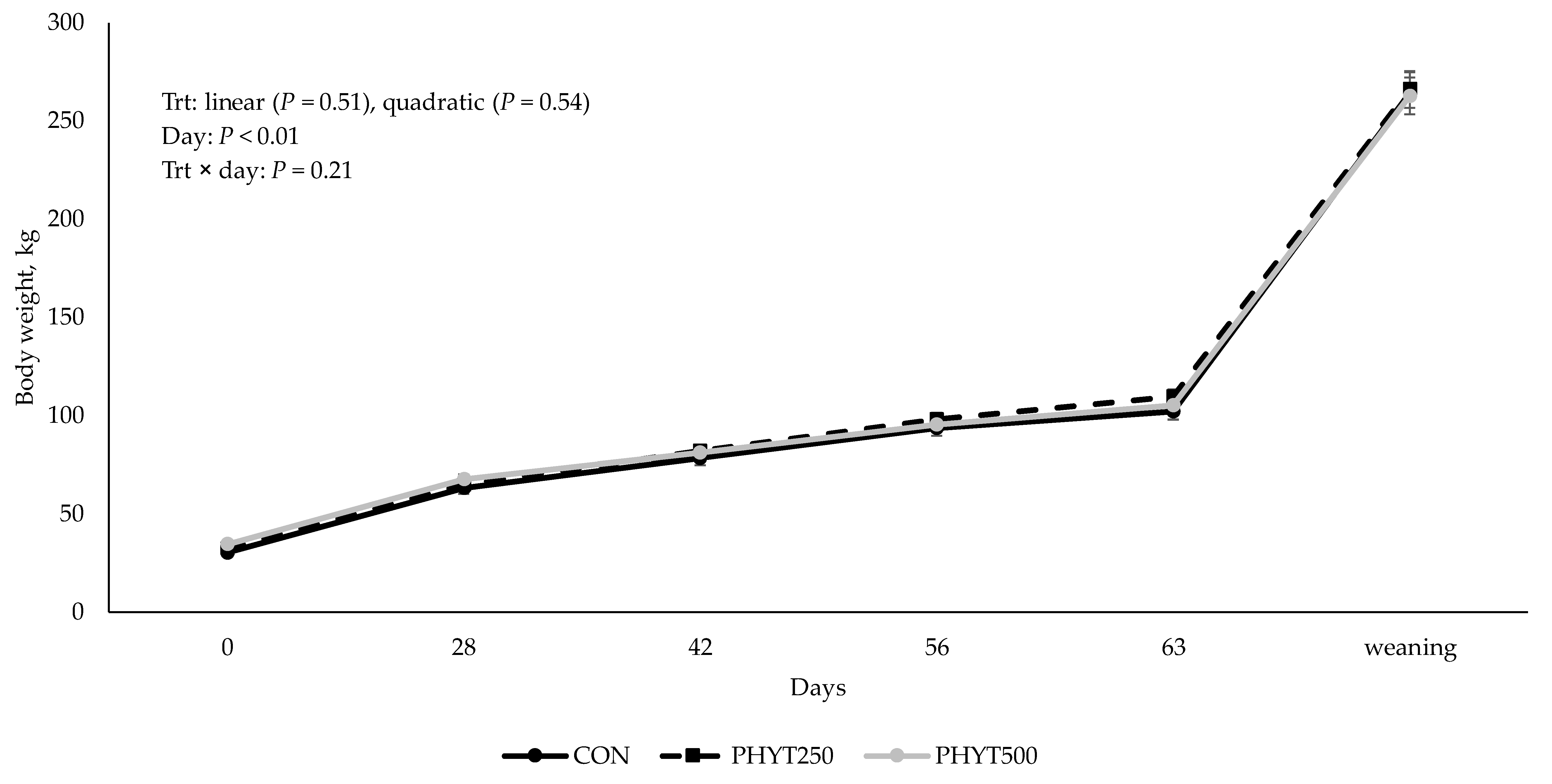
2.4. Cow–Calf Bodyweights
2.5. Radial Immunodiffusion Assays
2.6. Feed Sampling and Analyses
2.7. Statistical Analyses
3. Results
3.1. Colostrum and Milk Composition and Volume
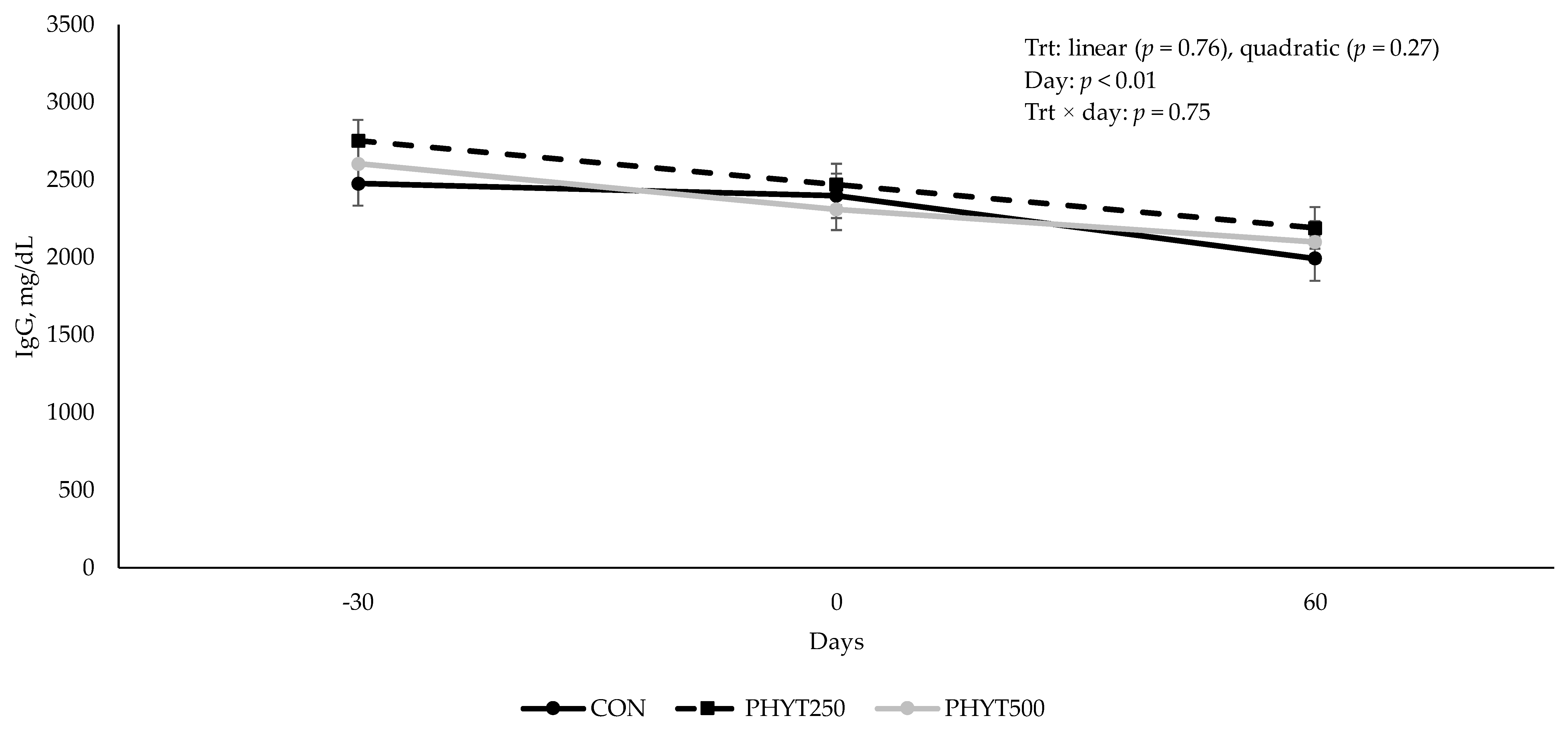
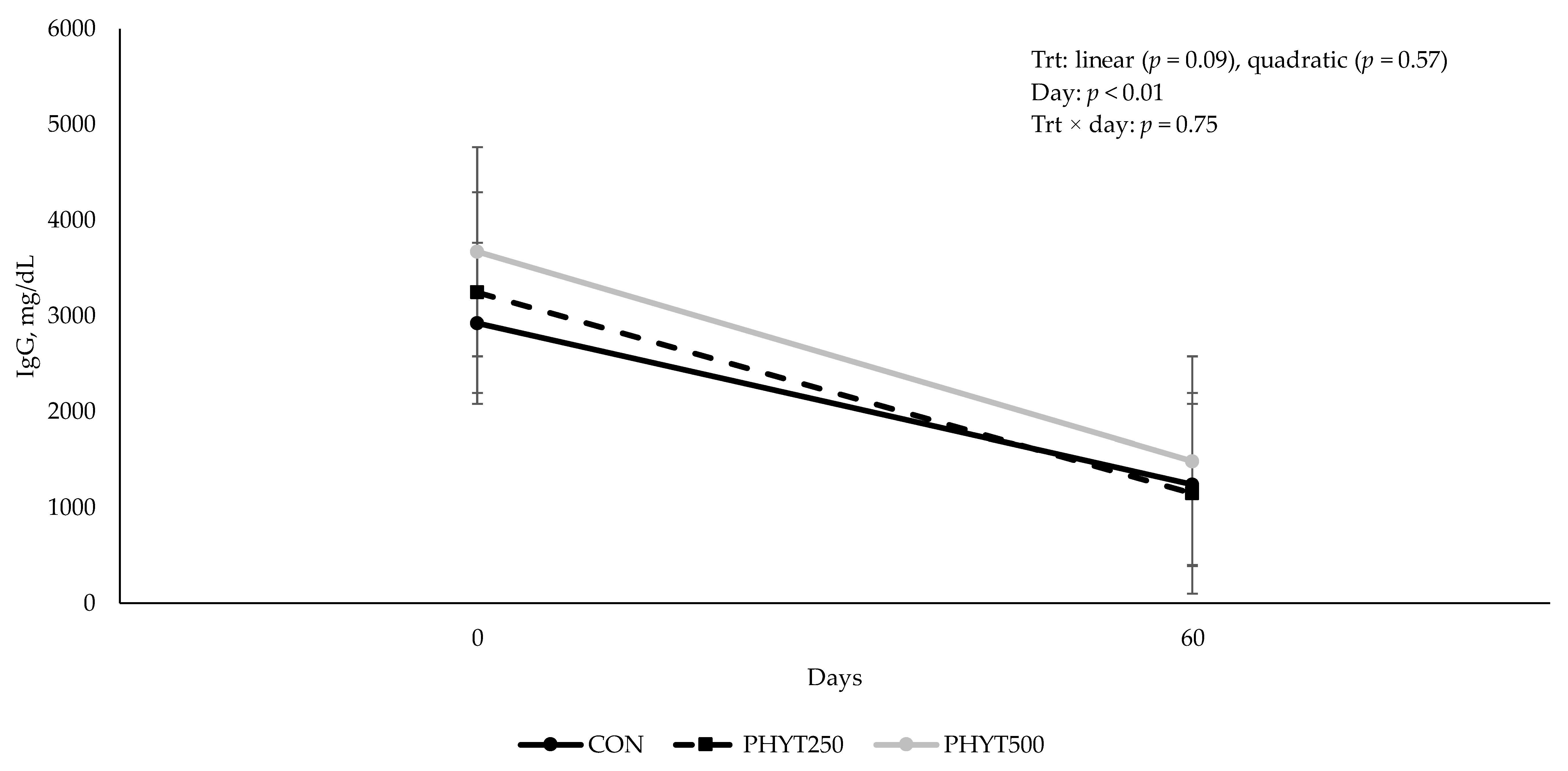
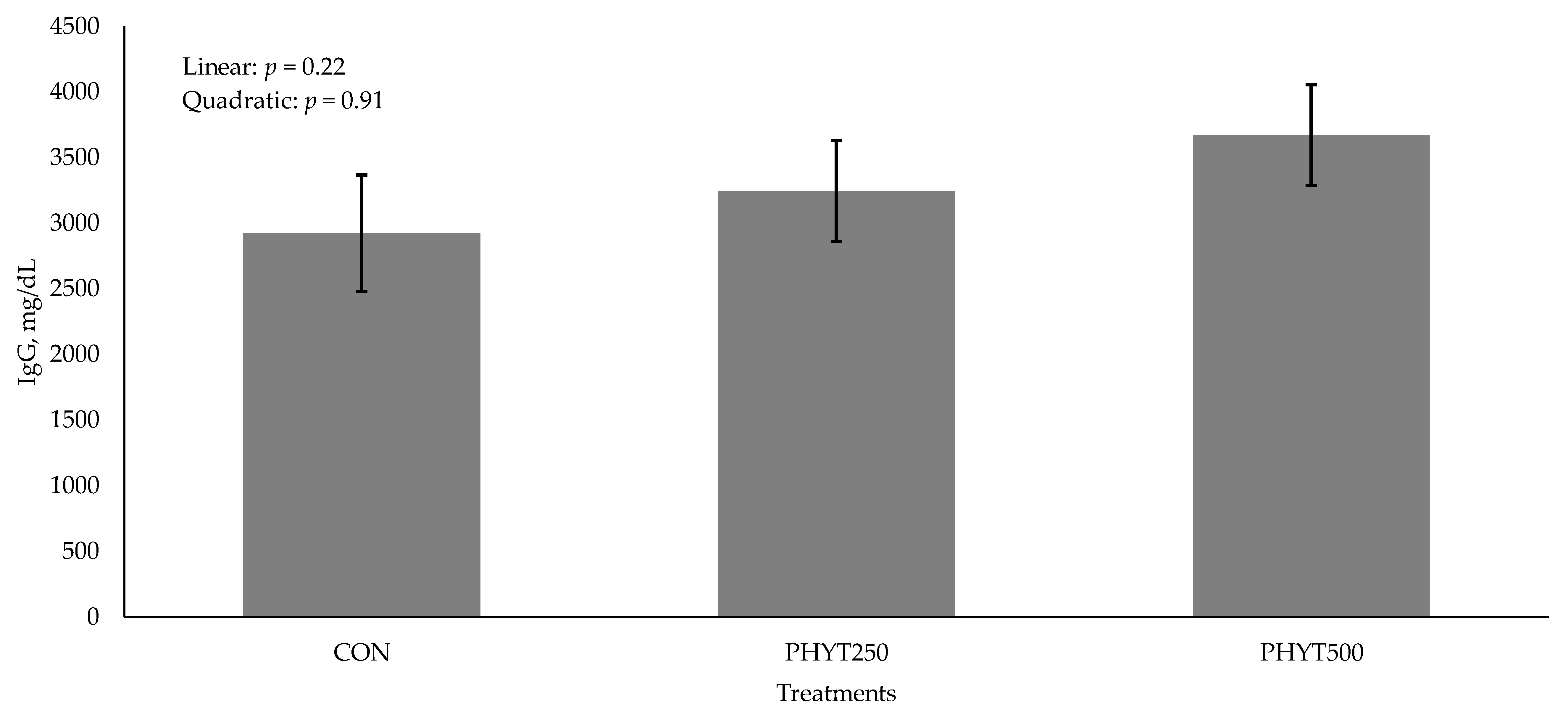
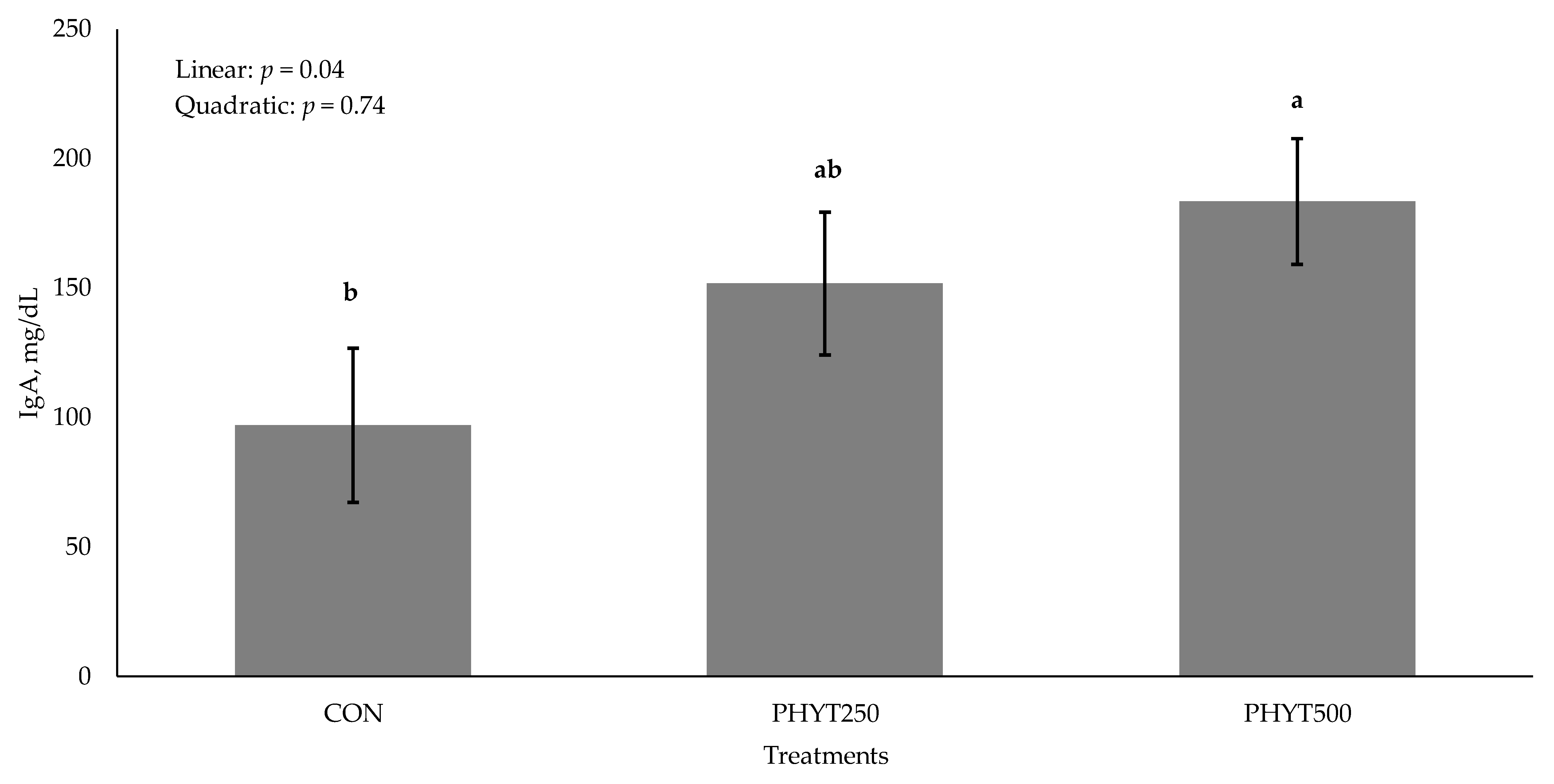
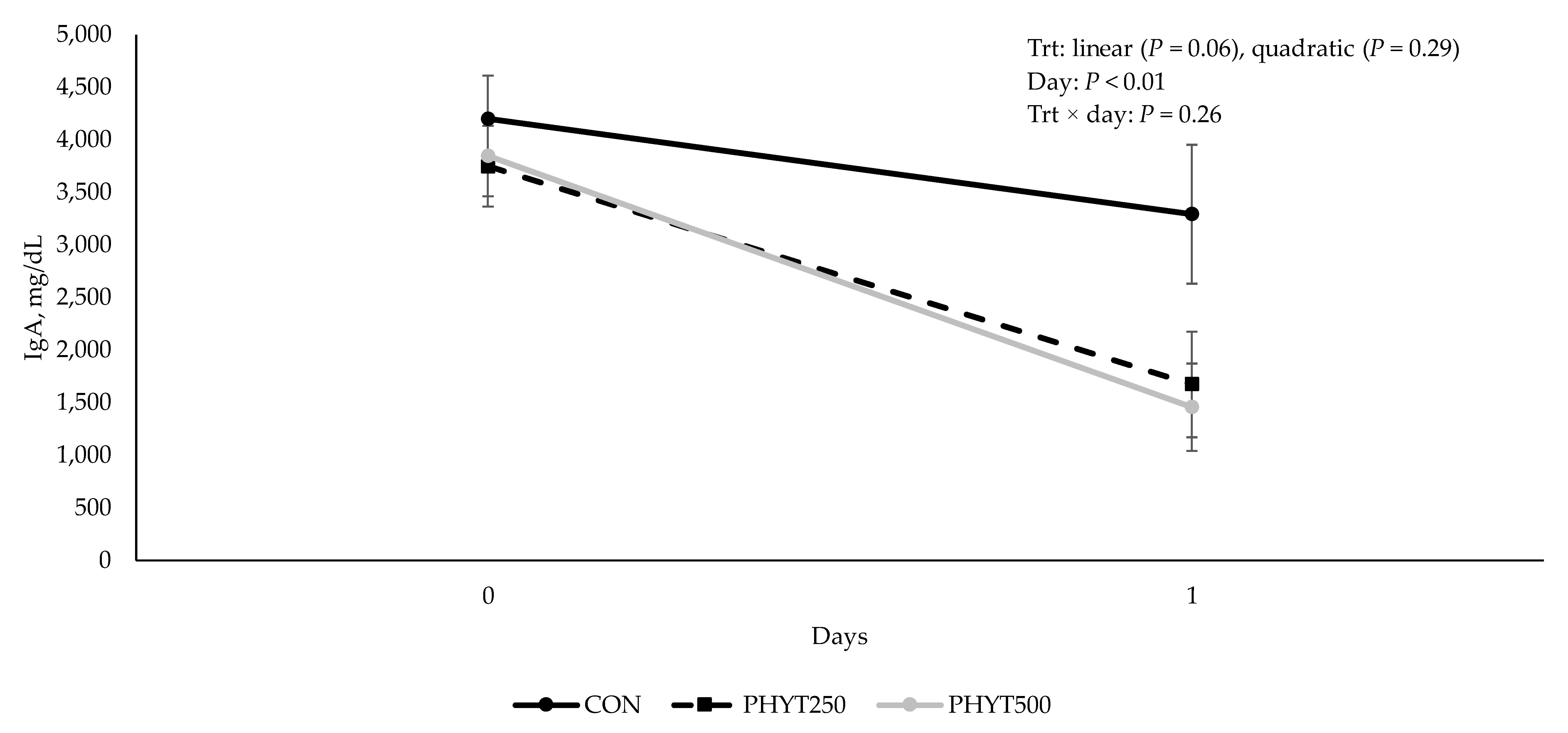
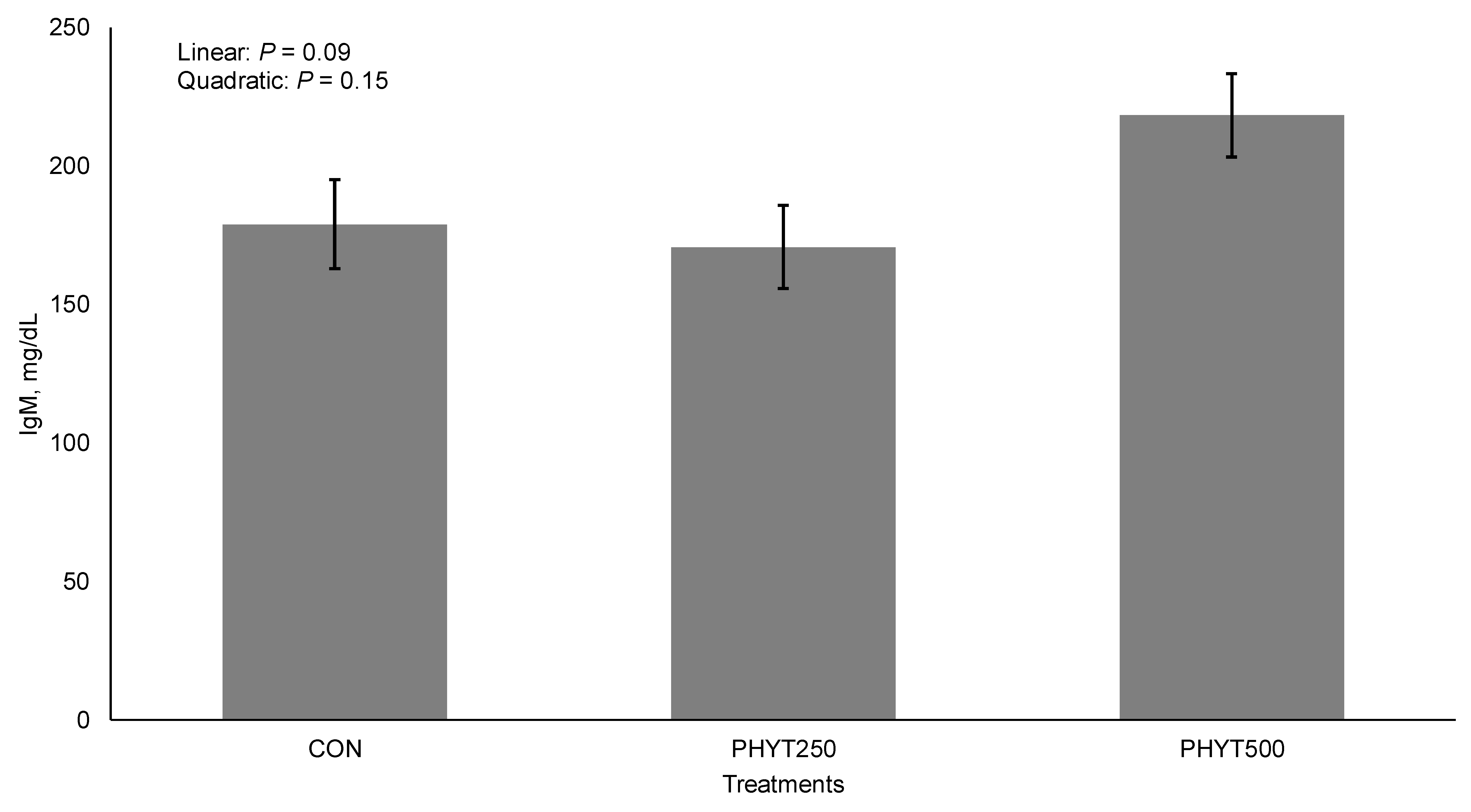
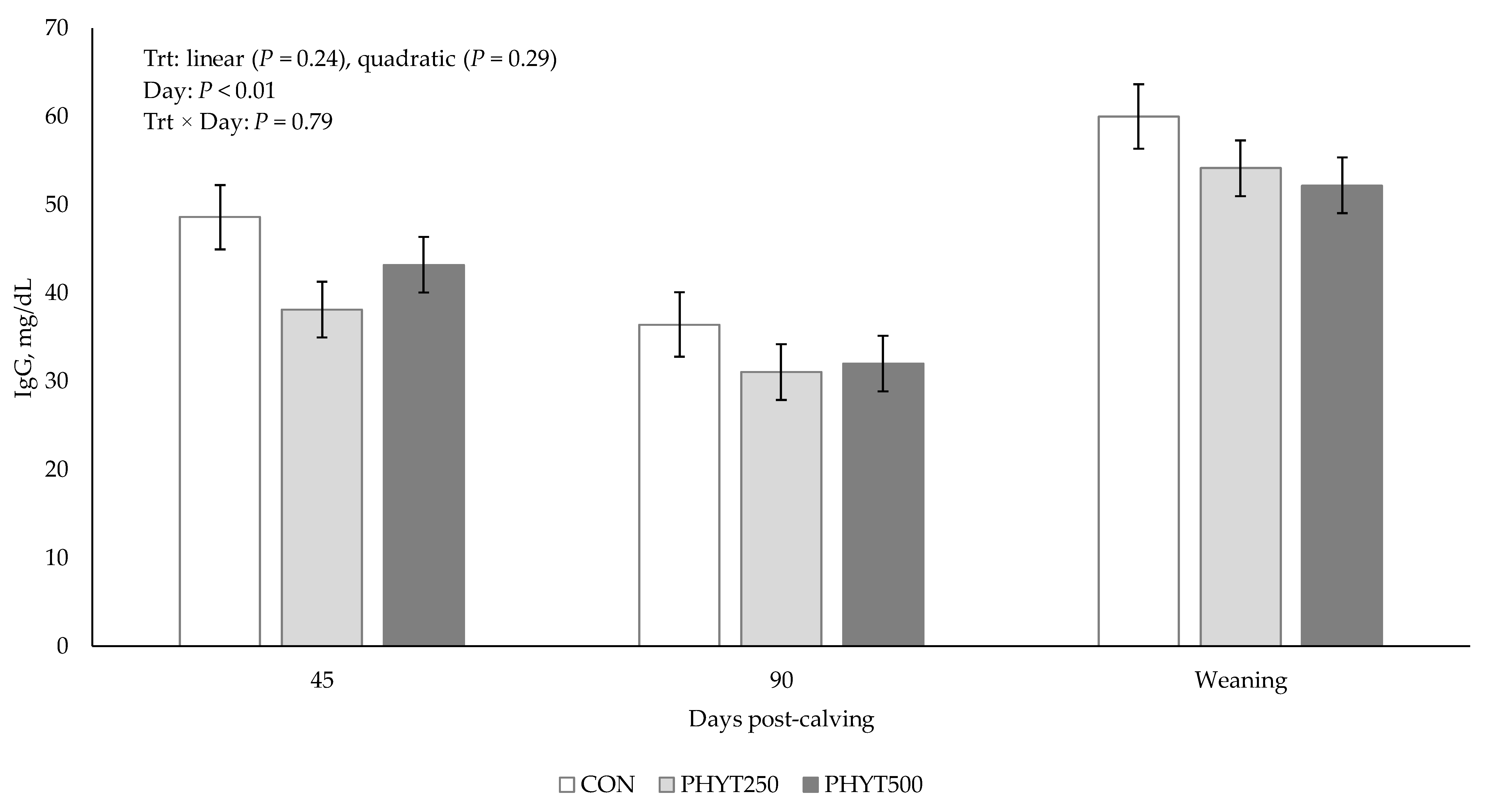
3.2. Immunoglobulin Concentrations in Serum, Colostrum, and Milk
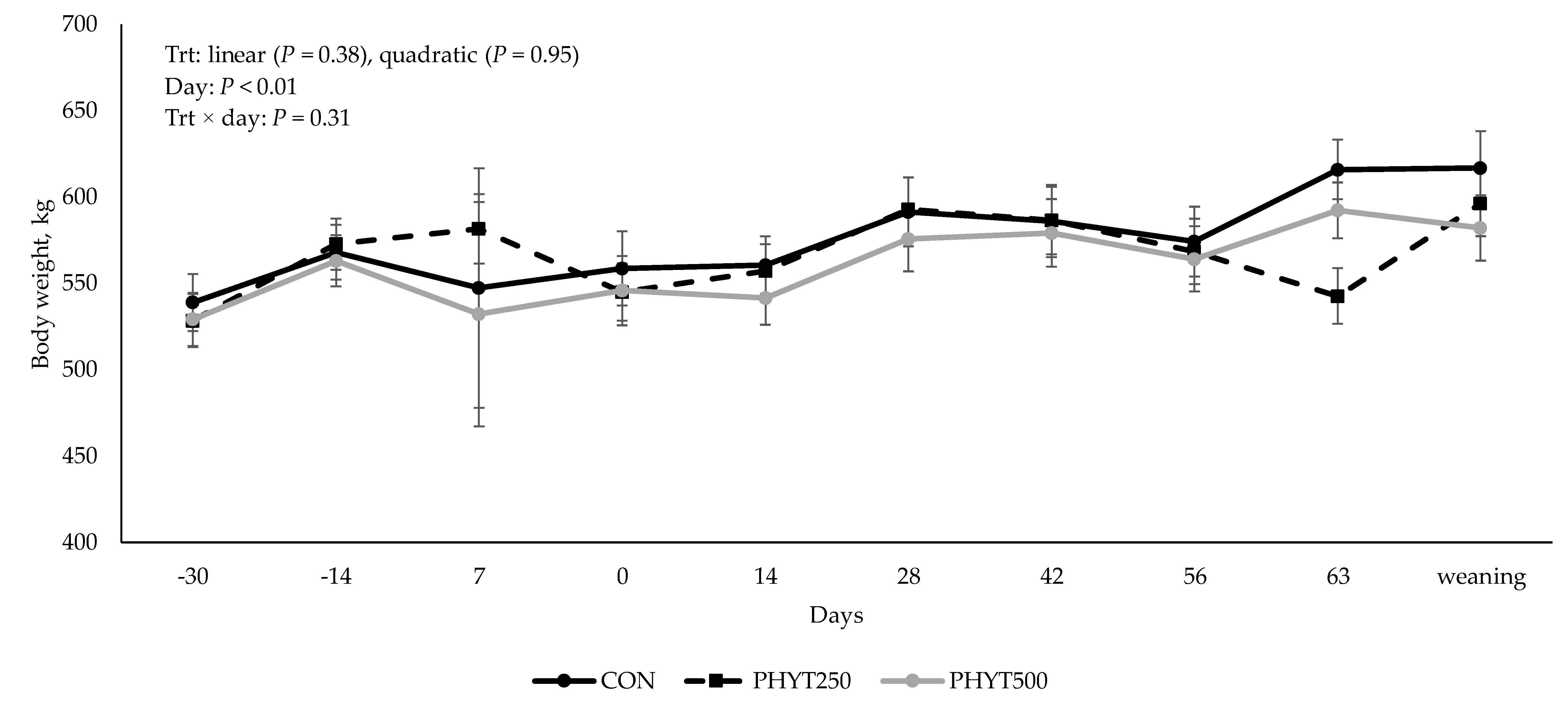
3.3. Cow and Calf Performance
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADG | Average daily gain |
| BW | Body weight |
| DDGS | Dried distiller grains with solubles |
| DM | Dry matter |
| Ig | Immunoglobulin |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| MUN | Milk urea nitrogen |
| RID | Radial immunodiffusion |
| TLR4 | Toll-like receptor 4 |
| TMR | Total mixed ration |
References
- McGee, M.; Earley, B. Passive immunity in beef-suckler calves. Animal 2019, 13, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Syed, B. Phytogenic feed additives in animal nutrition. In Medicinal and Aromatic Plants of the World: Scientific, Production, Commercial and Utilization Aspects; Springer: Dordrecht, The Netherlands, 2015; pp. 403–423. [Google Scholar]
- Fernandes, L.D.; Vasconcelos, A.B.; Lobo, A.R.; Rosado, G.L.; Bento, C.B. Effects of different additives on cattle feed intake and performance—A systematic review and meta-analysis. An. Acad. Bras. Ciênc. 2024, 96, e20230172. [Google Scholar] [CrossRef] [PubMed]
- Piran Filho, F.A.; Turner, T.D.; Mueller, I.; Daniel, J.L.P. Influence of phytogenic feed additive on performance of feedlot cattle. Front. Anim. Sci. 2021, 2, 767034. [Google Scholar] [CrossRef]
- Hassan, F.-u.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.-u.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet–microbe interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef]
- Rivera-Chacon, R.; Castillo-Lopez, E.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Supplementing a phytogenic feed additive modulates the risk of subacute rumen acidosis, rumen fermentation and systemic inflammation in cattle fed acidogenic diets. Animals 2022, 12, 1201. [Google Scholar] [CrossRef]
- van de Ligt, C.P.; Ravichandran, S.; Wall, E.H. PSVI-13 Formulated botanical preparation improves passive immunity from sows to piglets. J. Anim. Sci. 2024, 102 (Suppl. S2), 323–324. [Google Scholar] [CrossRef]
- Riley, D.; Chase Jr, C.; Olson, T.; Coleman, S.; Hammond, A. Genetic and nongenetic influences on vigor at birth and preweaning mortality of purebred and high-percentage Brahman calves. J. Anim. Sci. 2004, 82, 1581–1588. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds using refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef]
- Detmann, E.; Valadares Filho, S. On the estimation of non-fibrous carbohydrates in feeds and diets. Arq. Med. Vet. Zootec. 2010, 62, 980–984. [Google Scholar] [CrossRef]
- Oltner, R.; Wiktorsson, H. Urea concentrations in milk and blood as influenced by feeding varying amounts of protein and energy to dairy cows. Livest. Prod. Sci. 1983, 10, 457–467. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine colostrum: Its constituents and uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and factors affecting quality of bovine colostrum: A review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef]
- Brandon, M.R.; Watson, D.L.; Lascelles, A.K. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust. J. Exp. Biol. Med. Sci. 1971, 49, 613–623. [Google Scholar] [CrossRef]
- Barrington, G.M.; McFadden, T.B.; Huyler, M.T.; Besser, T.E. Regulation of colostrogenesis in cattle. Livest. Prod. Sci. 2001, 70, 95–104. [Google Scholar] [CrossRef]
- Redifer, C.A.; Wichman, L.G.; Rathert-Williams, A.R.; Freetly, H.C.; Meyer, A.M. Late gestational nutrient restriction in primiparous beef females: Nutrient partitioning among the dam, fetus, and colostrum during gestation. J. Anim. Sci. 2023, 101, skad195. [Google Scholar] [CrossRef]
- Hurlbert, J.L.; Baumgaertner, F.; Menezes, A.C.B.; Bochantin, K.A.; Diniz, W.J.; Underdahl, S.R.; Dorsam, S.T.; Kirsch, J.D.; Sedivec, K.K.; Dahlen, C.R. Supplementing vitamins and minerals to beef heifers during gestation: Impacts on mineral status in the dam and offspring, and growth and physiological responses of female offspring from birth to puberty. J. Anim. Sci. 2024, 102, skae002. [Google Scholar] [CrossRef]
- Reyes-Camacho, D.; Vinyeta, E.; Pérez, J.F.; Aumiller, T.; Criado, L.; Palade, L.M.; Taranu, I.; Folch, J.M.; Calvo, M.A.; Van der Klis, J.D. Phytogenic actives supplemented in hyperprolific sows: Effects on maternal transfer of phytogenic compounds, colostrum and milk features, performance and antioxidant status of sows and their offspring, and piglet intestinal gene expression. J. Anim. Sci. 2020, 98, skz390. [Google Scholar] [CrossRef]
- Ariza-Nieto, C.; Bandrick, M.; Baidoo, S.K.; Anil, L.; Molitor, T.W.; Hathaway, M. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J. Anim. Sci. 2011, 89, 1079–1089. [Google Scholar] [CrossRef]
- Okazaki, Y.; Han, Y.; Kayahara, M.; Watanabe, T.; Arishige, H.; Kato, N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2010, 56, 68–71. [Google Scholar] [CrossRef]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The emerging role of curcumin in the modulation of TLR-4 signaling pathway: Focus on neuroprotective and anti-rheumatic properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Mao, D. Effect of curcumin supplement in summer diet on blood metabolites, antioxidant status, immune response, and testicular gene expression in Hu sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lee, K.W.; Bravo, D.; Lillehoj, E.P. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet. Parasitol. 2011, 181, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26827/ (accessed on 29 January 2025).
- Brand, T.; Hünerberg, M.; McAllister, T.A.; He, M.; Saleem, A.M.; Shen, Y.; Miller, B.; Yang, W. Impact of a phytogenic feed additive on growth performance, feed intake, and carcass traits of beef cattle. Animals 2019, 9, 847. [Google Scholar] [CrossRef]
- Rivaroli, D.C.; Prado, R.M.D.; Ornaghi, M.G.; Mottin, C.; Ramos, T.R.; Barrado, A.G.; Jorge, A.M.; Prado, I.N.D. Essential oils in the diet of crossbred (½ Angus × ½ Nellore) bulls finished in feedlot on animal performance, feed efficiency, and carcass characteristics. J. Agric. Sci. 2017, 9, 205–212. [Google Scholar] [CrossRef]
- Rasby, R. Early Weaning: Effects on Cow and Calf Performance. Beef Cattle Production, 2024. Available online: https://beef.unl.edu/cattleproduction/earlyweaning (accessed on 19 February 2025).
- Maia Ribeiro, T.L.; Francis, B.B.G.; Ross, C.R.; Delver, J.J.; Francis, F.L.; Heldt, J.S.; Wall, E.H.; Rusche, W.C.; Smith, Z.K. Evaluation of a phytogenic blend fed with monensin on post-weaning growth performance, health, and sera metabolite responses during the initial 56 d feedlot receiving period in steer calves. Acta Agric. Scand. A Anim. Sci. 2024, 74, 18–26. [Google Scholar] [CrossRef]


| Experimental Diets | ||
|---|---|---|
| Item 1 | Pre-Calving | Post-Calving |
| Ingredients, % DM | ||
| Corn silage | 71 | 70 |
| Grass hay | 22 | - |
| Dried distiller grains plus solubles | 7 | - |
| Alfalfa hay | - | 30 |
| Chemical composition, g/kg DM | ||
| Organic matter | 943.4 | 939.7 |
| Crude protein | 90.9 | 110.1 |
| Ether extract | 23.9 | 19.9 |
| Neutral detergent fiber | 449.9 | 417.6 |
| Non-fiber carbohydrates | 378.8 | 392.0 |
| Item | Treatment 1 | Days | TRT Average | SEM 2 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Linear | Quadratic | Day | TRT × Day | ||||
| Fat, % | CON | 3.81 | 3.10 | 3.46 | 2.77 | 3.29 x | |||||
| PHYT250 | 5.14 | 3.92 | 4.40 | 3.44 | 4.23 y | 0.77 | 0.05 | 0.19 | 0.50 | 0.26 | |
| PHYT500 | 4.32 | 3.71 | 2.86 | 5.8 | 4.17 y | ||||||
| day average | 4.42 | 3.58 | 3.58 | 4.00 | |||||||
| Protein, % | CON | 14.65 | 6.97 | 5.03 | 4.56 | 7.80 | |||||
| PHYT250 | 13.77 | 6.19 | 4.94 | 4.40 | 7.33 | 0.59 | 0.36 | 0.09 | <0.01 | 0.16 | |
| PHYT500 | 15.7 | 7.23 | 5.42 | 4.64 | 8.24 | ||||||
| day average | 14.71 a | 6.79 b | 5.13 c | 4.53 d | |||||||
| Other solids, % | CON | 4.13 | 4.7 | 4.91 | 5.07 | 4.71 | |||||
| PHYT250 | 4.28 | 4.35 | 4.68 | 5.09 | 4.60 | 0.14 | 0.16 | 0.89 | <0.01 | 0.28 | |
| PHYT500 | 4.25 | 4.4 | 4.67 | 4.77 | 4.52 | ||||||
| day average | 4.22 d | 4.48 c | 4.76 b | 4.98 a | |||||||
| Lactose, % | CON | 2.88 | 3.67 | 3.96 | 4.14 | 3.66 | |||||
| PHYT250 | 2.98 | 3.34 | 3.75 | 4.19 | 3.57 | 0.15 | 0.21 | 0.96 | <0.01 | 0.45 | |
| PHYT500 | 3.01 | 3.36 | 3.69 | 3.87 | 3.48 | ||||||
| day average | 2.96 d | 3.45 c | 3.79 b | 4.07 a | |||||||
| Colostrum, mL | CON | 358.57 | 343.57 | 384.29 | 355.00 | 360.36 | |||||
| (left rear quarter) | PHYT250 | 457.50 | 200.00 | 272.50 | 351.25 | 320.31 | 60.93 | 0.48 | 0.33 | 0.25 | 0.42 |
| PHYT500 | 395.00 | 385.00 | 460.00 | 443.75 | 420.94 | ||||||
| day average | 403.69 | 309.52 | 372.26 | 383.33 | |||||||
| Component | Treatment 1 | Days | TRT Average | SEM 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 90 | Weaning | Linear | Quadratic | Day | TRT × Day | ||||
| Fat, % | CON | 4.07 | 3.66 | 3.79 | 3.84 | |||||
| PHYT250 | 3.95 | 3.87 | 4.33 | 4.05 | 1.04 | 0.07 | 0.56 | 0.85 | 0.83 | |
| PHYT500 | 4.68 | 4.82 | 4.64 | 4.71 | ||||||
| day average | 4.23 | 4.12 | 4.26 | |||||||
| Protein, % | CON | 3.19 | 3.18 | 3.70 | 3.36 | |||||
| PHYT250 | 3.26 | 3.13 | 3.97 | 3.45 | 0.18 | 0.95 | 0.29 | <0.01 | 0.14 | |
| PHYT500 | 3.27 | 3.13 | 3.69 | 3.36 | ||||||
| day average | 3.24 b | 3.15 c | 3.79 a | |||||||
| MUN mg/100 g | CON | 16.67 | 15.97 | 18.11 | 16.92 | |||||
| PHYT250 | 15.25 | 16.61 | 16.83 | 16.23 | 1.16 | 0.06 | 0.73 | 0.08 | 0.23 | |
| PHYT500 | 12.75 | 16.70 | 15.53 | 14.99 | ||||||
| day average | 14.89 | 16.43 | 16.82 | |||||||
| Other solids, % | CON | 5.67 | 5.60 | 4.55 | 5.27 | |||||
| PHYT250 | 5.65 | 5.61 | 4.44 | 5.23 | 0.27 | 0.55 | 0.97 | <0.01 | 0.30 | |
| PHYT500 | 5.63 | 5.26 | 4.66 | 5.18 | ||||||
| day average | 5.65 a | 5.49 b | 4.55 c | |||||||
| Lactose, % | CON | 4.76 | 4.71 | 3.59 | 4.35 | |||||
| PHYT250 | 4.74 | 4.71 | 3.48 | 4.31 | 0.28 | 0.67 | 0.96 | <0.01 | 0.36 | |
| PHYT500 | 4.73 | 4.42 | 3.71 | 4.28 | ||||||
| day average | 4.74 a | 4.61 b | 3.59 c | |||||||
| Volume, mL | CON | 5925.00 | 4501.67 | 3491.67 | 4639.44 | |||||
| PHYT250 | 6072.50 | 6321.25 | 4456.25 | 5616.67 | 604.06 | 0.12 | 0.28 | <0.01 | 0.50 | |
| PHYT500 | 6345.00 | 5922.00 | 4426.25 | 5564.58 | ||||||
| day average | 6114.17 a | 5581.81 a | 4124.72 b | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardon, G.H.; Kovarna, M.R.; Heldt, J.S.; Wall, E.H.; Menezes, A.C.B. Effects of Botanical Blend of Turmeric, Capsicum, and Pepper Extracts on Colostrum and Milk Yield and Quality, Passive Transfer of Immunity, and Performance of Beef Cow–Calf Pairs. Vet. Sci. 2025, 12, 250. https://doi.org/10.3390/vetsci12030250
Jardon GH, Kovarna MR, Heldt JS, Wall EH, Menezes ACB. Effects of Botanical Blend of Turmeric, Capsicum, and Pepper Extracts on Colostrum and Milk Yield and Quality, Passive Transfer of Immunity, and Performance of Beef Cow–Calf Pairs. Veterinary Sciences. 2025; 12(3):250. https://doi.org/10.3390/vetsci12030250
Chicago/Turabian StyleJardon, Grace H., Madison R. Kovarna, Jeff S. Heldt, Emma H. Wall, and Ana Clara B. Menezes. 2025. "Effects of Botanical Blend of Turmeric, Capsicum, and Pepper Extracts on Colostrum and Milk Yield and Quality, Passive Transfer of Immunity, and Performance of Beef Cow–Calf Pairs" Veterinary Sciences 12, no. 3: 250. https://doi.org/10.3390/vetsci12030250
APA StyleJardon, G. H., Kovarna, M. R., Heldt, J. S., Wall, E. H., & Menezes, A. C. B. (2025). Effects of Botanical Blend of Turmeric, Capsicum, and Pepper Extracts on Colostrum and Milk Yield and Quality, Passive Transfer of Immunity, and Performance of Beef Cow–Calf Pairs. Veterinary Sciences, 12(3), 250. https://doi.org/10.3390/vetsci12030250






