Post-Vaccination Assessment of Peste Des Petits Ruminants in Sheep and Goats in the United Arab Emirates
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Collection and Vaccination
2.3. Serology
2.4. Vaccine Efficacy and Vaccination Effectiveness
2.5. Annual Growth Rate of Immuno-Protection Threshold
2.6. Statistical Analysis
3. Results
3.1. PPR Antibody Seroprevalence Before Vaccination
3.2. PPR Post-Vaccination Seroprevalence
3.3. Assessing Effectiveness of Vaccination Campaign
3.4. Estimation of Time to Reach Immuno-Protection Threshold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balamurugan, V.; Vinod Kumar, K.; Dheeraj, R.; Kurli, R.; Suresh, K.P.; Govindaraj, G.; Shome, B.R.; Roy, P. Temporal and spatial epidemiological analysis of Peste des petits ruminants outbreaks from the past 25 years in sheep and goats and its control in India. Viruses 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Gargadennec, L.; Lalanne, A. La peste des petits ruminants. Bull. Serv. Zoo. AOF 1942, 5, 15–21. [Google Scholar]
- PPR Free Members. Available online: https://www.woah.org/en/disease/peste-des-petits-ruminants/#ui-id-5 (accessed on 25 August 2025).
- Parida, S.; Yusuf, J.; Njeumi, F. Update on peste des petits ruminants in Europe. Vet. Rec. 2024, 195, 211. [Google Scholar] [PubMed]
- FAO. Global Control and Eradication of Peste des Petits Ruminants: Investing in Veterinary Systems, Food Security and Poverty Alleviation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015.
- Zhao, H.; Njeumi, F.; Parida, S.; Benfield, C.T. Progress towards eradication of peste des petits ruminants through vaccination. Viruses 2021, 13, 59. [Google Scholar] [CrossRef]
- Furley, C.; Taylor, W.; Obi, T. An outbreak of peste des petits ruminants in a zoological collection. Vet. Rec. 1987, 121, 443–447. [Google Scholar]
- Kinne, J.; Kreutzer, R.; Kreutzer, M.; Wernery, U.; Wohlsein, P. Peste des petits ruminants in Arabian wildlife. Epidemiol. Infect. 2010, 138, 1211–1214. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Terab, A.M.A.; Eltahir, Y.M.; El Tigani-Asil, E.T.A.; Khalil, N.A.H.; Gasim, E.F.M.; Yuosf, M.F.; Al Yammahi, S.M.S.; Al Mansoori, A.M.A.; Al Muhairi, S.S.M.; et al. A Clinical, Pathological, Epidemiological and Molecular Investigation of Recent Outbreaks of Peste des Petits Ruminants Virus in Domestic and Wild Small Ruminants in the Abu Dhabi Emirate, United Arab Emirates. Vet. Sci. 2023, 10, 56. [Google Scholar]
- Fathelrahman, E.M.; Reeves, A.; Mohamed, M.S.; Ali, Y.M.E.; El Awad, A.I.; Bensalah, O.-K.; Abdalla, A.A. Epidemiology and cost of peste des petits ruminants (Ppr) eradication in small ruminants in the united arabemirates—Disease spread and control strategies simulations. Animals 2021, 11, 2649. [Google Scholar]
- World Organization for Animal Health WOAH World Animal Health Information System WOAH-WAHIS. Quantitative Data. Available online: https://wahis.woah.org/#/dashboards/qd-dashboard (accessed on 30 August 2025).
- Eltahir, Y.M.; Aburizq, W.; Bensalah, O.K.; Mohamed, M.S.; Al Shamisi, A.; AbdElkader, A.I.; Al-Majali, A. Modeling for Smart Vaccination against Peste des Petits Ruminants (PPR) in the Emirate of Abu Dhabi, United Arab Emirates. Animals 2023, 13, 3248. [Google Scholar] [CrossRef]
- WOAH World Organization for Animal Health, M.E.R., United Arab Emirates; Al-Qassimi, M., Ed.; WOAH: Paris, France, 2024; Available online: https://rr-middleeast.woah.org/en/about-us/regional-members-of-woah/the-united-arab-emirates-uae/ (accessed on 10 September 2025).
- Libeau, G.; Prehaud, C.; Lancelot, R.; Colas, F.; Guerre, L.; Bishop, D.; Diallo, A. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleobrotein. Res. Vet. Sci. 1995, 58, 50–55. [Google Scholar]
- Balamurugan, V.; Ojha, R.; Kumar, K.V.; Asha, A.; Ashraf, S.; Dsouza, A.H.; Pal, A.; Bokade, P.P.; Harshitha, S.K.; Deshpande, R. Post-vaccination sero-monitoring of peste des petits ruminants in sheep and goats in karnataka: Progress towards PPR eradication in India. Viruses 2024, 16, 333. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Muthuchelvan, D.; Govindaraj, G.; Roy, G.; Sharma, V.; Kumari, S.S.; Choudhary, D.; Mohanty, B.; Suresh, K.; Rajak, K. Serosurvey for assessing PPR vaccination status in rural system of Chhattisgarh state of India. Small Rumin. Res. 2018, 165, 87–92. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Sciences, 2nd ed.; Blackwell Publishing Asia Pty Ltd.: Victoria, Australia, 2006; pp. 49–52. [Google Scholar]
- Baron, M.; Parida, S.; Oura, C. Peste des petits ruminants: A suitable candidate for eradication? Vet. Rec. 2011, 169, 16–21. [Google Scholar] [CrossRef] [PubMed]
- FAO; WOAH. Overview of The Plan of Action Peste Petits Ruminants Global Eradication Programme II & III. In Together for Peste Petits Ruminants Global Eradication by 2030 Blueprint 2022; FAO: Rome, Italy; WOAH: Paris, France, 2022. [Google Scholar]
- Anderson, J.; Baron, M.; Cameron, A.; Kock, R.; Jones, B.; Pfeiffer, D.; Mariner, J.; McKeever, D.; Oura, C.; Roeder, P. Rinderpest eradicated; what next. Vet. Rec. 2011, 169, 10–11. [Google Scholar] [CrossRef]
- Lefèvre, P.C.; Diallo, A. Peste des petits ruminants. Rev. Sci. Tech. 1990, 9, 935–981. [Google Scholar] [CrossRef]
- Mariner, J.C.; Jones, B.A.; Rich, K.M.; Thevasagayam, S.; Anderson, J.; Jeggo, M.; Cai, Y.; Peters, A.R.; Roeder, P.L. The opportunity to eradicate peste des petits ruminants. J. Immunol. 2016, 196, 3499–3506. [Google Scholar] [CrossRef]
- Taylor, W. The distribution and epidemiology of peste des petits ruminants. Prev. Vet. Med. 1984, 2, 157–166. [Google Scholar] [CrossRef]
- Fine, A.E.; Pruvot, M.; Benfield, C.T.O.; Caron, A.; Cattoli, G.; Chardonnet, P.; Dioli, M.; Dulu, T.; Gilbert, M.; Kock, R.; et al. Eradication of Peste des Petits Ruminants Virus and the Wildlife-Livestock Interface. Front. Vet. Sci. 2020, 7, 50. [Google Scholar] [CrossRef]
- Benfield, C.T.O.; Legnardi, M.; Mayen, F.; Almajali, A.; Cinardi, G.; Wisser, D.; Chaka, H.; Njeumi, F. Peste Des Petits Ruminants in the Middle East: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Program (2017–2021). Animals 2023, 13, 1196. [Google Scholar] [CrossRef]
- Legnardi, M.; Raizman, E.; Beltran-Alcrudo, D.; Cinardi, G.; Robinson, T.; Falzon, L.C.; Djomgang, H.K.; Okori, E.; Parida, S.; Njeumi, F.; et al. Peste des Petits Ruminants in Central and Eastern Asia/West Eurasia: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Programme (2017–2021). Animals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Munir, M. Role of wild small ruminants in the epidemiology of peste des petits ruminants. Transbound Emerg. Dis. 2014, 61, 411–424. [Google Scholar] [CrossRef]
- Imanbayeva, D.; Pérez Aguirreburualde, M.S.; Knauer, W.; Tegzhanov, A.; Yustyniuk, V.; Arzt, J.; Perez, A.; Njeumi, F.; Parida, S. A Scoping Review on Progression Towards Freedom from Peste des Petits Ruminants (PPR) and the Role of the PPR Monitoring and Assessment Tool (PMAT). Viruses 2025, 17, 563. [Google Scholar] [CrossRef]
- Fournié, G.; Waret-Szkuta, A.; Camacho, A.; Yigezu, L.M.; Pfeiffer, D.U.; Roger, F. A dynamic model of transmission and elimination of peste des petits ruminants in Ethiopia. Proc. Natl. Acad. Sci. USA 2018, 115, 8454–8459. [Google Scholar] [CrossRef]
- WOAH; FAO. Manual on Global Strategy for the Control and Eradication of Pesti des Petits Ruminants; The World Organisation for Animal Health: Paris, France; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Kumbe, A.; Negussie, H.; Getachew, Y.; Alemu, B.; Alemayehu, G.; Girma, S.; Sibhatu, D.; Emiyu, K.; Waktole, H.; Leta, S. Epidemiology of peste des petits ruminants in selected districts of Borena zone, Ethiopia. BMC Vet. Res. 2024, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.E.; Hossain, M.M.; Ershaduzzaman, M.; Yousuf, M.A.; Islam, M.R. Sero-surveillance and sero-monitoring of locally produced PPR vaccine in the field and experimental level. Asian J. Med. Biol. Res. 2016, 2, 33–37. [Google Scholar]
- Mandefro, M.; Ibrahim, S.M.; Sibhatu, D.; Kassa, N.; Emiyu, K.; Debebe, K.; Dessalegn, B.; Birhan, M.; Bitew, M. Vaccine sero-monitoring and sero-surveillance of Peste des petits ruminants in small ruminants in West Gojjam zone, Amhara region, Ethiopia. Front. Vet. Sci. 2024, 11, 1392893. [Google Scholar] [CrossRef] [PubMed]
- Yirga, A.; Jemberu, W.T.; Lyons, N.; Gebru, A.; Akililu, F.; Rushton, J. Post-vaccination herd immunity against peste des petits ruminants and inter-vaccination population turnover in small ruminant flocks in northwest Ethiopia. Prev. Vet. Med. 2020, 174, 104850. [Google Scholar] [CrossRef]
- Savagar, B.; Jones, B.A.; Arnold, M.; Walker, M.; Fournié, G. Modelling flock heterogeneity in the transmission of peste des petits ruminants virus and its impact on the effectiveness of vaccination for eradication. Epidemics 2023, 45, 100725. [Google Scholar] [CrossRef]
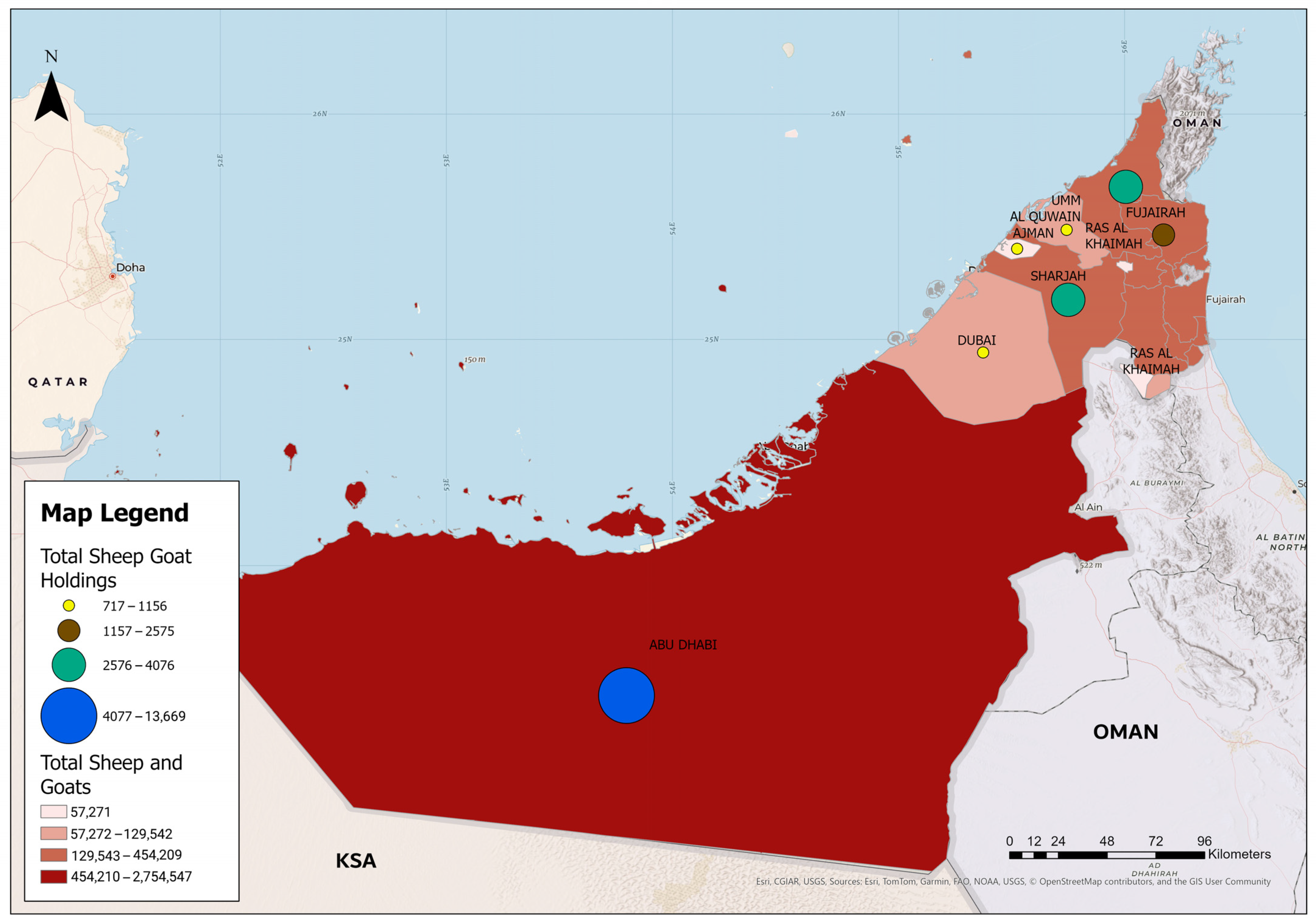

| Emirate | Total Sheep and Goats | Total Sheep and Goats Holdings | Percentage of Animals from Total Livestock Population |
|---|---|---|---|
| Abu Dhabi | 2,754,547 | 13,669 | 66.64 |
| Dubai | 129,542 | 717 | 3.13 |
| Sharjah | 454,209 | 4076 | 10.99 |
| Ajman | 57,271 | 780 | 1.39 |
| Umm Al-Quwain | 104,766 | 1156 | 2.54 |
| Ras Al-Khaimah | 357,032 | 3716 | 8.64 |
| Fujairah | 276,184 | 2575 | 6.68 |
| Total | 4,133,551 | 26,689 | 100 |
| Emirates | No. of Holdings Sampled | No. of Samples Screened | Total Goats | Total Sheep | No. of ELISA Positive Goats | No. of ELISA Positive Sheep | Totals ELISA Positive | Average PPR Seroprevalence in Goats (%) | Average PPR Seroprevalence in Sheep (%) | Average PPR Seroprevalence in Sheep and Goats (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abu Dhabi | 76 | 727 | 404 | 323 | 186 | 115 | 301 | 46.04 | 35.60 | 41.40 |
| Dubai | 9 | 90 | 45 | 45 | 21 | 21 | 42 | 46.67 | 46.67 | 46.67 |
| Sharjah | 29 | 290 | 145 | 145 | 80 | 80 | 160 | 55.17 | 55.17 | 55.17 |
| Ajman | 4 | 40 | 20 | 20 | 14 | 14 | 28 | 70.00 | 70.00 | 70.00 |
| Umm Al-Quwain | 7 | 70 | 35 | 35 | 14 | 21 | 35 | 40.00 | 60.00 | 50.00 |
| Ras Al-Khaimah | 21 | 205 | 105 | 100 | 85 | 73 | 158 | 80.95 | 73.00 | 77.07 |
| Fujairah | 17 | 170 | 85 | 85 | 61 | 48 | 109 | 71.76 | 56.47 | 64.12 |
| Total | 163 | 1592 | 839 | 753 | 461 | 372 | 833 | 54.95 | 49.40 | 52.32 |
| Row Labels | No. of Holdings Sampled | No. of Samples Screened | Total Goats | Total Sheep | No. of ELISA Positive Goats | No. of ELISA Positive Sheep | Totals ELISA Positive | Average PPR Seroprevalence in Goats (%) | Average PPR Seroprevalence in Sheep (%) | Average PPR Seroprevalence in Sheep and Goats (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abu Dhabi | 76 | 731 | 395 | 336 | 348 | 306 | 654 | 88.10 | 91.07 | 89.47 |
| Dubai | 9 | 90 | 45 | 45 | 42 | 45 | 87 | 93.33 | 100.00 | 96.67 |
| Sharjah | 29 | 288 | 140 | 148 | 134 | 149 | 283 | 95.71 | 100.68 | 98.26 |
| Ajman | 4 | 40 | 20 | 20 | 19 | 19 | 38 | 95.00 | 95.00 | 95.00 |
| Umm Al-Quwain | 7 | 70 | 35 | 35 | 35 | 35 | 70 | 100.00 | 100.00 | 100.00 |
| Ras Al-Khaimah | 21 | 200 | 105 | 95 | 102 | 90 | 192 | 97.14 | 94.74 | 96.00 |
| Fujairah | 17 | 170 | 85 | 85 | 84 | 82 | 166 | 98.82 | 96.47 | 97.65 |
| Total | 163 | 1589 | 825 | 764 | 764 | 726 | 1490 | 92.61 | 95.03 | 93.77 |
| Emirates | PPR Seroprevalence Pre-Vaccination (%) | PPR Seroprevalence Post-Vaccination (%) | Annual Increase (%) | Predicted Years to 70% | Predicted Years to 80% | Predicted Years to 90% | Predicted Years to 100% |
|---|---|---|---|---|---|---|---|
| Abu Dhabi | 41.40 | 89.47 | 48.06 | 0.6 | 0.8 | 1.0 | 1.2 |
| Dubai | 46.67 | 96.67 | 50.00 | 0.5 | 0.7 | 0.9 | 1.1 |
| Sharjah | 55.17 | 98.26 | 43.09 | 0.3 | 0.6 | 0.8 | 1.0 |
| Ajman | 70.00 | 95.00 | 25.00 | 0.0 | 0.4 | 0.8 | 1.2 |
| Umm Al-Quwain | 50.00 | 100.00 | 50.00 | 0.4 | 0.6 | 0.8 | 1.0 |
| Ras Al-Khaimah | 77.07 | 96.00 | 18.93 | 0.0 | 0.2 | 0.7 | 1.2 |
| Fujairah | 64.12 | 97.65 | 33.53 | 0.2 | 0.5 | 0.8 | 1.1 |
| All-UAE | 52.32 | 93.77 | 41.45 | 0.4 | 0.7 | 0.9 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltahir, Y.M.; Al Nuaimat, M.M.; Bensalah, O.K.; Osman, E.; Al-Ramamneh, D.S.; Khan, R.A.; Alsuwaidi, N.A.; Mohamed, M.S.; Kayaf, K.; Ismaeil, S.; et al. Post-Vaccination Assessment of Peste Des Petits Ruminants in Sheep and Goats in the United Arab Emirates. Vet. Sci. 2025, 12, 991. https://doi.org/10.3390/vetsci12100991
Eltahir YM, Al Nuaimat MM, Bensalah OK, Osman E, Al-Ramamneh DS, Khan RA, Alsuwaidi NA, Mohamed MS, Kayaf K, Ismaeil S, et al. Post-Vaccination Assessment of Peste Des Petits Ruminants in Sheep and Goats in the United Arab Emirates. Veterinary Sciences. 2025; 12(10):991. https://doi.org/10.3390/vetsci12100991
Chicago/Turabian StyleEltahir, Yassir M., Mervat Mari. Al Nuaimat, Oum Keltoum Bensalah, Ebrahim Osman, Diya S. Al-Ramamneh, Rashid A. Khan, Naema A. Alsuwaidi, Meera Saeed. Mohamed, Kaltham Kayaf, Sameera Ismaeil, and et al. 2025. "Post-Vaccination Assessment of Peste Des Petits Ruminants in Sheep and Goats in the United Arab Emirates" Veterinary Sciences 12, no. 10: 991. https://doi.org/10.3390/vetsci12100991
APA StyleEltahir, Y. M., Al Nuaimat, M. M., Bensalah, O. K., Osman, E., Al-Ramamneh, D. S., Khan, R. A., Alsuwaidi, N. A., Mohamed, M. S., Kayaf, K., Ismaeil, S., Yaaqeib, F., Abdelfatah, M., Tharwat, A., Antar, M., Kheir, M. A. E., Abdelazim, A. S., Koliyan, R., & Abdelhalim, M. M. (2025). Post-Vaccination Assessment of Peste Des Petits Ruminants in Sheep and Goats in the United Arab Emirates. Veterinary Sciences, 12(10), 991. https://doi.org/10.3390/vetsci12100991









