STEAP3 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Regulating Fatty Acid and Lipid Droplet Synthesis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
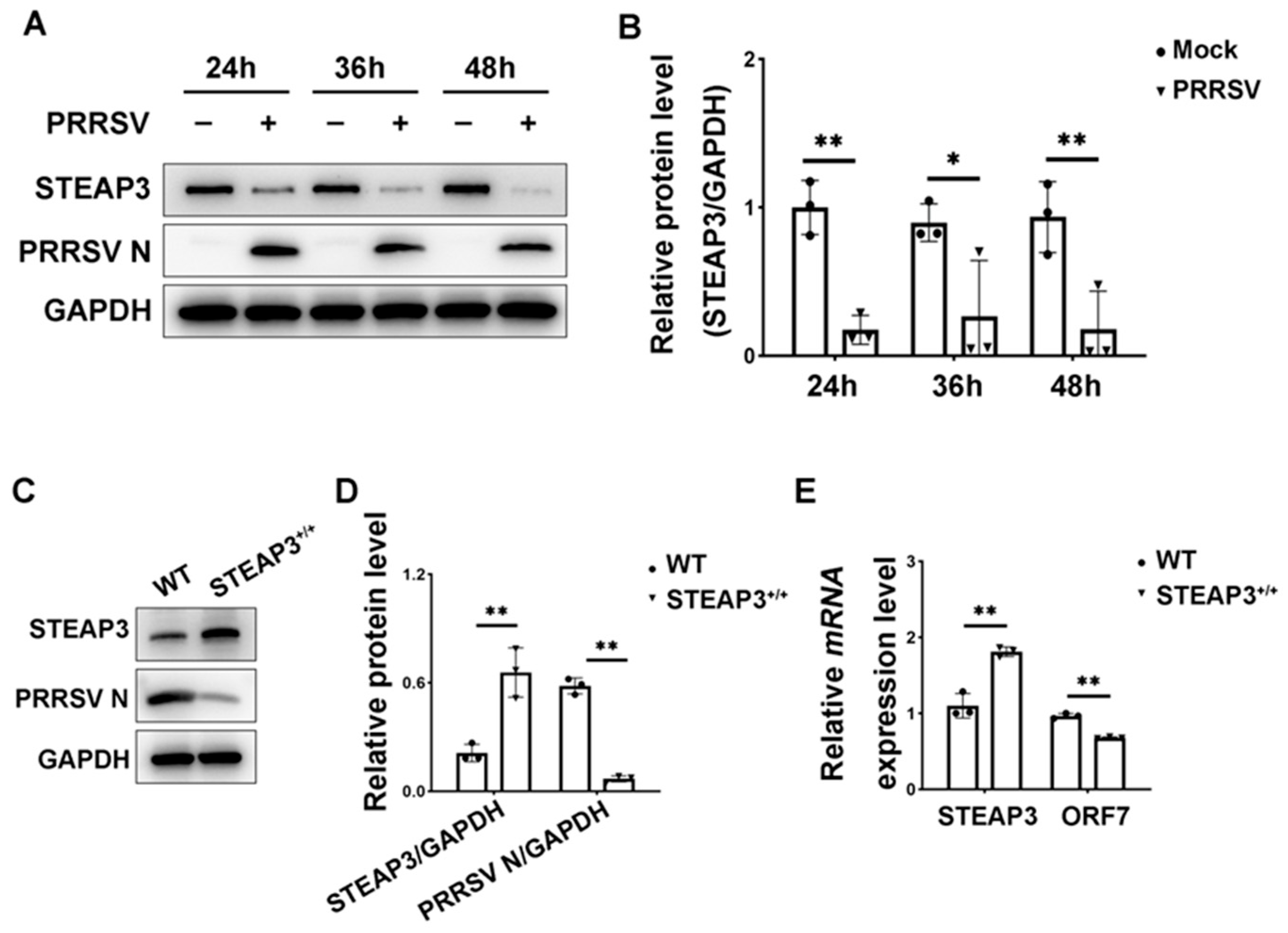
3.1. STEAP3 Suppresses PRRSV Replication
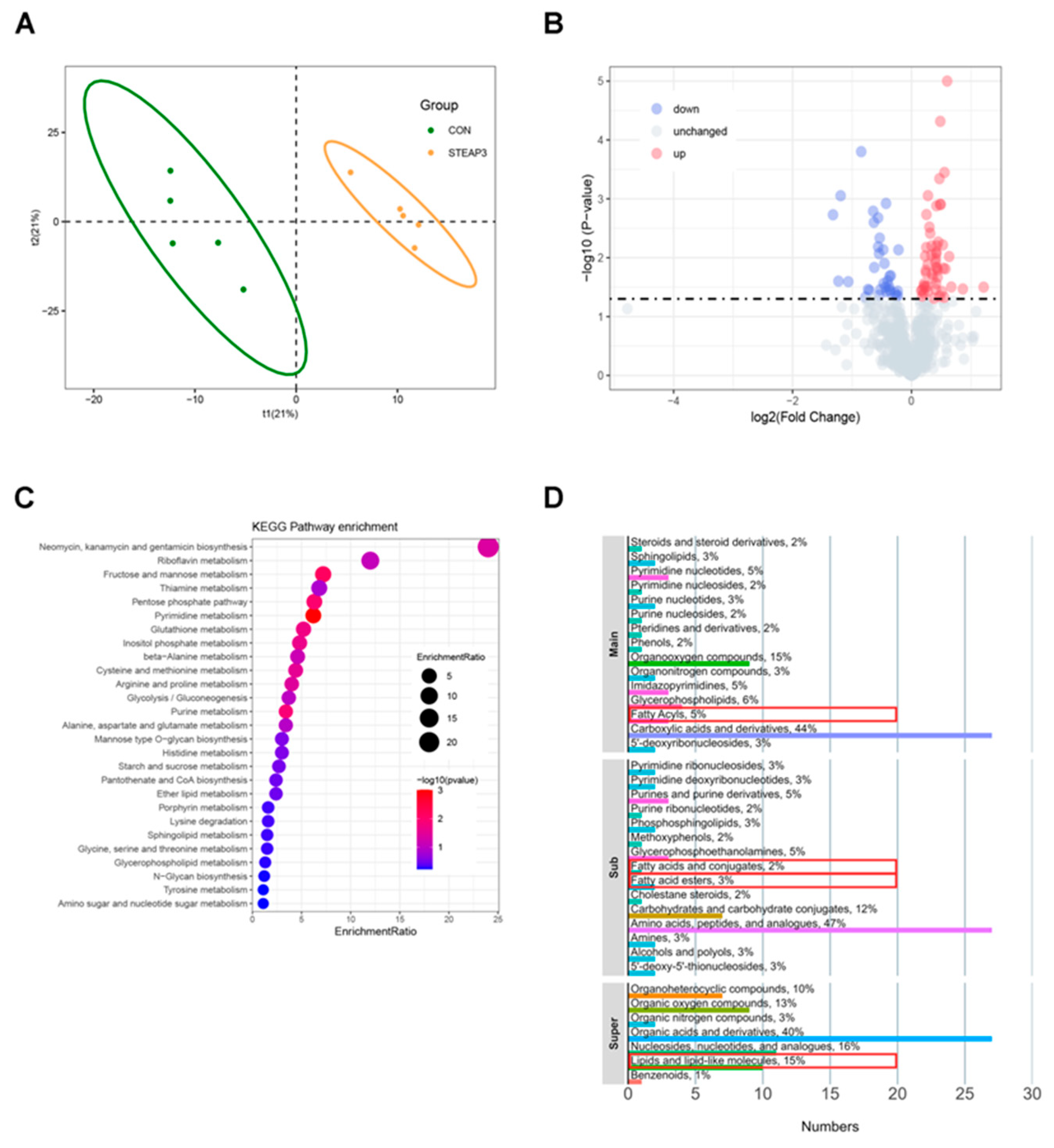
3.2. Transcriptomics Sequencing and Analysis
3.3. Metabolomics and Analysis
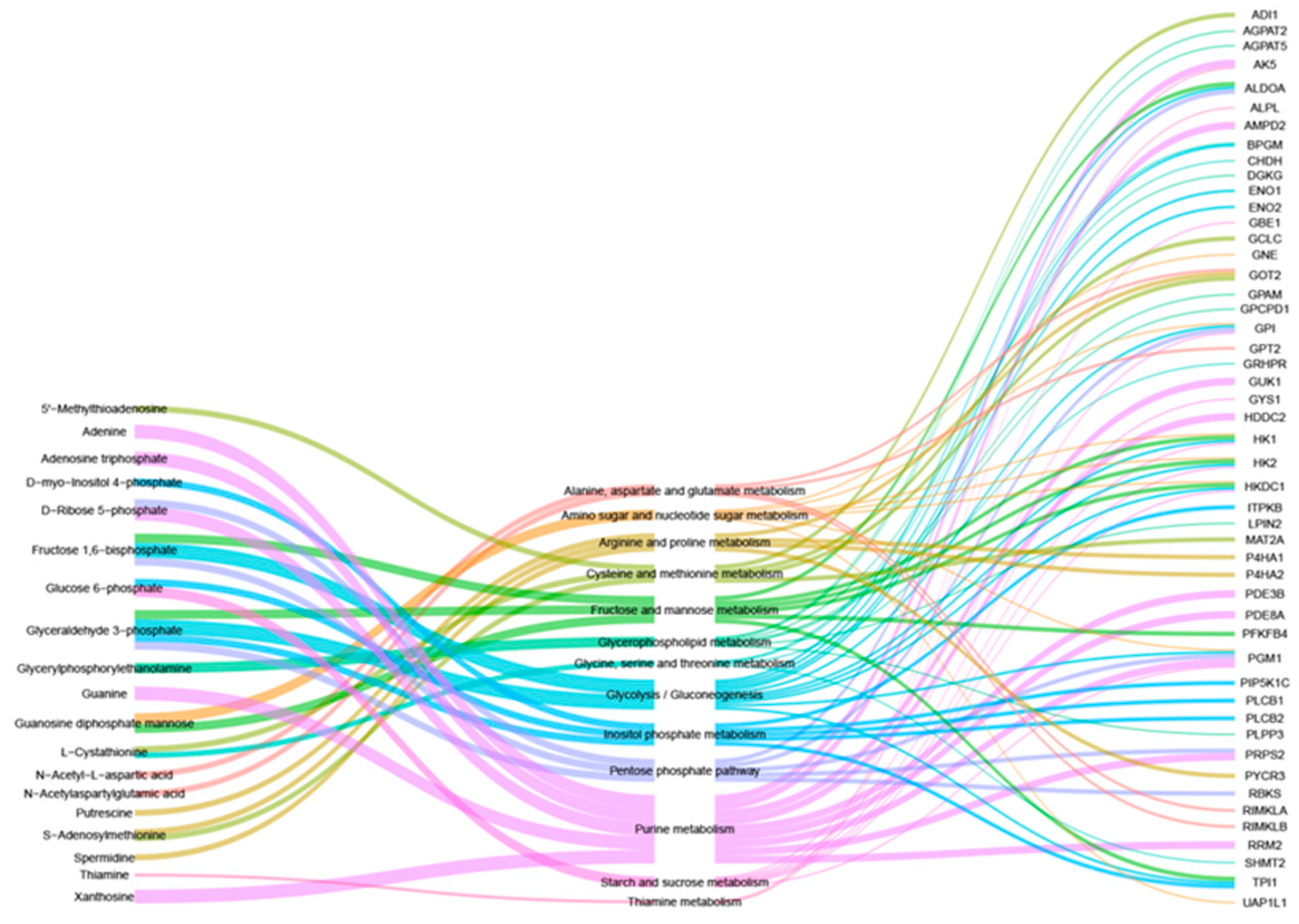
3.4. Combined Analysis of Transcriptomics and Metabolomics
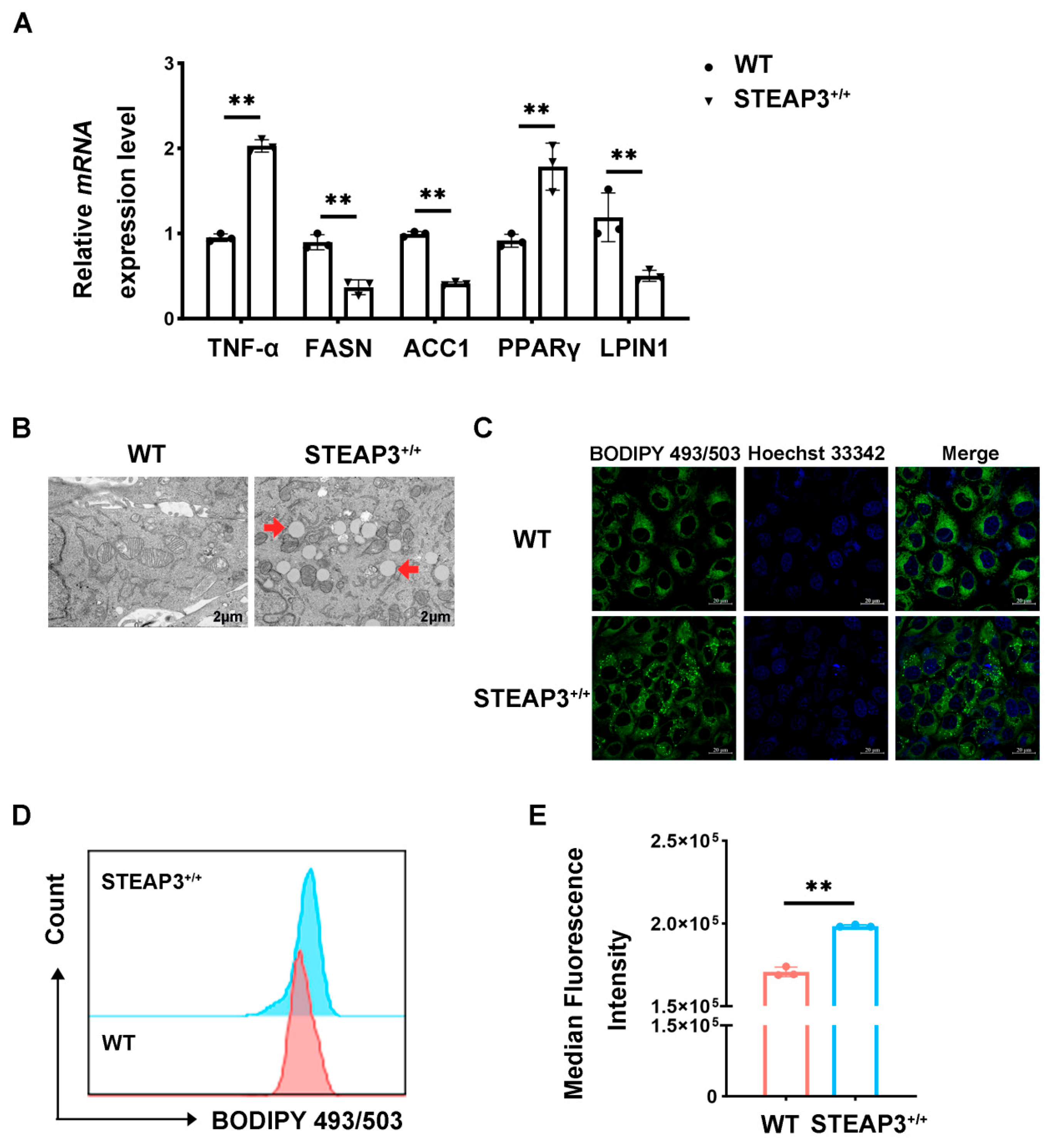
3.5. STEAP3 Inhibits FASN Expression and Promotes LD Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Z.; Chen, X.X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: Comparison of the North American and European isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef]
- Zheng, Z.; Ling, X.; Li, Y.; Qiao, S.; Zhang, S.; Wu, J.; Ma, Z.; Li, M.; Guo, X.; Li, Z.; et al. Host cells reprogram lipid droplet synthesis through YY1 to resist PRRSV infection. mBio 2024, 15, e0154924. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Han, Y.Q.; Wang, P.Z.; Wang, M.Y.; Yang, G.Y.; Li, J.L.; Wang, J.; Chu, B.B. Porcine reproductive and respiratory syndrome virus activates lipid synthesis through a ROS-dependent AKT/PCK1/INSIG/SREBPs axis. Int. J. Biol. Macromol. 2024, 282, 136720. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.A.; Bickel, P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef]
- Stapleford, K.A.; Miller, D.J. Role of cellular lipids in positive-sense RNA virus replication complex assembly and function. Viruses 2010, 2, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Kräusslich, H.G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef]
- Li, Q.; Pène, V.; Krishnamurthy, S.; Cha, H.; Liang, T.J. Hepatitis C virus infection activates an innate pathway involving IKK-α in lipogenesis and viral assembly. Nat. Med. 2013, 19, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yang, L.; Meng, X.; Xu, Q.; Zhou, X.; Liu, B. Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection. Vet. Sci. 2024, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Zhao, Q.; Wen, Y.P.; Wu, R.; Du, S.Y.; Huang, X.B.; Wen, X.T.; Cao, S.J.; Zeng, L.; Yan, Q.G. Porcine reproductive and respiratory syndrome virus upregulates SMPDL3B to promote viral replication by modulating lipid metabolism. iScience 2023, 26, 107450. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Stenson, B.M.; Dungner, E.; Näslund, E.; Hoffstedt, J.; Ryden, M.; Dahlman, I. Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. J. Clin. Endocrinol. Metab. 2008, 93, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, C.; Ji, C.; Zhao, Y.; Zhang, C.; Chen, F.; Gao, C.; Zhu, J.; Qian, L.; Guo, X. STEAP4, a gene associated with insulin sensitivity, is regulated by several adipokines in human adipocytes. Int. J. Mol. Med. 2010, 25, 361–367. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Q.; Tang, Y.D.; Zhai, J.; Hu, W.; Zheng, C. When ferroptosis meets pathogenic infections. Trends Microbiol. 2023, 31, 468–479. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Ding, T.; Chen, S.; Xiao, W.; Liu, Z.; Tu, J.; Yu, Y.; Dong, B.; Chen, W.; Zeng, Y. Six-Transmembrane Epithelial Antigen of Prostate 3 Promotes Hepatic Insulin Resistance and Steatosis. J. Lipid Res. 2023, 64, 100318. [Google Scholar] [CrossRef]
- Li, Y.; Shang, W.; Xiao, G.; Zhang, L.K.; Zheng, C. A Comparative Quantitative Proteomic Analysis of HCMV-Infected Cells Highlights pUL138 as a Multifunctional Protein. Molecules 2020, 25, 2520. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Duarte, L.F.; Farías, M.A.; Tognarelli, E.; Kalergis, A.M.; Bueno, S.M.; González, P.A. Impact of Hypoxia over Human Viral Infections and Key Cellular Processes. Int. J. Mol. Sci. 2021, 22, 7954. [Google Scholar] [CrossRef]
- Quarleri, J.; Cevallos, C.; Delpino, M.V. Apoptosis in infectious diseases as a mechanism of immune evasion and survival. Adv. Protein Chem. Struct. Biol. 2021, 125, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Farías, M.A.; Diethelm-Varela, B.; Kalergis, A.M.; González, P.A. Interplay between lipid metabolism, lipid droplets and RNA virus replication. Crit. Rev. Microbiol. 2024, 50, 515–539. [Google Scholar] [CrossRef]
- Cui, L.; Liu, P. Two Types of Contact Between Lipid Droplets and Mitochondria. Front. Cell Dev. Biol. 2020, 8, 618322. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, W.; Xiao, M.Z.X.; Zheng, Y.; Liang, Q. The interplay between lipid droplets and virus infection. J. Med. Virol. 2023, 95, e28967. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Carneiro, F.A.; Martins, I.C.; Assunção-Miranda, I.; Faustino, A.F.; Pereira, R.M.; Bozza, P.T.; Castanho, M.A.; Mohana-Borges, R.; Da Poian, A.T.; et al. Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins. J. Virol. 2012, 86, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.W.; Fu, P.F.; Zeng, L.; Qi, Y.L.; Li, X.Q.; Wang, Q.; Yang, G.Y.; Li, H.W.; Wang, J.; Chu, B.B.; et al. EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism. Microbiol. Spectr. 2022, 10, e0227621. [Google Scholar] [CrossRef]
- Howie, H.L.; Hay, A.M.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.D.; Ranson, M.S.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef]
- Zhang, F.; Tao, Y.; Zhang, Z.; Guo, X.; An, P.; Shen, Y.; Wu, Q.; Yu, Y.; Wang, F. Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica 2012, 97, 1826–1835. [Google Scholar] [CrossRef]
- Faustino, A.F.; Guerra, G.M.; Huber, R.G.; Hollmann, A.; Domingues, M.M.; Barbosa, G.M.; Enguita, F.J.; Bond, P.J.; Castanho, M.A.; Da Poian, A.T.; et al. Understanding dengue virus capsid protein disordered N-Terminus and pep14-23-based inhibition. ACS Chem. Biol. 2015, 10, 517–526. [Google Scholar] [CrossRef]
- Kim, Y.; George, D.; Prior, A.M.; Prasain, K.; Hao, S.; Le, D.D.; Hua, D.H.; Chang, K.O. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur. J. Med. Chem. 2012, 50, 311–318. [Google Scholar] [CrossRef] [PubMed]

| Primers | Primer Sequences (5′→3′) |
|---|---|
| ORF7 (N)-F | AAAACCAGTCCAGAGGCAAG |
| ORF7 (N)-R | CGGATCAGACGCACAGTATG |
| STEAP3-F | CCAATGCTGAGTACCTGGC |
| STEAP3-R | ATCTCCGAGACAGCACGC |
| FASN-qF | CACATCGTTCGAGCAGCATG |
| FASN-qR | AATTTCCAGGAAGCGACCGT |
| ACC1-qF | TAGTCTGCCACGGATCCAGA |
| ACC1-qR | GGGAGGGATCTCTGAGGGTT |
| PPARγ-qF | TGAGTTCGCTGTGAAGTT |
| PPARγ-qR | CAATCTGTCTGAGGTCTGT |
| TNF-α-qF | CTGTAGGTTGCTCCCACCTG |
| TNF-α-qR | CCAGTAGGGCGGTTACAGAC |
| LPIN1-qF | CAAGCAAGTAGGAGTGTCT |
| LPIN1-qR | GCGGAGGCAGAATGAATA |
| GAPDH-F | TGACAACAGCCTCAAGATCG |
| GAPDH-R | GTCTTCTGGGTGGCAGTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Guan, K.; Zhang, G. STEAP3 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Regulating Fatty Acid and Lipid Droplet Synthesis. Vet. Sci. 2025, 12, 147. https://doi.org/10.3390/vetsci12020147
Yuan C, Guan K, Zhang G. STEAP3 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Regulating Fatty Acid and Lipid Droplet Synthesis. Veterinary Sciences. 2025; 12(2):147. https://doi.org/10.3390/vetsci12020147
Chicago/Turabian StyleYuan, Chenyang, Kaifeng Guan, and Gaiping Zhang. 2025. "STEAP3 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Regulating Fatty Acid and Lipid Droplet Synthesis" Veterinary Sciences 12, no. 2: 147. https://doi.org/10.3390/vetsci12020147
APA StyleYuan, C., Guan, K., & Zhang, G. (2025). STEAP3 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Regulating Fatty Acid and Lipid Droplet Synthesis. Veterinary Sciences, 12(2), 147. https://doi.org/10.3390/vetsci12020147






