Network Pharmacology and Molecular Docking: Exploring the Mechanism of Peppermint in Mastitis Prevention and Treatment in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Active Ingredients and Targets of Peppermint
2.2. Screening of Potential Targets for Mint Treatment of Bovine Mastitis
2.3. Construction of Mint–Cow Mastitis Targets Protein–Protein Interaction Network
2.4. Gene Ontology (GO) Enrichment and KEGG Pathway Enrichment Analysis
2.5. Molecular Docking Analysis
3. Results
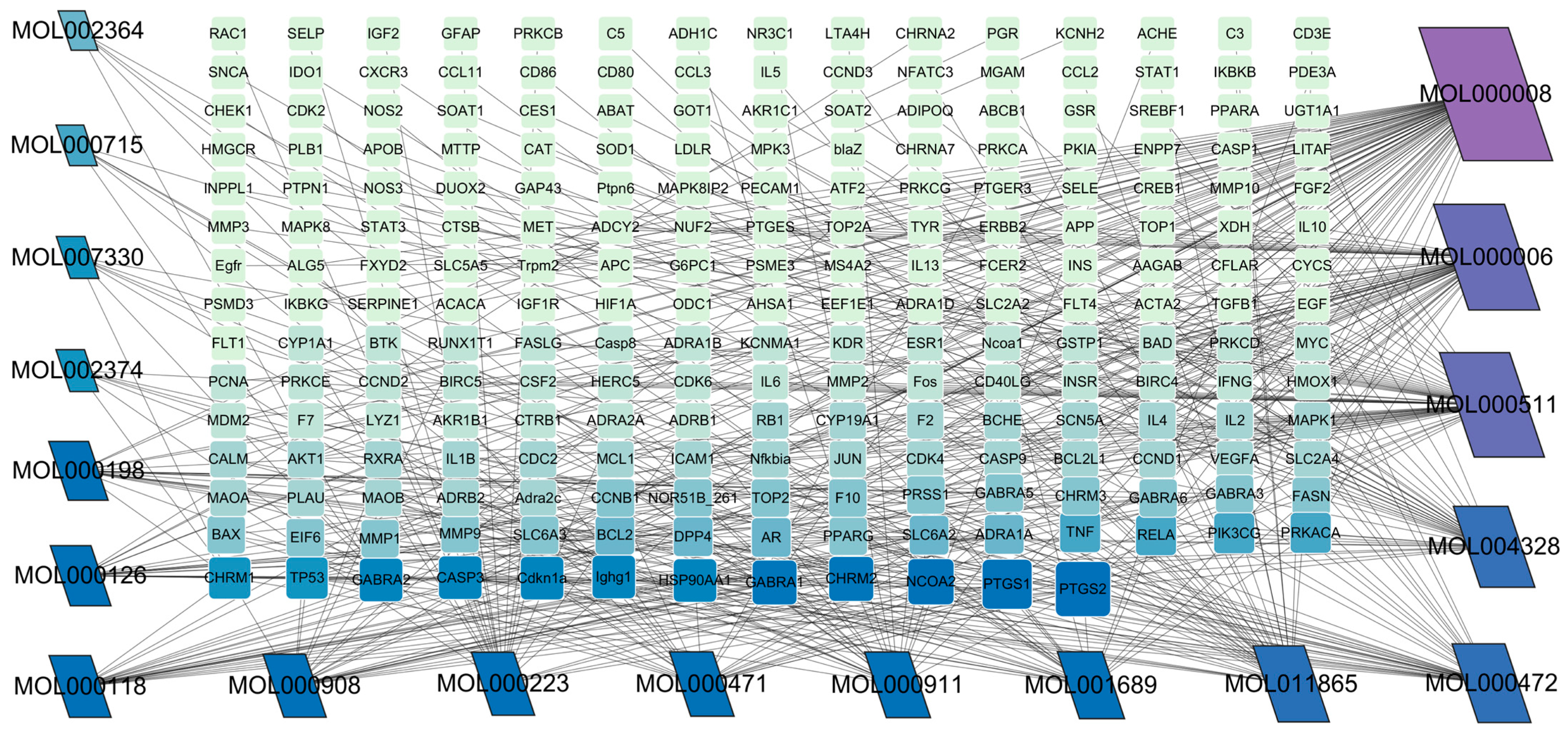
3.1. The Active Ingredients of Peppermint and Their Corresponding Targets
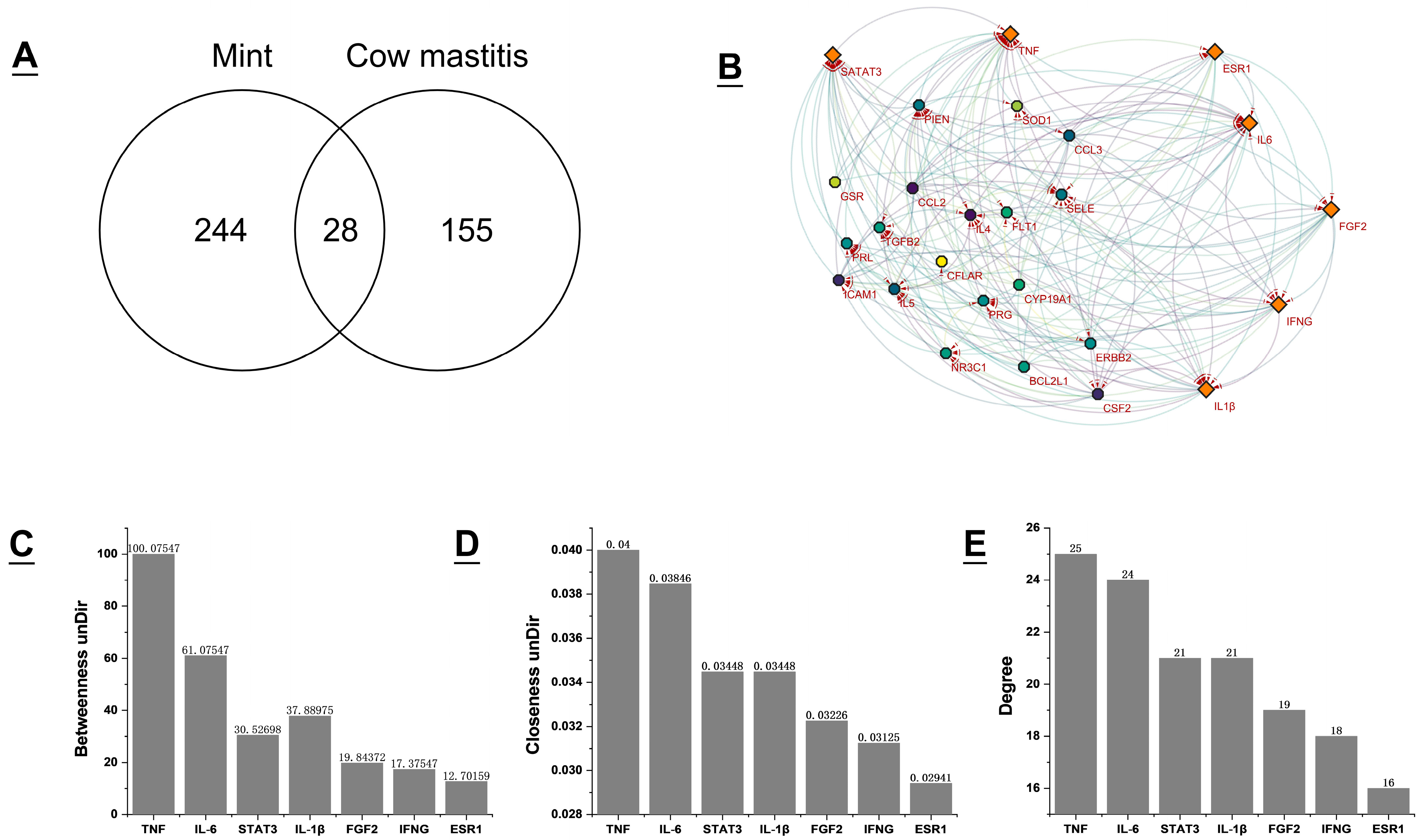
3.2. Acquisition of Potential Targets for Mint Treatment of Bovine Mastitis and Construction of PPI Network
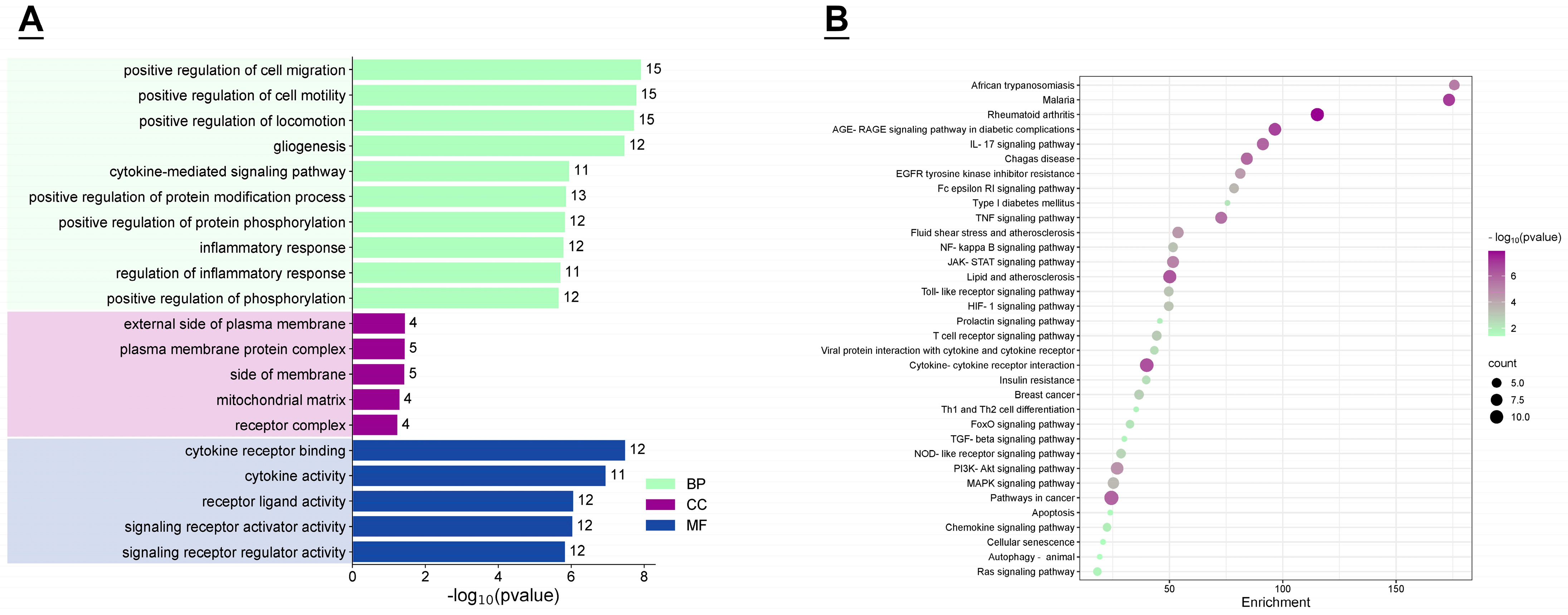
3.3. GO Function and KEGG Enrichment Analysis
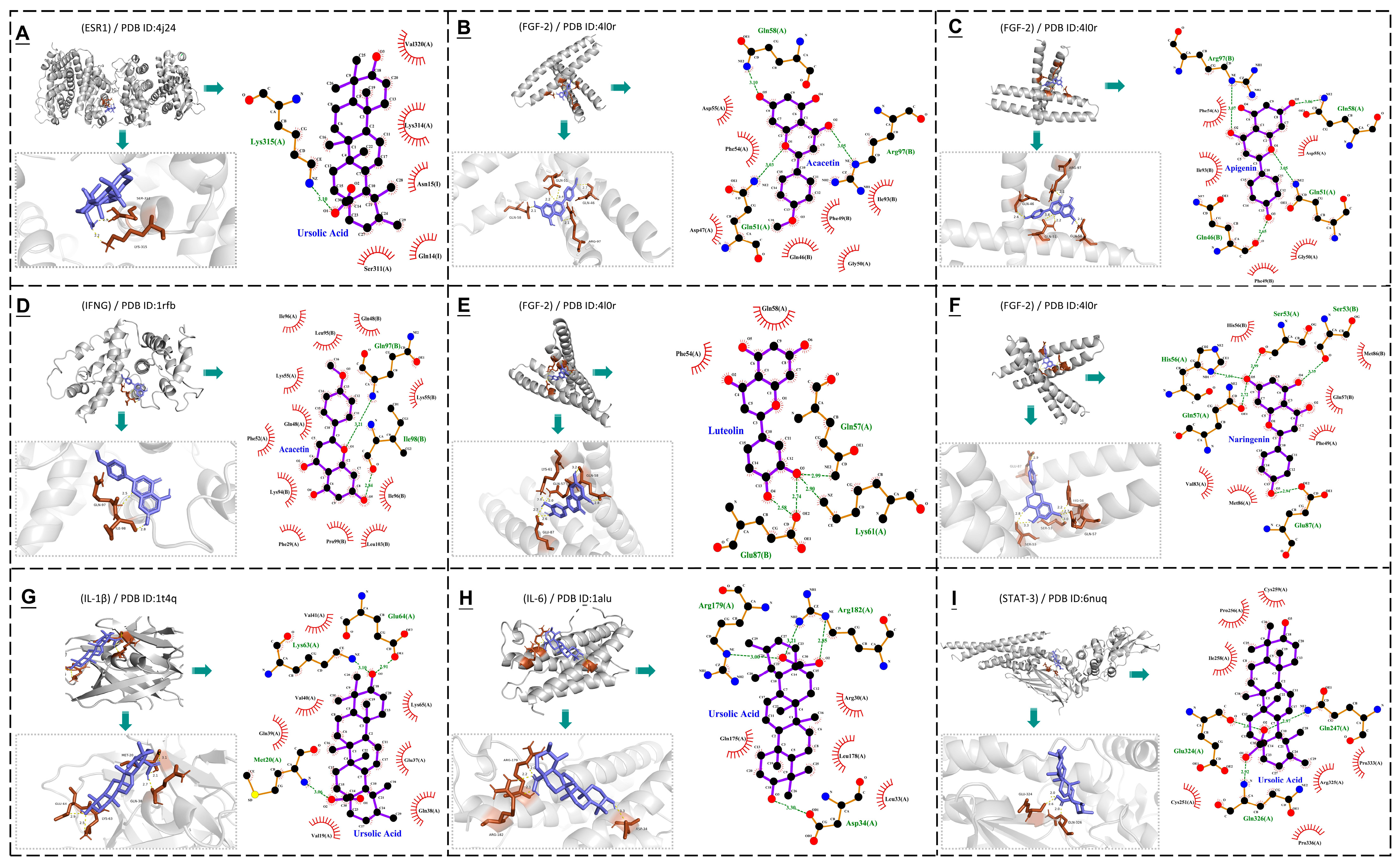
3.4. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECD/FAO. Agricultural Outlook 2022–2031. Available online: https://www.fao.org/4/CA4076EN/CA4076EN_Chapter7_Dairy.pdf (accessed on 12 December 2024).
- Tricarico, J.M.; Kebreab, E.; Wattiaux, M.A. MILK Symposium review: Sustainability of dairy production and consumption in low–income countries with emphasis on productivity and environmental impact. J. Dairy Sci. 2020, 103, 9791–9802. [Google Scholar] [CrossRef] [PubMed]
- Dalanezi, F.M.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.S.; Cerri, R.L.A.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, G.; Cambay, Z.; Yilmaz, S.; Aytekin, O.; Zigo, F.; Ozçelik, M.; Otlu, B. Identification of bacterial species in milk by MALDI–TOF and assessment of some oxidant–antioxidant parameters in blood and milk from cows with different health status of the udder. Pol. J. Vet. Sci. 2022, 25, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dyson, R.; Charman, N.; Hodge, A.; Rowe, S.M.; Taylor, L.F. A survey of mastitis pathogens including antimicrobial susceptibility in southeastern Australian dairy herds. J. Dairy Sci. 2022, 105, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Zigo, F.; Farkašová, Z.; Výrostková, J.; Regecová, I.; Ondrašovičová, S.; Vargová, M.; Sasáková, N.; Pecka–Kielb, E.; Bursová, Š.; Kiss, D.S. Dairy Cows’ Udder Pathogens and Occurrence of Virulence Factors in Staphylococci. Animals 2022, 12, 470. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Bano, I.; Li, K.; Ul Hassan, I.; Wang, W.; Qadeer, A.; Zhang, J. Evaluation of in vitro antibacterial effect of essential oil and some herbal plant extract used against mastitis pathogens. Vet. Med. Sci. 2022, 8, 2655–2661. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Hoang Le, Q.; Devanesan, S.; Sayed, S.R.M.; Rajeswari, V.D.; Liu, X.; Jhanani, G.K. Mint leaves (Mentha arvensis) mediated CaO nanoparticles in dye degradation and their role in anti–inflammatory, anti–cancer properties. Environ. Res. 2023, 236 Pt 1, 116718. [Google Scholar] [CrossRef]
- Azad, A.K.; Doolaanea, A.A.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro–hydrodynamic assisted synthesis of lecithin–stabilized peppermint oil–loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kačániová, M. In Vitro Antimicrobial Activity of Lavender, Mint, and Rosemary Essential Oils and the Effect of Their Vapours on Growth of Penicillium spp. in a Bread Model System. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.A.; Soares, P.M.; de Almeida, A.N.; Maia, A.R.; de Souza, E.P.; Assreuy, A.M. Antispasmodic effect of Mentha piperita essential oil on tracheal smooth muscle of rats. J. Ethnopharmacol. 2010, 130, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Chemical Composition and In Vitro Antibacterial Activity of Mentha spicata Essential Oil against Common Food–Borne Pathogenic Bacteria. J. Pathog. 2015, 2015, 916305. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008, 14, 3141–3150. [Google Scholar] [CrossRef]
- Ranjbar, M.; Kiani, M.; Nikpay, A. Antioxidant and scolicidal activities of four Iranian Mentha species (Lamiaceae) in relation to phenolic element. J. Herbmed. Pharmacol. 2020, 26, 200–208. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In vitro antiviral, anti–inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Lang, M.; Ferron, P.J.; Bursztyka, J.; Montjarret, A.; Duteil, E.; Bazire, A.; Bedoux, G. Evaluation of immunomodulatory activities of essential oils by high content analysis. J. Biotechnol. 2019, 303, 65–71. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of, U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti–inflammatory effects: A promising treatment strategy against COVID–19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Sun, L.C.; Zhang, H.B.; Gu, C.D.; Guo, S.D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.Q. Protective effect of acacetin on sepsis–induced acute lung injury via its anti–inflammatory and antioxidative activity. Arch. Pharm. Res. 2018, 41, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Negm, W.A.; Alexiou, A.; Batiha, G.E. Ursolic acid and SARS-CoV-2 infection: A new horizon and perspective. Inflammopharmacology 2022, 30, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in anti–inflammatory Activity, Mechanism and Therapeutic Application of Ursolic Acid. Mini Rev. Med. Chem. 2022, 22, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan—Rosmarinic acid conjugates with antioxidant, anti–inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. anti–oxidant and anti–inflammatory effects of caffeic acid: In vivo evidences in a model of noise–induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Weerts, Z.Z.R.M.; Keszthelyi, D.; Vork, L.; Aendekerk, N.C.P.; Frijlink, H.W.; Brouwers, J.R.B.J.; Neef, C.; Jonkers, D.M.A.E.; Masclee, A.A.M. A Novel Ileocolonic Release Peppermint Oil Capsule for Treatment of Irritable Bowel Syndrome: A Phase I Study in Healthy Volunteers. Adv. Ther. 2018, 35, 1965–1978. [Google Scholar] [CrossRef]
- Akhavan Amjadi, M.; Mojab, F.; Kamranpour, S.B. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran. J. Pharm. Res. 2012, 11, 1073–1077. [Google Scholar]
- Karsten, M.; Prince, D.; Robinson, R.; Stout–Aguilar, J. Effects of Peppermint Aromatherapy on Postoperative Nausea and Vomiting. J. PeriAnesth. Nurs. 2020, 35, 615–618. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.; Felix, C.T. Diet and functional abdominal pain in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.A.E.; Mohamed, A.G.; Bahgaat, W.K. Natural peppermint–flavored cheese. Acta Sci. Pol. Technol. Aliment. 2019, 18, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint Oils: In Vitro Ability to Perform anti–Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants 2021, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lopez, E.; Rivera-Chacon, R.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Short–term screening of multiple phytogenic compounds for their potential to modulate chewing behavior, ruminal fermentation profile, and pH in cattle fed grain–rich diets. J. Dairy Sci. 2021, 104, 4271–4289. [Google Scholar] [CrossRef]
- Saha, S.; Lachance, S. Effect of essential oils on cattle gastrointestinal nematodes assessed by egg hatch, larval migration and mortality testing. J. Helminthol. 2019, 94, e111. [Google Scholar] [CrossRef]
- Ali, F.; Rahul, N.F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2016, 20, 1197–1238. [Google Scholar] [CrossRef]
- Vinh, L.B.; Jang, H.J.; Phong, N.V.; Cho, K.; Park, S.S.; Kang, J.S.; Kim, Y.H.; Yang, S.Y. Isolation, structural elucidation, and insights into the anti–inflammatory effects of triterpene saponins from the leaves of Stauntonia hexaphylla. Bioorg. Med. Chem. Lett. 2019, 29, 965–969. [Google Scholar] [CrossRef]
- Mantawy, E.M.; Said, R.S.; Abdel-Aziz, A.K. Mechanistic approach of the inhibitory effect of chrysin on inflammatory and apoptotic events implicated in radiation–induced premature ovarian failure: Emphasis on TGF–β/MAPKs signaling pathway. Biomed. Pharmacother. 2019, 109, 293–303. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Meng, H.; Hu, C.; Li, J.; Luo, M.; Dong, J.; Wang, X.; Wang, J.; Deng, Y.; et al. Impact of luteolin on the production of alpha–toxin by Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 238–243. [Google Scholar] [CrossRef]
- Guo, Y.F.; Xu, N.N.; Sun, W.; Zhao, Y.; Li, C.Y.; Guo, M.Y. Luteolin reduces inflammation in Staphylococcus aureus–induced mastitis by inhibiting NF–kB activation and MMPs expression. Oncotarget 2017, 8, 28481–28493. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Yan, S.; Liu, M.; Sun, B.; Lu, Y.; Shao, Y. Ursolic acid alleviates inflammation and against diabetes–induced nephropathy through TLR4–mediated inflammatory pathway. Mol. Med. Rep. 2018, 18, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ni, Y.; Zhou, Q.; Liu, H.; Xiang, H.; Sui, H.; Shang, D. Emodin Attenuates Severe Acute Pancreatitis via Antioxidant and anti–inflammatory Activity. Inflammation 2019, 42, 2129–2138. [Google Scholar] [CrossRef]
- Hu, Z.N.; Huang, L.J.; Chen, W.P. The inhibitory effects of rosmarinic acid on catabolism induced by IL–1β in rat chondrocyte. Acta Biochim. Pol. 2018, 65, 535–538. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin–rich extract and rosmarinic acid, alone and in combination, protect against DSS–induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. anti–inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide–Induced Mastitis in Mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated–gene expression of pro–inflammatory cytokines (TNF–α, IL–1β and IL–6) via TLRs following NF–κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Johnzon, C.F.; Dahlberg, J.; Gustafson, A.M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The Effect of Lipopolysaccharide–Induced Experimental Bovine Mastitis on Clinical Parameters, Inflammatory Markers, and the Metabolome: A Kinetic Approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Liu, S.; Guo, W.; Jia, Y.; Ye, B.; Liu, S.; Fu, S.; Liu, J.; Hu, G. Menthol Targeting AMPK Alleviates the Inflammatory Response of Bovine Mammary Epithelial Cells and Restores the Synthesis of Milk Fat and Milk Protein. Front. Immunol. 2021, 12, 782989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, R.; Wang, Z. Oral supplementation with ursolic acid ameliorates sepsis–induced acute kidney injury in a mouse model by inhibiting oxidative stress and inflammatory responses. Mol. Med. Rep. 2018, 17, 7142–7148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Lu, J.; Wu, D.M.; Zheng, Z.H.; Zheng, Y.L.; Wang, X.H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.F. Ursolic acid attenuates lipopolysaccharide–induced cognitive deficits in mouse brain through suppressing p38/NF–κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor. Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Song, Z.; Ren, D.; Xu, X.; Wang, Y. Molecular cross–talk of IL–6 in tumors and new progress in combined therapy. Thorac. Cancer 2018, 9, 669–675. [Google Scholar] [CrossRef]
- Wu, X.; Wei, S.; Chen, M.; Li, J.; Wei, Y.; Zhang, J.; Dong, W. P2RY13 Exacerbates Intestinal Inflammation by Damaging the Intestinal Mucosal Barrier via Activating IL–6/STAT3 Pathway. Int. J. Biol. Sci. 2022, 18, 5056–5069. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Zhou, Y.; Tang, X.; Wang, K.; Ren, Y.; He, J. Sinomenine hydrochloride inhibits the progression of plasma cell mastitis by regulating IL–6/JAK2/STAT3 pathway. Int. Immunopharmacol. 2020, 81, 106025. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Zhou, H.Q.; Lin, Y.J.; Yi, L.T.; Chen, Z.G.; Cao, Q.D.; Guo, Y.R.; Wang, Z.N.; Chen, S.D.; Li, Y.; et al. FGF2 is overexpressed in asthma and promotes airway inflammation through the FGFR/MAPK/NF–κB pathway in airway epithelial cells. Mil. Med. Res. 2022, 9, 7. [Google Scholar] [CrossRef]
- Sheffield, L.G. Mastitis increases growth factor messenger ribonucleic acid in bovine mammary glands. J. Dairy Sci. 1997, 80, 2020–2024. [Google Scholar] [CrossRef]
- Lee, E.; Chanamara, S.; Pleasure, D.; Soulika, A.M. IFN–gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J. Neuroinflamm. 2012, 9, 7. [Google Scholar] [CrossRef]
- Legroux, L.; Arbour, N. Multiple Sclerosis and T Lymphocytes: An Entangled Story. J. Neuroimmune Pharmacol. 2015, 10, 528–546. [Google Scholar] [CrossRef] [PubMed]
- Kuchroo, V.K.; Anderson, A.C.; Waldner, H.; Munder, M.; Bettelli, E.; Nicholson, L.B. T cell response in experimental autoimmune encephalomyelitis (EAE): Role of self and cross–reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 2002, 20, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Vitenberga-Verza, Z.; Pilmane, M.; Šerstņova, K.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto–Simón, B. Identification of Inflammatory and Regulatory Cytokines IL–1α–, IL–4–, IL–6–, IL–12–, IL–13–, IL–17A–, TNF–α–, and IFN–γ–Producing Cells in the Milk of Dairy Cows with Subclinical and Clinical Mastitis. Pathogens 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Che, Y.; Zhang, M.; Ren, W.; Xia, X.; Liu, H.; Huang, T.; Huang, J.; Lei, L. IFN–γ Activates the TLR4–CCL5 Signaling Through Reducing Arginine Level, Leading to Enhanced Susceptibility of Bovine Mammary Epithelial Cells to Staphylococcus aureus. Inflammation 2020, 43, 2209–2221. [Google Scholar] [CrossRef]
- Wang, M.; Bissonnette, N.; Laterrière, M.; Dudemaine, P.L.; Gagné, D.; Roy, J.P.; Sirard, M.A.; Ibeagha–Awemu, E.M. Gene co–expression in response to Staphylococcus aureus infection reveals networks of genes with specific functions during bovine subclinical mastitis. J. Dairy Sci. 2023, 106, 5517–5536. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Nilsson, I.M.; Tarkowski, A. The dual role of interferon–gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 1998, 93, 80–85. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Furuya, Y.; Roberts, S.; Metzger, D.W. Role of Interleukin–12 in Protection against Pulmonary Infection with Methicillin–Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 6308–6316. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Nauseef, W.M. IFN–γ targets macrophage–mediated immune responses toward Staphylococcus aureus. J. Leukoc. Biol. 2017, 101, 751–758. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs–RAGE axis: Implications for inflammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Rubino, S.J.; Geddes, K.; Girardin, S.E. Innate IL–17 and IL–22 responses to enteric bacterial pathogens. Trends Immunol. 2012, 33, 112–118. [Google Scholar] [CrossRef]
- Bechara, R.; McGeachy, M.J.; Gaffen, S.L. The metabolism–modulating activity of IL–17 signaling in health and disease. J. Exp. Med. 2021, 218, e20202191. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Mallard, B. Differentially expressed genes associated with Staphylococcus aureus mastitis of Canadian Holstein cows. Vet. Immunol. Immunopathol. 2007, 120, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, G.; Moroni, P.; Genini, S.; Stella, A.; Boettcher, P.J.; Cremonesi, P.; Scaccabarozzi, L.; Giuffra, E.; Castiglioni, B. Differentially expressed genes associated with Staphylococcus aureus mastitis in dairy goats. Vet. Immunol. Immunopathol. 2010, 135, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bidère, N.; Ngo, V.N.; Lee, J.; Collins, C.; Zheng, L.; Wan, F.; Davis, R.E.; Lenz, G.; Anderson, D.E.; Arnoult, D.; et al. Casein kinase 1alpha governs antigen–receptor–induced NF–kappaB activation and human lymphoma cell survival. Nature 2009, 458, 92–96. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF–kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Boulanger, D.; Bureau, F.; Mélotte, D.; Mainil, J.; Lekeux, P. Increased nuclear factor kappaB activity in milk cells of mastitis–affected cows. J. Dairy Sci. 2003, 86, 1259–1267. [Google Scholar] [CrossRef]
- Boutet, P.; Sulon, J.; Closset, R.; Detilleux, J.; Beckers, J.F.; Bureau, F.; Lekeux, P. Prolactin–induced activation of nuclear factor kappaB in bovine mammary epithelial cells: Role in chronic mastitis. J. Dairy Sci. 2007, 90, 155–164. [Google Scholar] [CrossRef]
- Bao, L.; Sun, H.; Zhao, Y.; Feng, L.; Wu, K.; Shang, S.; Xu, J.; Shan, R.; Duan, S.; Qiu, M.; et al. Hexadecanamide alleviates Staphylococcus aureus–induced mastitis in mice by inhibiting inflammatory responses and restoring blood–milk barrier integrity. PLoS Pathog. 2023, 19, e1011764. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, W.; Wen, H.; Su, Q.; Hao, Z.; Liu, J.; Gao, Y.; Zhang, H.; Ge, B.; Tong, C.; et al. Esculetin improves murine mastitis induced by streptococcus isolated from bovine mammary glands by inhibiting NF–κB and MAPK signaling pathways. Microb. Pathog. 2023, 185, 106393. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, G.; Liu, W.; Dou, M.; Wang, X.; Shi, W.; Bao, Y. Salvia miltiorrhiza polysaccharides ameliorates Staphylococcus aureus–induced mastitis in rats by inhibiting activation of the NF–κB and MAPK signaling pathways. BMC Vet. Res. 2022, 18, 201. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Ma, N.; Chang, G.; Shen, X. β–carotene alleviates LPS–induced inflammation through regulating STIM1/ORAI1 expression in bovine mammary epithelial cells. Int. Immunopharmacol. 2022, 113 Pt A, 109377. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Chen, K.; Wang, Y.; Yang, F.; Wang, G. UFL1 Alleviates Lipopolysaccharide–Induced Cell Damage and Inflammation via Regulation of the TLR4/NF–κB Pathway in Bovine Mammary Epithelial Cells. Oxidative Med. Cell Longev. 2019, 2019, 6505373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, X.; Zhao, G.; Wu, H.; Mi, J.; Qiu, C.; Peng, X.; Deng, G. IFN–τ Plays an anti–Inflammatory Role in Staphylococcus aureus–Induced Endometritis in Mice Through the Suppression of NF–κB Pathway and MMP9 Expression. J. Interferon Cytokine Res. 2017, 37, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y.; Lin, C.; Yan, R.; Li, Z.; Chen, Q.; Zhang, H.; Xu, H.; Chen, X.; Chen, Y.; et al. Regularity of Toll–Like Receptors in Bovine Mammary Epithelial Cells Induced by Mycoplasma bovis. Front. Vet. Sci. 2022, 9, 846700. [Google Scholar] [CrossRef] [PubMed]
- Maurić Maljković, M.; Vlahek, I.; Piplica, A.; Ekert Kabalin, A.; Sušić, V.; Stevanović, V. Prospects of toll–like receptors in dairy cattle breeding. Anim. Genet. 2023, 54, 425–434. [Google Scholar] [CrossRef]
- Méndez-Samperio, P.; Belmont, L.; Miranda, E. Mycobacterium bovis BCG Toll–like receptors 2 and 4 cooperation increases the innate epithelial immune response. Arch. Med. Res. 2008, 39, 33–39. [Google Scholar] [CrossRef]
- Lan, R.; Zhou, Y.; Wang, Z.; Fu, S.; Gao, Y.; Gao, X.; Zhang, J.; Han, X.; Phouthapane, V.; Xu, Y.; et al. Reduction of ROS–HIF1α–driven glycolysis by taurine alleviates Streptococcus uberis infection. Food Funct. 2022, 13, 1774–1784. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Wang, X.; Li, X.; Qi, S.; Zhang, Y.; Gao, M.Q. TGF–β1 Induces EMT in Bovine Mammary Epithelial Cells Through the TGFβ1/Smad Signaling Pathway. Cell Physiol. Biochem. 2017, 43, 82–93. [Google Scholar] [CrossRef]
- Geng, N.; Liu, K.; Lu, J.; Xu, Y.; Wang, X.; Wang, R.; Liu, J.; Liu, Y.; Han, B. Autophagy of bovine mammary epithelial cell induced by intracellular Staphylococcus aureus. J. Microbiol. 2020, 58, 320–329. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Teuber, S.; Cárdenas, S.; Carretta, M.D.; Morán, G.G.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A. D–Lactate Increases Cytokine Production in Bovine Fibroblast–Like Synoviocytes via MCT1 Uptake and the MAPK, PI3K/Akt, and NFκB Pathways. Animals 2020, 10, 2105. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, F.; Ma, W.; Li, X.; Zhou, X. DAD3 targets ACE2 to inhibit the MAPK and NF–κB signalling pathways and protect against LPS–induced inflammation in bovine mammary epithelial cells. Vet. Res. 2022, 53, 104. [Google Scholar] [CrossRef]


| MOL ID | Active Ingredient | Degree | Molecule Structure |
|---|---|---|---|
| MOL000008 | Apigenin | 77 |  |
| MOL000006 | Luteolin | 56 | 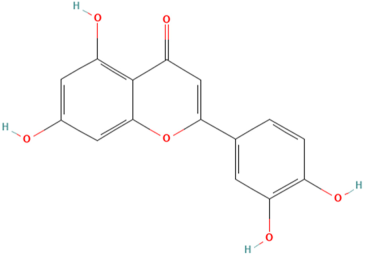 |
| MOL000511 | Ursolic Acid | 55 | 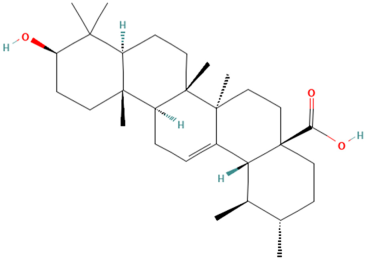 |
| MOL004328 | Naringenin | 37 | 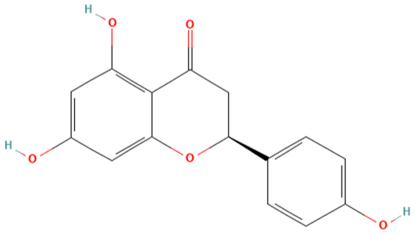 |
| MOL000472 | Emodin | 35 |  |
| MOL011865 | Rosmarinic Acid | 32 | 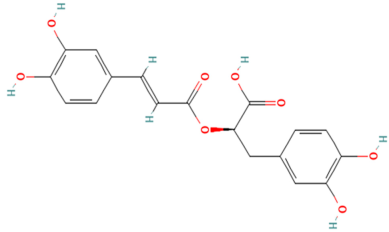 |
| MOL001689 | Acacetin | 26 |  |
| MOL000715 | Menthone | 12 | 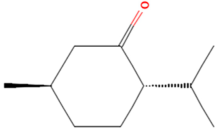 |
| MOL002374 | Neomenthol | 7 | 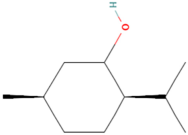 |
| MOL007330 | L–Menthol | 7 |  |
| MOL002364 | Isomenthol | 5 |  |
| Mint Components | Core Target (PDB ID) | ||||||
|---|---|---|---|---|---|---|---|
| TNF (1u5y) | IL–6 (1alu) | STAT–3 (6nuq) | IL–1β (1t4q) | FGF–2 (4l0r) | IFNG (1rfb) | ESR–1 (4j24) | |
| Apigenin | −4.61/3 | −4.3/2 | −5.05/2 | −5.5/2 | −6.71/4 | −5.63/2 | −5.27/3 |
| Luteolin | −4.27/3 | −3.75/1 | −4.41/3 | −5.3/4 | −6.32/5 | −4.27/2 | −4.16/3 |
| Ursolic Acid | −5.86/2 | −6.13/3 | −7.45/2 | −7.72/2 | −5.84/1 | −5.78/1 | −7.16/1 |
| Naringenin | −5.13/2 | −4.09/1 | −5.06/2 | −5.23/2 | −6.51/3 | −5.38/1 | −5.29/0 |
| Emodin | −4.6/1 | −4.81/2 | −4.37/1 | −5.35/3 | −5.53/4 | −4.56/1 | −4.29/2 |
| Rosmarinic Acid | −2.01/4 | −3.12/3 | −2.17/1 | −2.76/2 | −3.12/3 | −4.59/2 | −2.76/1 |
| Acacetin | −4.64/2 | −4.38/1 | −4.58/2 | −5.35/2 | −6.85/3 | −6.67/1 | −4.38/2 |
| Menthone | −4.72/1 | −4.03/1 | −4.55/1 | −4.22/0 | −5.18/1 | −5.56/0 | −4.06/1 |
| Neomenthol | −4.43/2 | −4.19/2 | −4.68/2 | −4.63/1 | −4.97/1 | −5.41/2 | −5.13/2 |
| L–Menthol | −4.58/1 | −3.81/1 | −4.33/2 | −4.4/1 | −5.54/2 | −5.35/1 | −4.98/1 |
| Isomenthol | −4.39/2 | −3.82/2 | −4.84/2 | −4.23/2 | −4.96/1 | −5.28/2 | −4.79/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lai, J.; Xu, F.; Liu, M. Network Pharmacology and Molecular Docking: Exploring the Mechanism of Peppermint in Mastitis Prevention and Treatment in Dairy Cows. Vet. Sci. 2025, 12, 129. https://doi.org/10.3390/vetsci12020129
Wang X, Lai J, Xu F, Liu M. Network Pharmacology and Molecular Docking: Exploring the Mechanism of Peppermint in Mastitis Prevention and Treatment in Dairy Cows. Veterinary Sciences. 2025; 12(2):129. https://doi.org/10.3390/vetsci12020129
Chicago/Turabian StyleWang, Xinyu, Jiaxin Lai, Fei Xu, and Mingchun Liu. 2025. "Network Pharmacology and Molecular Docking: Exploring the Mechanism of Peppermint in Mastitis Prevention and Treatment in Dairy Cows" Veterinary Sciences 12, no. 2: 129. https://doi.org/10.3390/vetsci12020129
APA StyleWang, X., Lai, J., Xu, F., & Liu, M. (2025). Network Pharmacology and Molecular Docking: Exploring the Mechanism of Peppermint in Mastitis Prevention and Treatment in Dairy Cows. Veterinary Sciences, 12(2), 129. https://doi.org/10.3390/vetsci12020129






