Disopyramide Therapy in Cats with Obstructive Hypertrophic Cardiomyopathy Non-Responsive to Carvedilol
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Standard Echocardiography
2.3. Two-Dimensional Speckle Tracking Echocardiography
2.4. Cardiac Troponin I Measurement
2.5. Electrocardiography
2.6. Statistical Analysis
3. Results
3.1. Clinical Profiles
3.2. Echocardiography
3.3. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kittleson, M.D.; Côté, E. The Feline Cardiomyopathies: 2. Hypertrophic Cardiomyopathy. J. Feline Med. Surg. 2021, 23, 1028–1051. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Holmes, D.R. Hypertrophic Obstructive Cardiomyopathy. N. Engl. J. Med. 2004, 350, 1320–1327. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of Left Ventricular Outflow Tract Obstruction on Clinical Outcome in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Steve, R.O.; Carolyn, Y.H.; Irfan, M.A.; Seshadri, B.; Michael, A.B.; Sharlene, M.D.; Joseph, A.D.; Kelly, C.E.; Lauren, E.; José, A.J.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Kitaoka, H.; Tsutsui, H.; Kubo, T.; Ide, T.; Chikamori, T.; Fukuda, K.; Fujino, N.; Higo, T.; Isobe, M.; Kamiya, C.; et al. JCS/JHFS 2018 Guideline on the Diagnosis and Treatment of Cardiomyopathies. Circ. J. 2021, 85, 1590–1689. [Google Scholar] [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM Consensus Statement Guidelines for the Classification, Diagnosis, and Management of Cardiomyopathies in Cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Gilbert, E.M.; Abraham, W.T.; Adams, K.F.; Fowler, M.B.; Hershberger, R.E.; Kubo, S.H.; Narahara, K.A.; Ingersoll, H.; Krueger, S.; et al. Carvedilol Produces Dose-Related Improvements in Left Ventricular Function and Survival in Subjects with Chronic Heart Failure. Circulation 1996, 94, 2807–2816. [Google Scholar] [CrossRef]
- Nishiyama, K.; Tsutamoto, T.; Yamaji, M.; Kawahara, C.; Yamamoto, T.; Fujii, M.; Horie, M. Dose-Dependent Prognostic Effect of Carvedilol in Patients With Chronic Heart Failure Special Reference to Ranscardiac Gradient of Norepinephrine. Circ. J. 2009, 73, 2270–2275. [Google Scholar] [CrossRef][Green Version]
- Ostman-Smith, I.; Wettrell, G.; Riesenfeld, T. A Cohort Study of Childhood Hypertrophic Cardiomyopathy: Improved Survival Following High-Dose Beta-Adrenoceptor Antagonist Treatment. J. Am. Coll. Cardiol. 1999, 34, 1813–1822. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Suzuki, R.; Yasumura, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Left Ventricular Geometric Characteristics Predict Response to Carvedilol in Cats with Asymptomatic Hypertrophic Obstructive Cardiomyopathy Caused by Systolic Anterior Motion of the Mitral Valve. J. Vet. Med. Sci. 2019, 81, 734–738. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yuchi, Y.; Yasumura, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Assessment of Myocardial Function in Obstructive Hypertrophic Cardiomyopathy Cats with and without Response to Medical Treatment by Carvedilol. BMC Vet. Res. 2019, 15, 376. [Google Scholar] [CrossRef]
- Güler, A.; Erata, Y.E.; Demirtola, A.İ.; Türkmen, İ.; Atmaca, S.; Şahin, H.; Aydın, S.; Almasri, M.; Tekin, M.; Coşkun, G.; et al. The Effect of Disopyramide Therapy on Functional Capacity Improvement in Patients with Obstructive Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2025, 421, 132913. [Google Scholar] [CrossRef]
- Sanchez-Nadales, A.; Anampa-Guzmán, A.; Khan, A. Disopyramide for Hypertrophic Cardiomyopathy. Cureus 2019, 11, e4526. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Barac, I.; McKenna, W.J.; Elliott, P.M.; Dickie, S.; Chojnowska, L.; Casey, S.; Maron, B.J. Multicenter Study of the Efficacy and Safety of Disopyramide in Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Fujimoto, E.; Nishikawa, Y.; Nakamura, T. Left Ventricular Outflow Tract Pressure Gradient Changes after Carvedilol–Disopyramide Cotherapy in a Cat with Hypertrophic Obstructive Cardiomyopathy. J. Vet. Cardiol. 2020, 29, 40–46. [Google Scholar] [CrossRef]
- Satomi, S.; Suzuki, R.; Yuchi, Y.; Yoshii, Y.; Kanno, H.; Teshima, T.; Matsumoto, H. Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats with or Without Left Ventricular Outflow Tract Obstruction. Animals 2025, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Capozza, P.; Donati, F.; Simioniuc, A.; Corciu, A.I.; Fontanive, P.; Pieroni, A.; Di Bello, V.; Marzilli, M. Patterns of Left Ventricular Remodeling in Chronic Heart Failure: Prevalence and Prognostic Implications. Am. Heart J. 2011, 161, 1088–1095. [Google Scholar] [CrossRef]
- Alam, M.; Zhang, L.; Stampehl, M.; Lakkis, N.; Dokainish, H. Usefulness of Speckle Tracking Echocardiography in Hypertensive Crisis and the Effect of Medical Treatment. Am. J. Cardiol. 2013, 112, 260–265. [Google Scholar] [CrossRef]
- Suchodolski, A.; Wójcik-Giertuga, M.; Kos-Kudła, B.; Szulik, M. Acromegaly in Speckle Tracking Echocardiography—A New Cardiac Hypertrophy Phenotype? Case Report and Review. Life 2024, 14, 1459. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Niina, A.; Teshima, T.; Matsumoto, H.; Koyama, H. Layer-Specific Myocardial Function in Asymptomatic Cats with Obstructive Hypertrophic Cardiomyopathy Assessed Using 2-Dimensional Speckle-Tracking Echocardiography. J. Vet. Intern. Med. 2019, 33, 37–45. [Google Scholar] [CrossRef]
- Ozawa, K.; Funabashi, N.; Takaoka, H.; Kamata, T.; Kanaeda, A.; Saito, M.; Nomura, F.; Kobayashi, Y. Characteristic Myocardial Strain Identified in Hypertrophic Cardiomyopathy Subjects with Preserved Left Ventricular Ejection Fraction Using a Novel Multi-Layer Transthoracic Echocardiography Technique. Int. J. Cardiol. 2015, 184, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yamada, S.; Iwano, H.; Nishino, H.; Nakabachi, M.; Yokoyama, S.; Abe, A.; Ichikawa, A.; Kaga, S.; Nishida, M.; et al. Myocardial Shortening in 3 Orthogonal Directions and Its Transmural Variation in Patients with Nonobstructive Hypertrophic Cardiomyopathy. Circulation J. 2015, 79, 2471–2479. [Google Scholar] [CrossRef]
- Bastos, R.F.; Tuleski, G.L.; Sousa, M.G. QT Interval Instability and QRS Interval Dispersion in Healthy Cats and Cats with a Hypertrophic Cardiomyopathy Phenotype. J. Feline Med. Surg. 2023, 25, 1098612X231151479. [Google Scholar] [CrossRef]
- Fridericia, L.S. Die Systolendauer Im Elektrokardiogramm Bei Normalen Menschen Und Bei Herzkranken. Acta Medica Scand. 1920, 53, 489–506. [Google Scholar] [CrossRef]
- Henry, W.L.; Clark, C.E.; Griffith, J.M.; Epstein, S.E. Mechanism of Left Ventricular Outflow Obstruction in Patients with Obstructive Asymmetric Septal Hypertrophy (Idiopathic Hypertrophic Subaortic Stenosis). Am. J. Cardiol. 1975, 35, 337–345. [Google Scholar] [CrossRef]
- Jiang, L.; Levine, R.A.; King, M.E.; Weyman, A.E. An Integrated Mechanism for Systolic Anterior Motion of the Mitral Valve in Hypertrophic Cardiomyopathy Based on Echocardiographic Observations. Am. Heart J. 1987, 113, 633–644. [Google Scholar] [CrossRef]
- Conklin, H.M.; Huang, X.; Davies, C.H.; Sahn, D.J.; Shively, B.K. Biphasic Left Ventricular Outflow and Its Mechanism in Hypertrophic Obstructive Cardiomyopathy. J. Am. Soc. Echocardiogr. 2004, 17, 375–383. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Chu, C.K.; Delia, E.; Mograder, A.; Dwyer, E.M. An Echocardiographic Study of the Fluid Machanics of Obstruction in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 1993, 22, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.X.; Shiota, T.; Lever, H.M.; Rubin, D.N.; Bauer, F.; Kim, Y.J.; Sitges, M.; Greenberg, N.L.; Drinko, J.K.; Martin, M.; et al. Impact of Left Ventricular Outflow Tract Area on Systolic Outflow Velocity in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 308–314. [Google Scholar] [CrossRef]
- Sherrid, M.V. Pathophysiology and Treatment of Hypertrophic Cardiomyopathy. Prog. Cardiovasc. Dis. 2006, 49, 123–151. [Google Scholar] [CrossRef] [PubMed]
- Coppini, R.; Ferrantini, C.; Pioner, J.M.; Santini, L.; Wang, Z.J.; Palandri, C.; Scardigli, M.; Vitale, G.; Sacconi, L.; Stefàno, P.; et al. Electrophysiological and Contractile Effects of Disopyramide in Patients With Obstructive Hypertrophic Cardiomyopathy: A Translational Study. JACC Basic Transl. Sci. 2019, 4, 795–813. [Google Scholar] [CrossRef]
- Saito, T.; Suzuki, R.; Yuchi, Y.; Fukuoka, H.; Satomi, S.; Teshima, T.; Matsumoto, H. Post-Carvedilol Myocardial Function in Cats with Obstructive Hypertrophic Cardiomyopathy. Front. Vet. Sci. 2025, 12, 1571850. [Google Scholar] [CrossRef]
- Dybro, A.M.; Rasmussen, T.B.; Nielsen, R.R.; Andersen, M.J.; Jensen, M.K.; Poulsen, S.H. Randomized Trial of Metoprolol in Patients With Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2505–2517. [Google Scholar] [CrossRef]
- Spirito, P.; Seidman, C.E.; McKenna, W.J.; Maron, B.J. The Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 1997, 336, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Massera, D.; Sherrid, M.V.; Adlestein, E.; Bokhari, N.; Alvarez, I.C.; Wu, W.Y.; Reuter, M.C.; Maron, M.S.; Maron, B.J.; Rowin, E.J. Disopyramide Revisited for Treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy: Efficacy and Safety in Patients Treated for at Least 5 Years. J. Am. Heart Assoc. 2025, 14, e037639. [Google Scholar] [CrossRef]
- Yedidya, I.; Elbaz Greener, G.; Vaturi, M.; Sagie, A.; Amir, O.; Carasso, S.; Monakier, D. Impact of Short-Acting Disopyramide on Left Ventricular Mechanics Evaluated by Strain Analysis in Patients with Hypertrophic Obstructive Cardiomyopathy. J. Clin. Med. 2022, 11, 7325. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Lester, S.J.; Solomon, S.D.; Michels, M.; Elliott, P.M.; Nagueh, S.F.; Choudhury, L.; Zemanek, D.; Zwas, D.R.; Jacoby, D.; et al. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients With Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2518–2532. [Google Scholar] [CrossRef]
- Jain, R.; Osranek, M.; Jan, M.F.; Kalvin, L.R.; Olet, S.; Allaqaband, S.Q.; Jahangir, A.; Khandheria, B.K.; Tajik, A.J. Marked Respiratory-Related Fluctuations in Left Ventricular Outflow Tract Gradients in Hypertrophic Obstructive Cardiomyopathy: An Observational Study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C.; Bois, J.P.; Masaki, M.; Yuasa, T.; Oh, J.K.; Ommen, S.R.; Nishimura, R.A.; Klarich, K.W. Postprandial Hemodynamics in Hypertrophic Cardiomyopathy. Echocardiography 2015, 32, 1614–1620. [Google Scholar] [CrossRef]
- Coats, C.J.; Masri, A.; Barriales-Villa, R.; Abraham, T.P.; Brinkley, D.M.; Claggett, B.L.; Hagege, A.; Hegde, S.M.; Ho, C.Y.; Kulac, I.J.; et al. Cardiac Biomarkers and Effects of Aficamten in Obstructive Hypertrophic Cardiomyopathy: The SEQUOIA-HCM Trial. Eur. Heart J. 2024, 45, 4464–4478. [Google Scholar] [CrossRef]
- Borgeat, K.; Sherwood, K.; Payne, J.R.; Luis Fuentes, V.; Connolly, D.J. Plasma Cardiac Troponin I Concentration and Cardiac Death in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2014, 28, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Langhorn, R.; Tarnow, I.; Willesen, J.L.; Kjelgaard-Hansen, M.; Skovgaard, I.M.; Koch, J. Cardiac Troponin I and T as Prognostic Markers in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2014, 28, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Fourey, D.; Weissler-Snir, A.; Hindieh, W.; Chan, R.H.; Gollob, M.H.; Rakowski, H. Safety of Outpatient Initiation of Disopyramide for Obstructive Hypertrophic Cardiomyopathy Patients. J. Am. Heart Assoc. 2017, 6, e005152. [Google Scholar] [CrossRef] [PubMed]
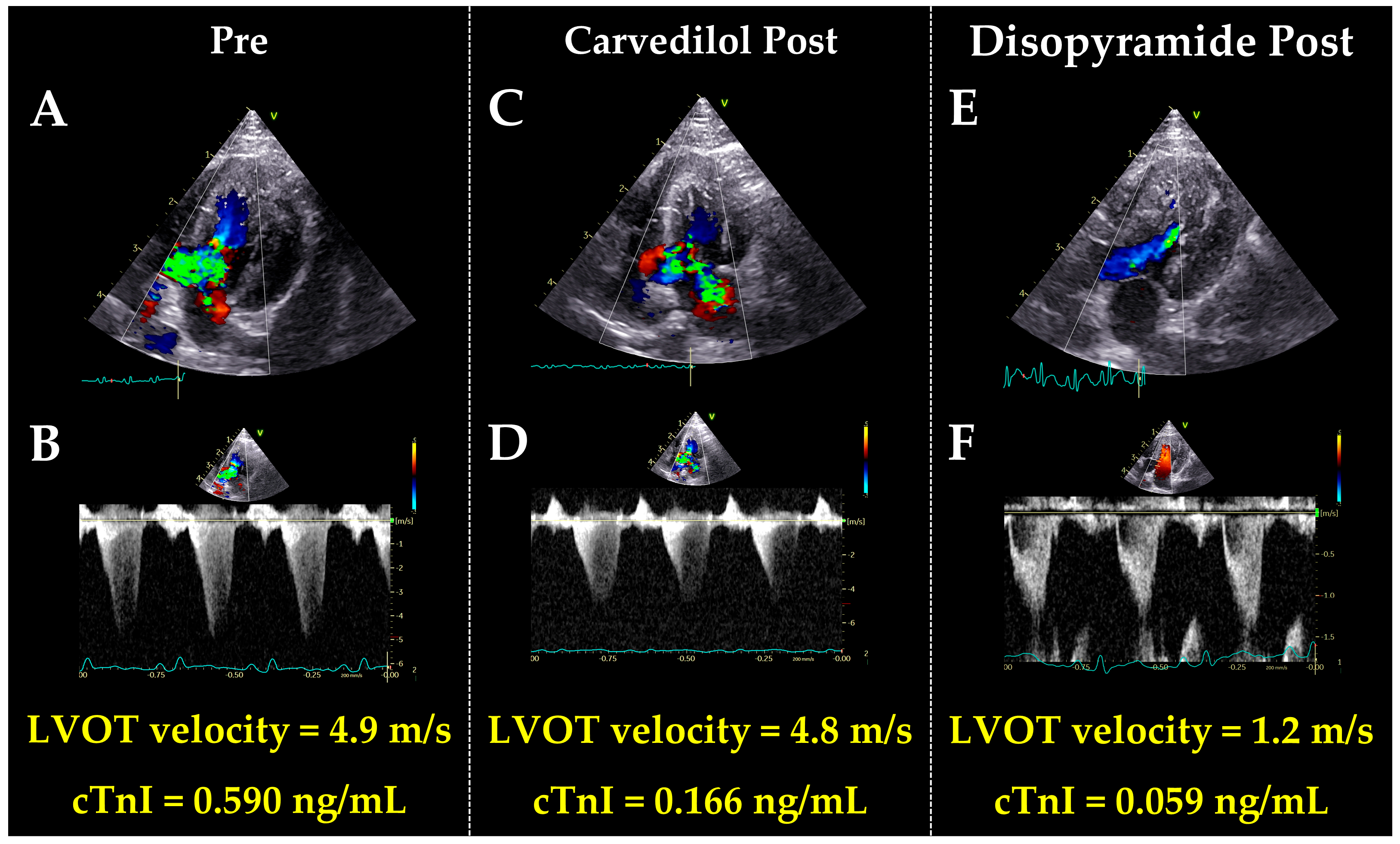
| Variables | Pre (n = 6) | Post (n = 6) | p Value |
|---|---|---|---|
| Nunber (Male/Female) | 6 (3/3) | ||
| Age (y) | 2.1 (1.1, 3.7) | 2.2 (1.4, 3.9) | 0.055 |
| Body weight (kg) | 3.3 (3.0, 4.5) | 3.3 (3.0, 4.4) | 0.673 |
| Systolic blood pressure (mmHg) | 133 (124, 139) | 125 (119, 131) | 0.137 |
| HR (bpm) | 174 (156, 188) | 180 (171, 189) | 0.513 |
| QT (ms) | 186 (166, 208) | 186 (168, 206) | 0.653 |
| QTc (ms) | 34.3 (28.6, 38.6) | 33.5 (29.1, 37.3) | 0.851 |
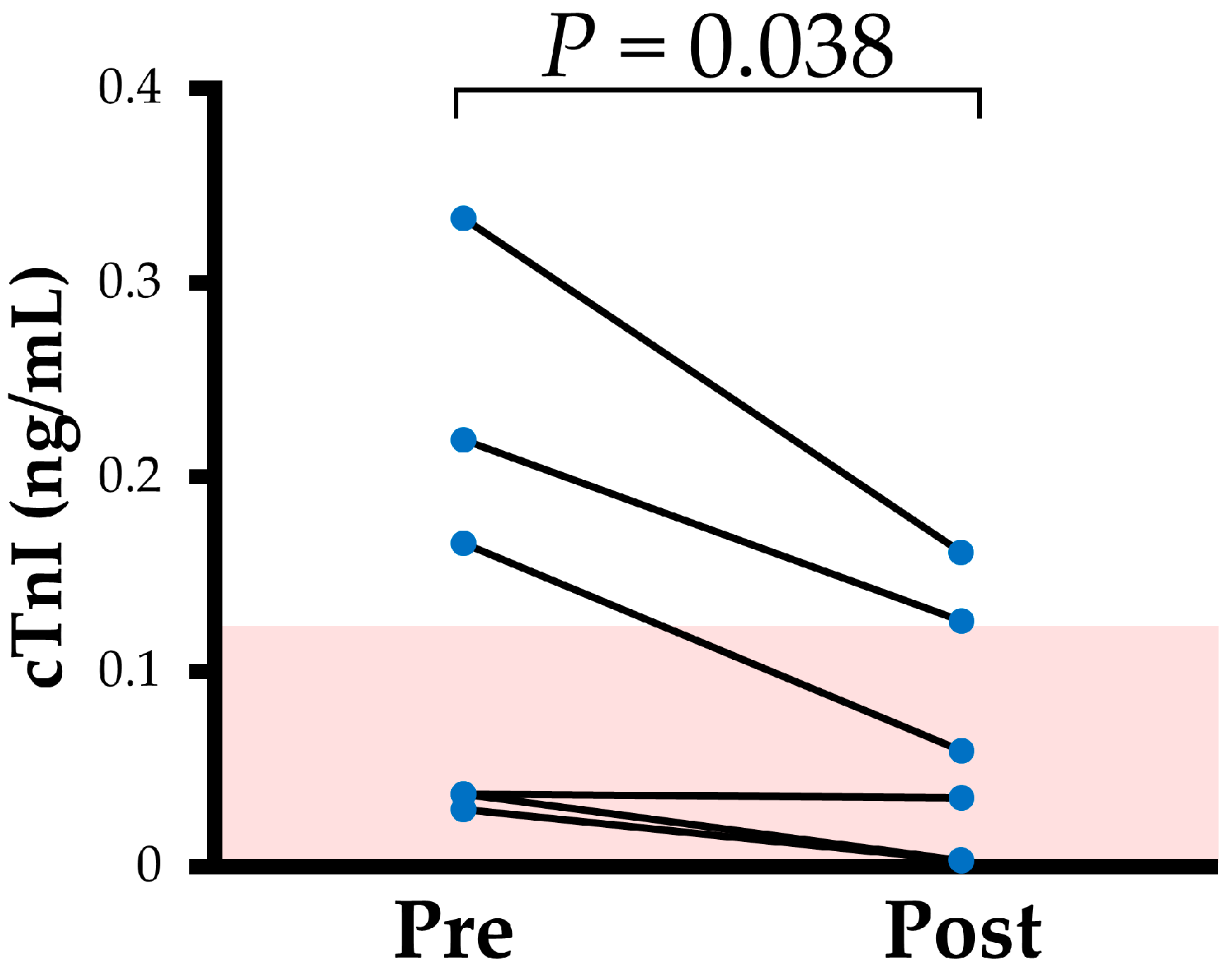
| cTnI (ng/mL) | 0.102 (0.035, 0.248) | 0.047 (0.003, 0.135) | 0.038 |
| Carvedilol dose (mg/kg q 12h) | 0.6 (0.6, 0.6) | 0.6 (0.5, 0.6) | |
| Disopyramide dose (mg/kg q 12h) | 11.4 (8.5, 12.5) | ||
| Duration of disopyramide (days) | 56 (19, 138) | ||
| Variables | Pre (n = 6) | Post (n = 6) | p Value |
|---|---|---|---|
| LA/Ao | 1.2 (1.1, 1.3) | 1.0 (0.9, 1.4) | 0.334 |
| IVSd (mm) | 5.5 (5.2, 6.5) | 5.3 (4.5, 6.1) | 0.028 |
| LVIDd (mm) | 12.1 (11.6, 13.0) | 12.9 (11.1, 13.9) | 0.642 |
| LVPWd (mm) | 5.8 (5.4, 5.9) | 5.1 (4.5, 5.7) | 0.293 |
| RWT | 0.9 (0.8, 1.0) | 0.7 (0.5, 0.7) | 0.518 |
| FS (%) | 40.1 (34.5, 43.8) | 44.0 (38.4, 52.4) | 0.464 |
| E-wave (m/s) | 0.7 (0.6, 0.8) | 0.7 (0.5, 0.7) | 0.462 |
| E/A | 1.1 (0.6, 0.8) n = 5 | 1.0 (0.9, 1.0) n = 5 | 0.144 |
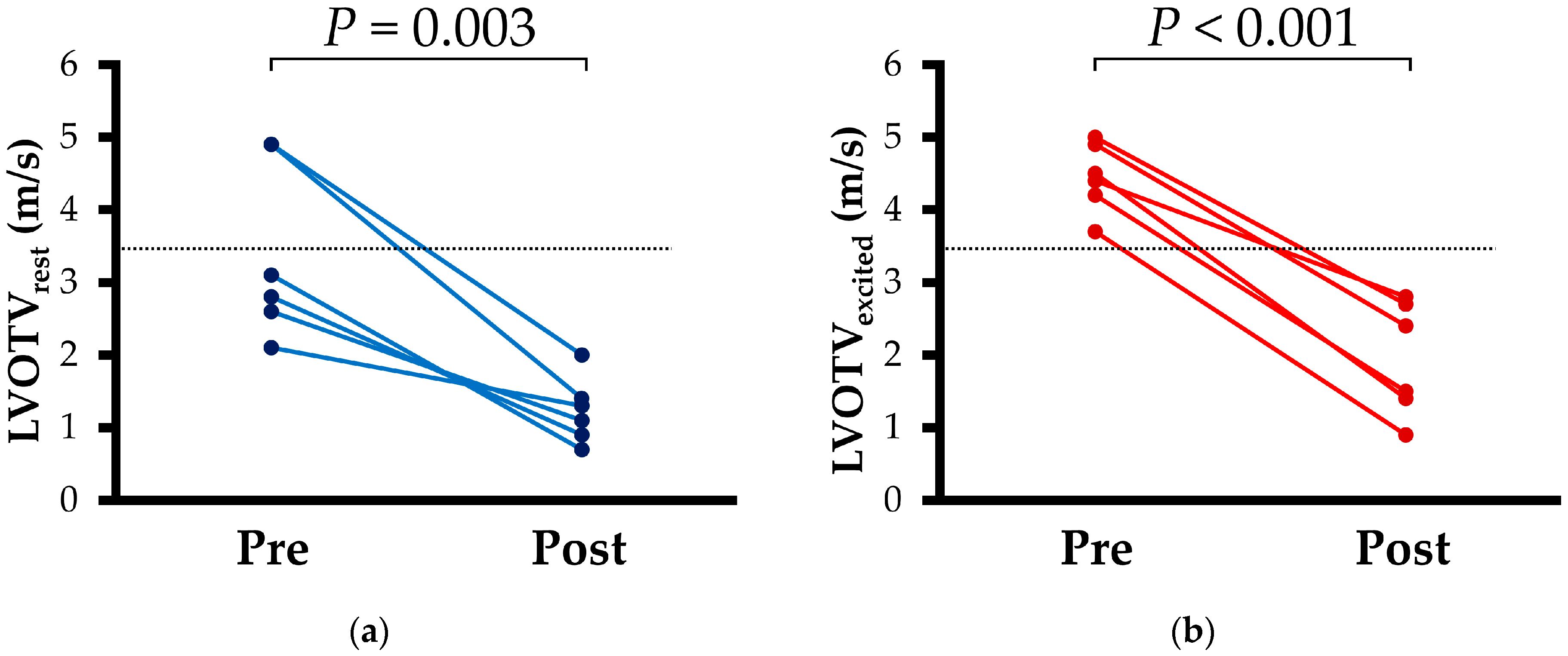
| LVOTVrest (m/s) | 3.0 (2.5, 4.9) | 1.2 (0.9, 1.6) * | 0.003 |
| LVOTVexcited (m/s) | 4.5 (4.1, 4.9) | 2.0 (1.3, 2.7) * | <0.001 |
| Variables | Pre (n = 6) | Post (n = 6) | p Value |
|---|---|---|---|
| Longitudinal strains (%) | |||
| Whole layer | 16.2 (13.4, 18.0) | 10.0 (9.2, 19.0) | 0.345 |
| Endocardial layer | 19.2 (14.3, 22.9) | 11.2 (10.3, 22.2) | 0.345 |
| Epicardial layer | 13.3 (11.2, 14.4) | 9.1 (8.3, 16.5) | 0.753 |
| Endo/Epi | 1.5 (1.1, 1.6) | 1.3 (1.2, 1.3) | 0.556 |
| Circumferential strains (%) | |||
| Whole layer | 16.0 (15.4, 20.6) | 19.9 (16.1, 20.8) | 0.380 |
| Endocardial layer | 32.9 (28.2, 36.9) | 36.2 (27.2, 39.3) | 0.752 |
| Epicardial layer | 8.0 (5.5, 8.8) | 8.3 (7.9, 8.9) | 0.249 |
| Endo/Epi | 4.5 (3.5, 6.0) | 3.9 (3.5, 4.7) | 0.265 |
| Variables | cTnI | LVOTVrest | LVOTVexcited | |||
|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | |
| cTnI | ― | ― | 0.62 | <0.01 | 0.66 | 0.020 |
| Longitudinal strains (%) | ||||||
| Whole layer | −0.53 | 0.074 | −0.03 | 0.936 | −0.01 | 0.985 |
| Endocardial layer | −0.52 | 0.081 | 0.01 | 0.970 | 0.01 | 0.980 |
| Epicardial layer | −0.45 | 0.143 | −0.07 | 0.836 | −0.08 | 0.808 |
| Endo/Epi | −0.33 | 0.301 | 0.19 | 0.552 | 0.12 | 0.719 |
| Circumferential strains (%) | ||||||
| Whole layer | −0.20 | 0.541 | −0.11 | 0.743 | −0.14 | 0.669 |
| Endocardial layer | −0.25 | 0.428 | 0.03 | 0.926 | −0.03 | 0.934 |
| Epicardial layer | −0.06 | 0.862 | −0.20 | 0.540 | −0.18 | 0.571 |
| Endo/Epi | −0.04 | 0.914 | 0.22 | 0.499 | 0.16 | 0.617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satomi, S.; Suzuki, R.; Yuchi, Y.; Kanno, H.; Nomura, M.; Teshima, T.; Matsumoto, H. Disopyramide Therapy in Cats with Obstructive Hypertrophic Cardiomyopathy Non-Responsive to Carvedilol. Vet. Sci. 2025, 12, 999. https://doi.org/10.3390/vetsci12100999
Satomi S, Suzuki R, Yuchi Y, Kanno H, Nomura M, Teshima T, Matsumoto H. Disopyramide Therapy in Cats with Obstructive Hypertrophic Cardiomyopathy Non-Responsive to Carvedilol. Veterinary Sciences. 2025; 12(10):999. https://doi.org/10.3390/vetsci12100999
Chicago/Turabian StyleSatomi, Shuji, Ryohei Suzuki, Yunosuke Yuchi, Haruka Kanno, Miyuki Nomura, Takahiro Teshima, and Hirotaka Matsumoto. 2025. "Disopyramide Therapy in Cats with Obstructive Hypertrophic Cardiomyopathy Non-Responsive to Carvedilol" Veterinary Sciences 12, no. 10: 999. https://doi.org/10.3390/vetsci12100999
APA StyleSatomi, S., Suzuki, R., Yuchi, Y., Kanno, H., Nomura, M., Teshima, T., & Matsumoto, H. (2025). Disopyramide Therapy in Cats with Obstructive Hypertrophic Cardiomyopathy Non-Responsive to Carvedilol. Veterinary Sciences, 12(10), 999. https://doi.org/10.3390/vetsci12100999







