Getah Virus: A New Contaminant in Veterinary Vaccines
Simple Summary
Abstract
1. Introduction
2. Evidence of Contamination with GETV in Live PRRSV Vaccines
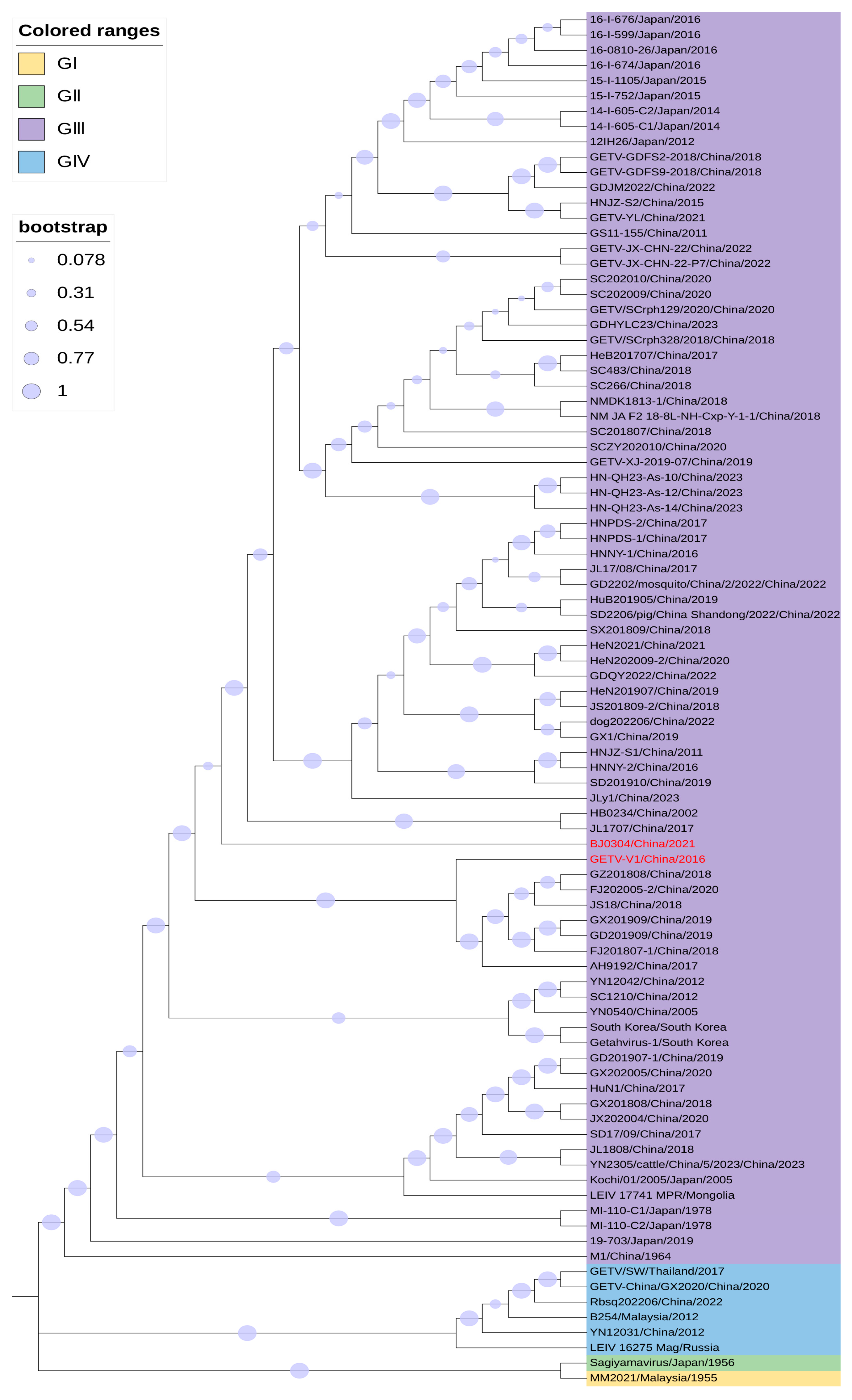
3. Genomic Comparison Between Vaccine-Contaminated GETV Strains and Other GETV Strains
3.1. Genomic Comparison of GETV-V1 and Other GETV Strains
3.2. Genomic Comparison of BJ0304 and Other GETV Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkema, H.W.; Bartels, C.J.; van Wuijckhuise, L.; Hesselink, J.W.; Holzhauer, M.; Weber, M.F.; Franken, P.; Kock, P.A.; Bruschke, C.J.; Zimmer, G.M. Outbreak of Bovine virus diarrhea on Dutch dairy farms induced by a bovine herpesvirus 1 marker vaccine contaminated with bovine virus diarrhea virus type 2. Tijdschr. Diergeneeskd. 2001, 126, 158–165. [Google Scholar] [PubMed]
- Su, Q.; Li, Y.; Zhang, Y.W.; Zhang, Z.H.; Meng, F.F.; Cui, Z.Z.; Chang, S.; Zhao, P. Newcastle disease virus-attenuated vaccine LaSota played a key role in the pathogenicity of contaminated exogenous virus. Vet. Res. 2018, 49, 80. [Google Scholar] [CrossRef]
- Thornton, D.H. A survey of mycoplasma detection in veterinary vaccines. Vaccine 1986, 4, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Harasawa, R.; Shintani, M.; Fujiwara, H.; Sasaki, Y.; Horino, A.; Kenri, T.; Asada, K.; Kato, I.; Chino, F. Application of PCR for detection of mycoplasma DNA and pestivirus RNA in human live viral vaccines. Biologicals 1996, 24, 371–375. [Google Scholar] [CrossRef]
- Kojima, A.; Takahashi, T.; Kijima, M.; Ogikubo, Y.; Nishimura, M.; Nishimura, S.; Harasawa, R.; Tamura, Y. Detection of Mycoplasma in avian live virus vaccines by polymerase chain reaction. Biologicals 1997, 25, 365–371. [Google Scholar] [CrossRef]
- Kojima, A.; Takahashi, T.; Kijima, M.; Ogikubo, Y.; Tamura, Y.; Harasawa, R. Detection of mycoplasma DNA in veterinary live virus vaccines by the polymerase chain reaction. J. Vet. Med. Sci. 1996, 58, 1045–1048. [Google Scholar] [CrossRef][Green Version]
- Awad, A.M.; Abd El-Hamid, H.S.; Abou Rawash, A.A.; Ibrahim, H.H. Detection of reticuloendotheliosis virus as a contaminant of fowl pox vaccines. Poult. Sci. 2010, 89, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Dong, X.; Yang, C.H.; Li, Q.H.; Cui, Z.Z.; Chang, S.; Zhao, P.; Yu, K.Z.; Yang, H.C. Isolation, identification, and whole genome sequencing of reticuloendotheliosis virus from a vaccine against Marek’s disease. Poult. Sci. 2015, 94, 643–649. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Cui, Z.Z.; Chang, S.; Zhao, P. Genome analysis and pathogenicity of reticuloendotheliosis virus isolated from a contaminated vaccine seed against infectious bursal disease virus: First report in China. J. Gen. Virol. 2016, 97, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.; Cheng, S. Detection and characterization of avian leukosis virus in Marek’s disease vaccines. Avian Dis. 2006, 50, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Shittu, I.; Adedeji, A.J.; Luka, P.D.; Asala, O.O.; Sati, N.M.; Nwagbo, I.O.; Chinyere, C.N.; Arowolo, O.O.; Adole, J.A.; Emennaa, P.; et al. Avian leukosis virus subgroup-J as a contaminant in live commercially available poultry vaccines distributed in Nigeria. Biologicals 2019, 57, 29–33. [Google Scholar] [CrossRef]
- Mao, Y.Q.; Su, Q.; Li, J.P.; Jiang, T.Z.; Wang, Y.X. Avian leukosis virus contamination in live vaccines: A retrospective investigation in China. Vet. Microbiol. 2020, 246, 108712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Cui, S.; Fu, J.Y.; Wang, Y.X.; Cui, Z.Z.; Fang, L.C.; Chang, S.; Zhao, P. Molecular characterization of chicken infectious anemia virus from contaminated live-virus vaccines. Poult. Sci. 2017, 96, 1045–1051. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.J.; Chen, L.F.; Wang, Q.; Zhou, M.; Zhao, H.; Chi, Z.N.; Wang, Y.X.; Chang, S.; Zhao, P. Genomic Characterization of CIAV Detected in Contaminated Attenuated NDV Vaccine: Epidemiological Evidence of Source and Vertical Transmission From SPF Chicken Embryos in China. Front. Vet. Sci. 2022, 9, 930887. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, N.; Velazquez-Salinas, L.; Ridpath, J.F.; Verdugo-Rodríguez, A.; Basurto-Alcántara, F.J. Detection and genotyping of bovine viral diarrhea virus found contaminating commercial veterinary vaccines, cell lines, and fetal bovine serum lots originating in Mexico. Arch. Virol. 2021, 166, 1999–2003. [Google Scholar] [CrossRef]
- Harasawa, R. Adventitious pestivirus RNA in live virus vaccines against bovine and swine diseases. Vaccine 1995, 13, 100–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.J.; Dong, Y.Q.; Wang, J.; Wu, F.X.; Liu, S.; Shao, W.X.; Li, X.C.; Wang, S.S. Detection and Analysis of Bovine Viral Diarrhea Virus Contamination in Classical Swine Fever and Porcine Reproductive and Respiratory Syndrome Vaccines. China Anim. Health Insp. 2015, 32, 76–79. [Google Scholar]
- Yoshikawa, R.; Shimode, S.; Sakaguchi, S.; Miyazawa, T. Contamination of live attenuated vaccines with an infectious feline endogenous retrovirus (RD-114 virus). Arch. Virol. 2014, 159, 399–404. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Sato, E.; Miyazawa, T. Contamination of infectious RD-114 virus in vaccines produced using non-feline cell lines. Biologicals 2011, 39, 33–37. [Google Scholar] [CrossRef]
- Zhai, S.L.; Lu, S.S.; Wei, W.K.; Lv, D.H.; Wen, X.H.; Zhai, Q.; Chen, Q.L.; Sun, Y.W.; Xi, Y. Reservoirs of Porcine Circoviruses: A Mini Review. Front. Vet. Sci. 2019, 19, 319. [Google Scholar] [CrossRef]
- Cui, X.Y.; Xia, D.S.; Luo, L.Z.; An, T.Q. Recombination of Porcine Reproductive and Respiratory Syndrome Virus: Features, Possible Mechanisms, and Future Directions. Viruses 2024, 16, 929. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, J.F.; Zhou, J.; Long, Y.P.; Xiao, L.; Fan, Y.D.; Yang, D.J.; Zhang, B.; Zhang, Z.D.; Liu, L. A novel NADC34-like porcine reproductive and respiratory syndrome virus 2 with complex genome recombination is highly pathogenic to piglets. Infect. Genet. Evol. 2023, 112, 105436. [Google Scholar] [CrossRef]
- Zhao, D.S.; Yang, B.; Yuna, X.G.; Shen, C.C.; Zhang, D.J.; Shi, X.J.; Zhang, T.; Cui, H.M.; Yang, J.K.; Chen, X.H.; et al. Advanced Research in Porcine Reproductive and Respiratory Syndrome Virus Co-infection With Other Pathogens in Swine. Front. Vet. Sci. 2021, 8, 699561. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Kumanomido, T.; Kamada, M. Getah virus as an equine pathogen. Vet. Clin. N. Am. Equine Pract. 2000, 16, 605–617. [Google Scholar] [CrossRef]
- Lu, G.; Chen, R.A.; Shao, R.; Dong, N.; Liu, W.Q.; Li, S.J. Getah virus: An increasing threat in China. J. Infect. 2020, 80, 350–371. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.Y.; Liang, G.D. Getah Virus (Alphavirus): An Emerging, Spreading Zoonotic Virus. Pathogens 2022, 11, 945. [Google Scholar] [CrossRef]
- Chu, P.P.; Guo, H.Y.; Zhou, X.; Chen, S.N.; Sun, X.R.; Tian, S.C.; Zou, Y.G.; Li, C.L.; Zhang, R.; Zhai, S.L. Emergence of a novel GIII Getah virus variant in pigs in Guangdong, China, 2023. Microbiol. Spectrum 2024, 12, e0048324. [Google Scholar] [CrossRef]
- Yang, T.; Li, R.; Hu, Y.; Yang, L.; Zhao, D.; Du, L.; Li, J.; Ge, M.; Yu, X. An outbreak of Getah virus infection among pigs in China, 2017. Transbound. Emerg. Dis. 2018, 65, 632–637. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.P.; Kuang, Z.P.; Lin, L.M.; Zhang, H.; Yin, L.J.; Hong, J.B.; Ren, B.H.; Li, Q.H.; Wang, L.X. Isolation and pathogenicity of a highly virulent group III porcine Getah virus in China. Front. Cell. Infect. Microbiol. 2024, 14, 1494654. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, A.J.; Chen, L.; Wang, X.G.; Cui, D.D.; Chang, H.T.; Wang, C.Q. Isolation and phylogenetic analysis of Getah virus from a commercial modified live vaccine against porcine reproductive and respiratory syndrome virus. Mol. Cell. Probes 2020, 53, 101650. [Google Scholar] [CrossRef]
- Gao, X.T.; Li, J.L.; Wu, T.; Dou, J.P.; Zhang, W.R.; Jia, H.; Zhang, Z.F.; Liu, X.J.; Li, Y.N. The Isolation and Characterization of a Novel Group III-Classified Getah Virus from a Commercial Modified Live Vaccine against PRRSV. Viruses 2023, 15, 2090. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Qiu, X.S.; Cao, X.Y.; Mai, Z.H.; Zhu, X.Y.; Li, N.; Zhang, H.; Zhang, J.Y.; Li, Z.X.; Shaya, N.; et al. Molecular and serological surveillance of Getah virus in the Xinjiang Uygur Autonomous Region, China, 2017–2020. Virol. Sin. 2022, 37, 229–237. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, Y.X.; Guan, Z.X.; Zhang, Y.; Li, Y.H.; Yang, Y.; Zhang, J.J.; Li, Z.J.; Qiu, Y.F.; Li, B.B.; et al. Seroprevalence of Getah virus in Pigs in Eastern China Determined with a Recombinant E2 Protein-Based Indirect ELISA. Viruses 2022, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Zhang, H.Q.; Chen, X.Q.; Zeng, M.Y.; Han, H.; Guo, Y.J.; Li, J.M.; Luo, S.C.; Yan, G.Z.; Chen, S.N.; et al. Evolutionary characterization and pathogenicity of Getah virus from pigs in Guangdong Province of China. Arch. Virol. 2023, 168, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dellicour, S.; Yan, Z.Q.; Veit, M.; Gill, M.S.; He, W.T.; Zhai, X.F.; Ji, X.; Suchard, M.A.; Lemey, P.; et al. Early Genomic Surveillance and Phylogeographic Analysis of Getah Virus, a Reemerging Arbovirus, in Livestock in China. J. Virol. 2023, 97, e0109122. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zhang, Q.Y.; Shan, H.; Cao, Z.; Huang, J. Genome characteristics of atypical porcine pestivirus from abortion cases in Shandong Province, China. Virol. J. 2023, 20, 282. [Google Scholar] [CrossRef]
- Lan, J.H.; Fang, M.T.; Duan, L.L.; Liu, Z.; Wang, G.G.; Wu, Q.; Fan, K.; Huang, D.Y.; Ye, Y.; Wan, G.; et al. Novel Porcine Getah Virus from Diarrheal Piglets in Jiangxi Province, China: Prevalence, Genome Sequence, and Pathogenicity. Animals 2024, 14, 2980. [Google Scholar] [CrossRef]
| Strains | GETV-V1 | BJ0304 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete Genome | 5′ UTR | Non-Structural Polyprotein | Structural Polyprotein | 3′ UTR | Complete Genome | 5′ UTR | Non-Structural Polyprotein | Structural Polyprotein | 3′ UTR | |||||
| nt | nt | nt | aa | nt | aa | nt | nt | nt | nt | aa | nt | aa | nt | |
| MM2021 | 95.2 | 97.4 | 95.4 | 98.7 | 95.1 | 98.1 | 93.5 | 95.3 | 98.7 | 95.5 | 98.5 | 95.2 | 98 | 94.2 |
| Sagiyamavirus | 97.1 | 98.7 | 97.4 | 99 | 97 | 98.4 | 94.6 | 97.3 | 100 | 97.6 | 99 | 97 | 98.4 | 95.3 |
| M1 | 98 | 98.7 | 98.2 | 99 | 97.9 | 98.8 | 97.2 | 98.2 | 100 | 98.3 | 99 | 98 | 98.8 | 98 |
| 16-I-676 | 98.8 | 98.7 | 98.9 | 99.4 | 98.8 | 99.6 | 98.2 | 98.9 | 100 | 98.9 | 99.2 | 98.9 | 99.6 | 99 |
| 15-I-752 | 98.8 | 98.7 | 99 | 99.5 | 98.8 | 99.6 | 98.2 | 98.9 | 100 | 98.9 | 99.3 | 98.9 | 99.6 | 99 |
| 14-I-605-C2 | 98.8 | 98.7 | 99 | 99.5 | 98.8 | 99.6 | 98 | 98.9 | 100 | 99 | 99.3 | 98.9 | 99.6 | 98.7 |
| 12IH26 | 98.9 | 98.7 | 99 | 99.6 | 98.9 | 99.6 | 98.2 | 99 | 100 | 99 | 99.4 | 98.9 | 99.6 | 99 |
| GETV-GDFS2-2018 | 98.7 | 98.7 | 98.8 | 99.4 | 98.7 | 99.4 | 98.2 | 98.8 | 100 | 98.8 | 99.2 | 98.8 | 99.4 | 99 |
| HNJZ-S2 | 98.8 | 98.7 | 98.9 | 99.6 | 98.9 | 99.6 | 98.5 | 98.9 | 100 | 98.9 | 99.4 | 99 | 99.6 | 99.2 |
| HNPDS-2 | 98.9 | 98.7 | 99.1 | 99.5 | 98.8 | 99.7 | 98 | 99.1 | 100 | 99.2 | 99.5 | 98.9 | 99.7 | 98.7 |
| HNNY-2 | 98.9 | 98.7 | 99.1 | 99.5 | 98.9 | 99.7 | 98.2 | 99.1 | 100 | 99.1 | 99.5 | 99 | 99.7 | 99 |
| JL17/08 | 98.9 | 98.7 | 99.1 | 99.5 | 98.9 | 99.6 | 98 | 99 | 100 | 99.1 | 99.4 | 98.9 | 99.6 | 98.7 |
| HNJZ-S1 | 98.9 | 98.7 | 99.1 | 99.4 | 98.9 | 99.7 | 98.2 | 99.1 | 100 | 99.1 | 99.2 | 99 | 99.7 | 99 |
| SC266 | 98.5 | 98.7 | 98.7 | 99.1 | 98.5 | 99.4 | 97.5 | 98.7 | 100 | 98.7 | 99 | 98.6 | 99.4 | 98.2 |
| SC483 | 98.5 | 98.7 | 98.7 | 99.2 | 98.4 | 99.4 | 97.5 | 98.7 | 100 | 98.8 | 99.1 | 98.5 | 99.4 | 98.2 |
| SC201807 | 98.7 | 97.4 | 98.9 | 99.5 | 98.7 | 99.6 | 95.5 | 98.8 | 98.7 | 98.9 | 99.4 | 98.8 | 99.6 | 96.2 |
| JL1707 | 98.9 | 98.7 | 99.1 | 99.5 | 98.8 | 99.3 | 98.5 | 99.1 | 100 | 99.1 | 99.3 | 98.9 | 99.3 | 99.2 |
| HB0234 | 98.9 | 98.7 | 99.1 | 99.3 | 98.8 | 99.3 | 98.5 | 99.1 | 100 | 99.2 | 99.2 | 98.9 | 99.3 | 99.2 |
| JS18 | / | / | 99.5 | 99.6 | 99.4 | 99.7 | / | 96.6 | / | 99 | 99.3 | 98.6 | 99.2 | / |
| AH9192 | 99.4 | 98.7 | 99.3 | 99.3 | 99.4 | 99.6 | 99.5 | 98.7 | 100 | 98.9 | 99.1 | 98.6 | 99.2 | 98.7 |
| GETV-V1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99 | 98.7 | 99.1 | 99.3 | 98.9 | 99.5 | 98.2 |
| YN12042 | 98.4 | 98.7 | 98.8 | 99.3 | 98.9 | 99.6 | 88.3 | 98.5 | 100 | 98.8 | 99.2 | 99 | 99.7 | 88.6 |
| SC1210 | 98.7 | 98.7 | 98.9 | 99.4 | 98.6 | 99.5 | 96.7 | 98.8 | 100 | 98.9 | 99.3 | 98.7 | 99.5 | 97.5 |
| YN0540 | 98.8 | 98.7 | 99 | 99.4 | 98.9 | 99.6 | 96.7 | 99 | 100 | 99 | 99.3 | 99 | 99.6 | 97.5 |
| South Korea | 98.2 | 98.7 | 99.2 | 99.5 | 99.2 | 99.6 | 70.2 | 98.4 | 100 | 99.3 | 99.4 | 99.3 | 99.8 | 70.6 |
| GX201808 | 97.3 | 97.4 | 97.3 | 99 | 97.3 | 99 | 98.5 | 97.5 | 98.7 | 97.4 | 99 | 97.5 | 99 | 98.2 |
| HuN1 | 97.5 | 98.7 | 97.4 | 99.2 | 97.8 | 99.2 | 97.7 | 97.7 | 100 | 97.5 | 99.1 | 97.9 | 99.2 | 98 |
| SD17/09 | 97.7 | 98.7 | 97.7 | 99.3 | 97.6 | 99.2 | 98.5 | 97.8 | 100 | 97.8 | 99.3 | 97.7 | 99.2 | 98.7 |
| JL1808 | 97.7 | 98.7 | 97.8 | 99.3 | 97.7 | 99.3 | 98.2 | 97.9 | 100 | 97.9 | 99.3 | 97.7 | 99.3 | 98.5 |
| LEIV 17741 MPR | 98.5 | 98.7 | 98.5 | 99.3 | 98.7 | 99.7 | 98.2 | 98.7 | 100 | 98.6 | 99.2 | 98.8 | 99.7 | 99 |
| MI-110-C1 | 98.6 | 98.7 | 98.7 | 99.5 | 98.6 | 99.6 | 98 | 98.7 | 100 | 98.7 | 99.3 | 98.7 | 99.6 | 98.7 |
| B254 | / | / | 96.3 | 98.4 | 96.3 | 98.4 | / | / | / | 96.4 | 98.2 | 96.1 | 98 | / |
| YN12031 | 96.2 | 97.4 | 96.3 | 98.6 | 96.4 | 98.8 | 95.5 | 96.1 | 98.7 | 96.3 | 98.5 | 96.3 | 98.4 | 96 |
| GETV/SW/Thailand/2017 | 96 | 98.7 | 96.3 | 98.8 | 95.8 | 98.6 | 94.7 | 95.9 | 100 | 96.3 | 98.7 | 95.6 | 98.3 | 95.2 |
| LEIV 16275 Mag | 97.4 | 97.4 | 97.6 | 99.2 | 97.5 | 99.2 | 96 | 97.4 | 98.7 | 97.7 | 99.1 | 97.6 | 99.2 | 96.7 |
| GETV-JX-CHN-22 | 98.6 | 97.4 | 98.8 | 99.5 | 98.7 | 99.5 | 98 | 98.8 | 98.7 | 98.8 | 99.3 | 98.7 | 99.5 | 98.7 |
| GETV-YL | 98.7 | 98.7 | 98.9 | 99.5 | 98.8 | 99.7 | 98.2 | 98.8 | 100 | 98.9 | 99.3 | 98.8 | 99.6 | 99 |
| GETV-XJ-2019-07 | 98.3 | 98.7 | 98.5 | 99.3 | 98.4 | 99.5 | 97.5 | 98.5 | 100 | 98.5 | 99.2 | 98.4 | 99.5 | 98.2 |
| GETV/SCrph328 | / | / | 98.9 | 99.6 | 98.5 | 99.6 | / | / | / | 98.9 | 99.5 | 98.7 | 99.6 | / |
| GS11-155 | 98.9 | 98.7 | 99.1 | 99.5 | 98.8 | 99.7 | 98.2 | 99.1 | 100 | 99.2 | 99.4 | 98.9 | 99.7 | 98.5 |
| dog202206 | 0 | 98.7 | 98.8 | 99.5 | 98.5 | 99.3 | 0 | 0 | 100 | 98.9 | 99.4 | 98.5 | 99.2 | 0 |
| Rbsq202206 | 96 | 98.7 | 96.3 | 98.7 | 95.9 | 98.5 | 94.7 | 95.9 | 100 | 96.4 | 98.6 | 95.7 | 98.2 | 95.2 |
| GDQY2022 | 98.8 | 98.7 | 99 | 99.6 | 98.9 | 99.7 | 98 | 98.9 | 100 | 99 | 99.4 | 98.9 | 99.7 | 98.7 |
| GDJM2022 | 98.5 | 97.4 | 98.7 | 99.5 | 98.5 | 99.3 | 98.2 | 98.6 | 98.7 | 98.6 | 99.3 | 98.6 | 99.3 | 99 |
| SCZY202010 | 98.6 | 98.7 | 98.6 | 99.1 | 98.8 | 99.6 | 97.5 | 98.7 | 100 | 98.7 | 98.9 | 98.8 | 99.6 | 98.2 |
| HeN2021 | 98.7 | 98.7 | 98.9 | 99.5 | 98.7 | 99.5 | 98 | 98.9 | 100 | 98.9 | 99.3 | 98.8 | 99.5 | 98.7 |
| GD201907-1 | / | / | 97.2 | 98.7 | 97.5 | 99.1 | / | / | / | 97.3 | 98.7 | 97.6 | 99.1 | / |
| GX201909 | / | / | 99.4 | 99.6 | 99.4 | 99.7 | 0 | / | / | 98.9 | 99.3 | 98.6 | 99.2 | / |
| HeB201707 | / | / | 98.6 | 99 | 98.4 | 99.4 | 97.5 | / | / | 98.6 | 98.9 | 98.5 | 99.4 | 98.2 |
| HeN201907 | / | / | 98.8 | 99.5 | 98.7 | 99.6 | 0 | / | / | 98.9 | 99.4 | 98.7 | 99.4 | / |
| BJ0304 | 99 | 98.7 | 99.1 | 99.3 | 98.9 | 99.5 | 98.2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 19-703 | / | / | 98.6 | 99.4 | 98.5 | 99.3 | 0 | / | / | 98.6 | 99.2 | 98.6 | 99.3 | / |
| NMDK1813-1 | 98.3 | 98.7 | 98.8 | 99.6 | 98.5 | 99.6 | 85.8 | 98.4 | 100 | 98.9 | 99.5 | 98.7 | 99.6 | 86.5 |
| GZ201808 | / | / | 99.4 | 99.5 | 99.4 | 99.6 | / | / | / | 99 | 99.3 | 98.6 | 99.2 | / |
| GDHYLC23 | 98.3 | 97.4 | 98.8 | 99.5 | 98.5 | 99.6 | 89.4 | 98.5 | 98.7 | 98.8 | 99.4 | 98.6 | 99.6 | 90 |
| GD2202/mosquito/China/2/2022 | 98.9 | 98.7 | 99.1 | 99.6 | 98.8 | 99.6 | 98 | 99 | 100 | 99.1 | 99.3 | 99 | 99.7 | 98.7 |
| GETV-GDFS9-2018 | 98.7 | 98.7 | 98.8 | 99.4 | 98.7 | 99.4 | 98.2 | 98.8 | 100 | 98.8 | 99.2 | 98.8 | 99.4 | 99 |
| HNPDS-1 | 98.9 | 98.7 | 99.1 | 99.5 | 98.9 | 99.7 | 97.5 | 99.1 | 100 | 99.2 | 99.5 | 98.9 | 99.7 | 98.2 |
| HNNY-1 | 98.9 | 98.7 | 74.3 | 74.5 | 98.9 | 99.7 | 98 | 99.1 | 100 | 74.3 | 74.5 | 99 | 99.7 | 98.7 |
| 15-I-1105 | 98.8 | 98.7 | 98.9 | 99.4 | 98.8 | 99.6 | 98 | 98.9 | 100 | 98.9 | 99.2 | 98.9 | 99.6 | 98.7 |
| 16-I-674 | 98.8 | 98.7 | 98.9 | 99.4 | 98.8 | 99.6 | 98.2 | 98.9 | 100 | 98.9 | 99.2 | 98.9 | 99.6 | 99 |
| 16-I-599 | 98.8 | 98.7 | 98.9 | 99.4 | 98.8 | 99.6 | 98 | 98.9 | 100 | 98.9 | 99.2 | 98.9 | 99.6 | 98.7 |
| 14-I-605-C1 | 98.8 | 98.7 | 99 | 99.5 | 98.8 | 99.6 | 98.2 | 98.9 | 100 | 99 | 99.3 | 98.9 | 99.6 | 99 |
| MI-110-C2 | 98.6 | 98.7 | 98.7 | 99.5 | 98.6 | 99.7 | 98 | 98.7 | 100 | 98.7 | 99.4 | 98.7 | 99.7 | 98.7 |
| Kochi/01/2005 | 97.8 | 98.7 | 97.9 | 99.3 | 97.8 | 99.3 | 98 | 97.9 | 100 | 97.9 | 99.3 | 97.9 | 99.3 | 98.2 |
| SC202010 | 98.4 | 98.7 | 98.6 | 99.1 | 98.4 | 99 | 97.5 | 98.6 | 100 | 98.6 | 98.9 | 98.5 | 99.2 | 98.2 |
| GD201909 | / | 98.7 | 99.3 | 99.5 | 99.4 | 99.7 | / | / | 100 | 98.8 | 99.3 | 98.6 | 99.2 | / |
| GX202005 | / | / | 97.3 | 98.9 | 97.6 | 98.8 | / | / | / | 97.4 | 98.8 | 97.6 | 98.7 | / |
| JS201809-2 | / | / | 98.8 | 99.4 | 98.7 | 99.6 | / | / | / | 98.8 | 99.3 | 98.7 | 99.4 | / |
| JX202004 | / | / | 97.2 | 98.9 | 97.4 | 99.1 | 98.5 | / | / | 97.3 | 98.9 | 97.5 | 99.1 | 98.7 |
| SX201809 | / | / | 99 | 99.5 | 98.7 | 99.7 | / | / | / | 99 | 99.3 | 98.8 | 99.7 | / |
| FJ202005-2 | / | / | 99.4 | 99.5 | 99.1 | 99.1 | / | / | / | 98.9 | 99.3 | 98.2 | 98.6 | / |
| HuB201905 | / | / | 99.1 | 99.6 | 98.9 | 99.7 | / | / | / | 99.1 | 99.4 | 99 | 99.7 | / |
| SD201910 | / | / | 98.9 | 99.4 | 98.9 | 99.7 | 97.7 | / | / | 98.9 | 99.2 | 99 | 99.7 | 98.5 |
| SC202009 | / | / | 98.8 | 99.4 | 98.5 | 99.5 | 97.5 | / | / | 98.8 | 99.3 | 98.6 | 99.6 | 98.2 |
| FJ201807-1 | / | / | 99.5 | 99.6 | 99.4 | 99.7 | / | / | / | 99 | 99.3 | 98.6 | 99.2 | / |
| HeN202009-2 | 98.8 | 98.7 | 98.9 | 99.5 | 98.9 | 99.7 | 98 | 98.9 | 100 | 98.9 | 99.3 | 98.9 | 99.7 | 98.7 |
| GX1 | / | / | 98.9 | 99.5 | 98.7 | 99.6 | / | / | / | 98.9 | 99.4 | 98.7 | 99.4 | / |
| GETV/SCrph129/2020 | / | / | 98.8 | 99.5 | 98.5 | 99.6 | / | / | / | 98.8 | 99.4 | 98.6 | 99.6 | / |
| NM,JA_F2_18-8L-NH-Cxp-Y-1-1 | 98.6 | 98.7 | 98.8 | 99.5 | 98.4 | 99.6 | 97.5 | 98.8 | 100 | 98.9 | 99.4 | 98.5 | 99.6 | 98.2 |
| Getahvirus-1 | 98.2 | 98.7 | 99.2 | 99.5 | 99.2 | 99.6 | 70.2 | 98.4 | 100 | 99.3 | 99.4 | 99.3 | 99.8 | 70.6 |
| 16-0810-26 | / | / | 99 | 99.4 | 98.8 | 99.6 | / | / | / | 98.9 | 99.2 | 98.9 | 99.6 | / |
| GETV-China/GX2020 | / | 96.1 | 95.6 | 98.3 | 95.1 | 98 | / | / | 97.4 | 95.6 | 98.2 | 95 | 97.7 | / |
| YN2305/cattle/China/5/2023 | 97.7 | 98.7 | 73.3 | 74.3 | 97.7 | 99.3 | 98.5 | 97.9 | 100 | 73.3 | 74.3 | 97.7 | 99.3 | 98.7 |
| JLy1 | 98.8 | 98.7 | 98.9 | 99.4 | 98.7 | 99.3 | 97.2 | 98.9 | 100 | 99 | 99.3 | 98.9 | 99.5 | 98 |
| SD2206 | 98.9 | 98.7 | 99.1 | 99.6 | 98.9 | 99.7 | 98 | 99.1 | 100 | 99.1 | 99.4 | 99 | 99.7 | 98.7 |
| HN-QH23-As-10 | / | / | 98.6 | 99.4 | 98.3 | 99.4 | / | / | / | 98.6 | 99.3 | 98.3 | 99.4 | / |
| HN-QH23-As-12 | / | / | 98.5 | 99.4 | 98.3 | 99.5 | / | / | / | 98.6 | 99.3 | 98.4 | 99.5 | / |
| HN-QH23-As-14 | / | / | 98.6 | 99.3 | 98.3 | 99.4 | / | / | / | 98.6 | 99.3 | 98.3 | 99.4 | / |
| GETV-JX-CHN-22-P7 | 98.6 | 97.4 | 98.7 | 99.4 | 98.8 | 99.7 | 98 | 98.8 | 98.7 | 98.7 | 99.3 | 98.8 | 99.7 | 98.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, P.-P.; Chen, S.-N.; Zhou, X.; Wei, Z.-Z.; Zhai, S.-L. Getah Virus: A New Contaminant in Veterinary Vaccines. Vet. Sci. 2025, 12, 82. https://doi.org/10.3390/vetsci12020082
Chu P-P, Chen S-N, Zhou X, Wei Z-Z, Zhai S-L. Getah Virus: A New Contaminant in Veterinary Vaccines. Veterinary Sciences. 2025; 12(2):82. https://doi.org/10.3390/vetsci12020082
Chicago/Turabian StyleChu, Pin-Pin, Sheng-Nan Chen, Xia Zhou, Zu-Zhang Wei, and Shao-Lun Zhai. 2025. "Getah Virus: A New Contaminant in Veterinary Vaccines" Veterinary Sciences 12, no. 2: 82. https://doi.org/10.3390/vetsci12020082
APA StyleChu, P.-P., Chen, S.-N., Zhou, X., Wei, Z.-Z., & Zhai, S.-L. (2025). Getah Virus: A New Contaminant in Veterinary Vaccines. Veterinary Sciences, 12(2), 82. https://doi.org/10.3390/vetsci12020082








