Diversity of Escherichia coli from Faecal Samples of Danish Calves with Diarrhoea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of E. coli
2.2. Multiple-Locus Variable-Number Tandem Repeat Analysis (MLVA)
2.3. Selection of Strains for Whole Genome Sequencing (WGS)
2.4. DNA Purification and WGS
2.5. De Novo Assembly and Typing
2.6. Pathotype Prediction
2.7. Statistical Analysis
3. Results
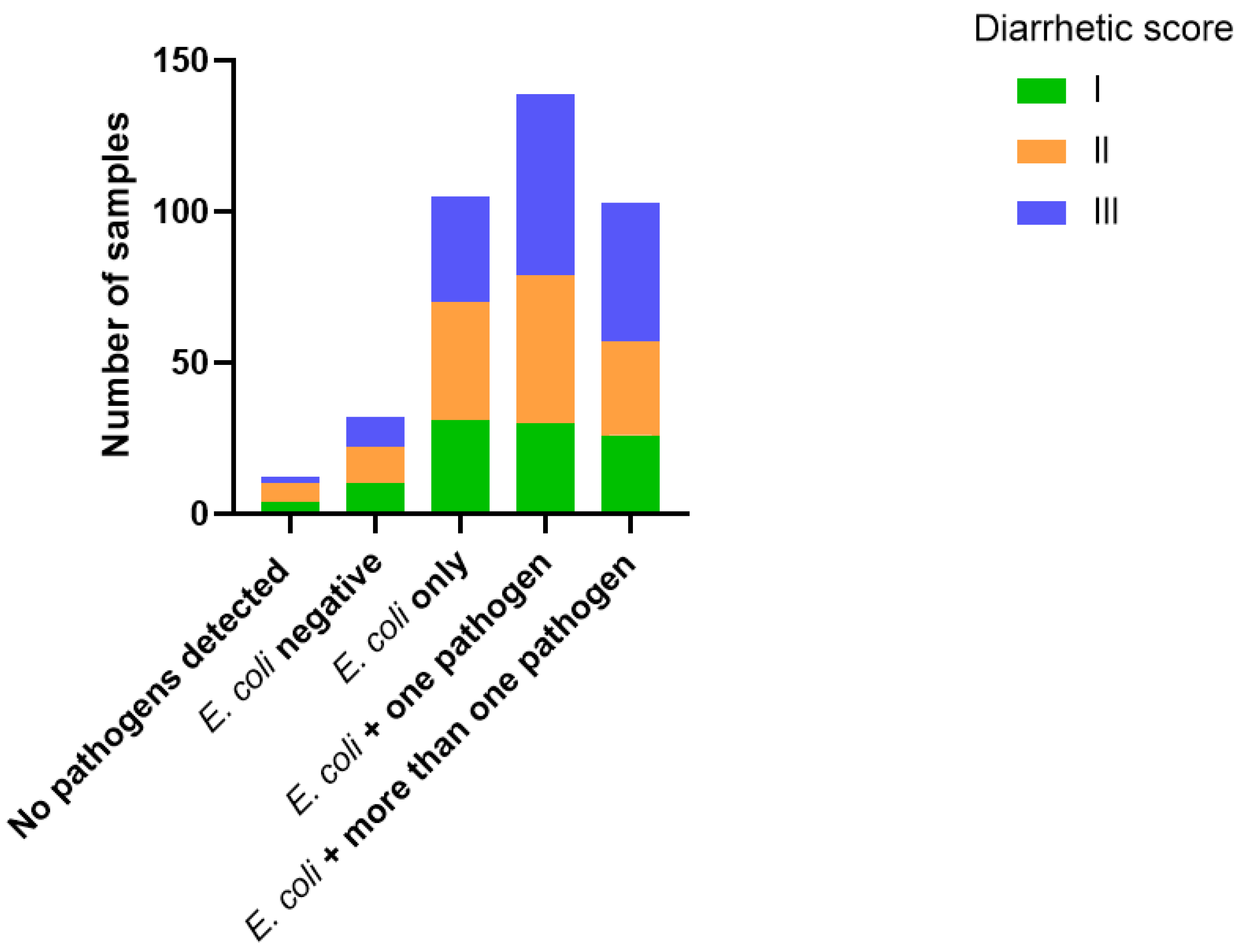
3.1. E. coli-Positive Faecal Samples and Diarrhetic Score
3.2. E. coli MLVA Typing and Diversity
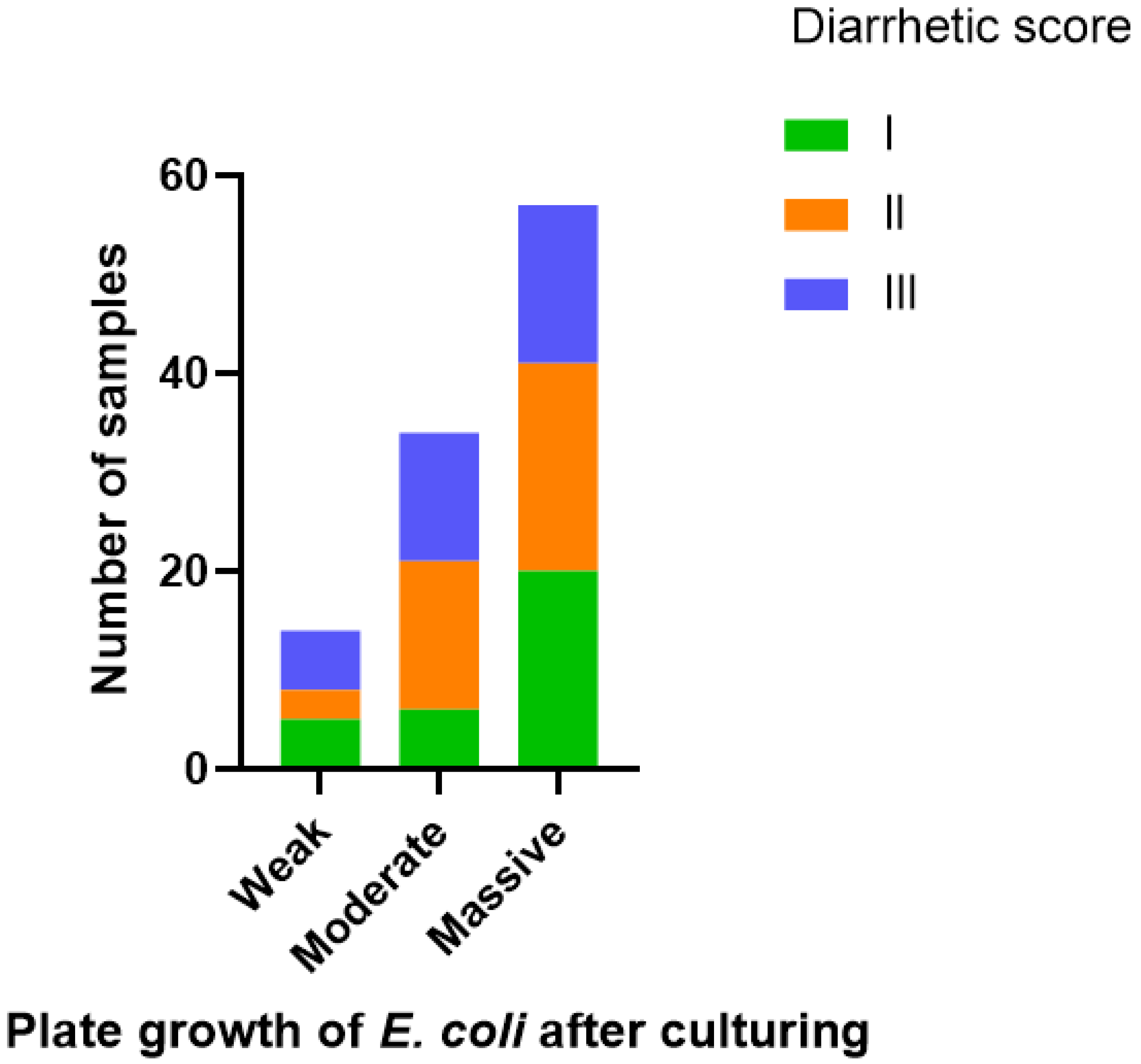
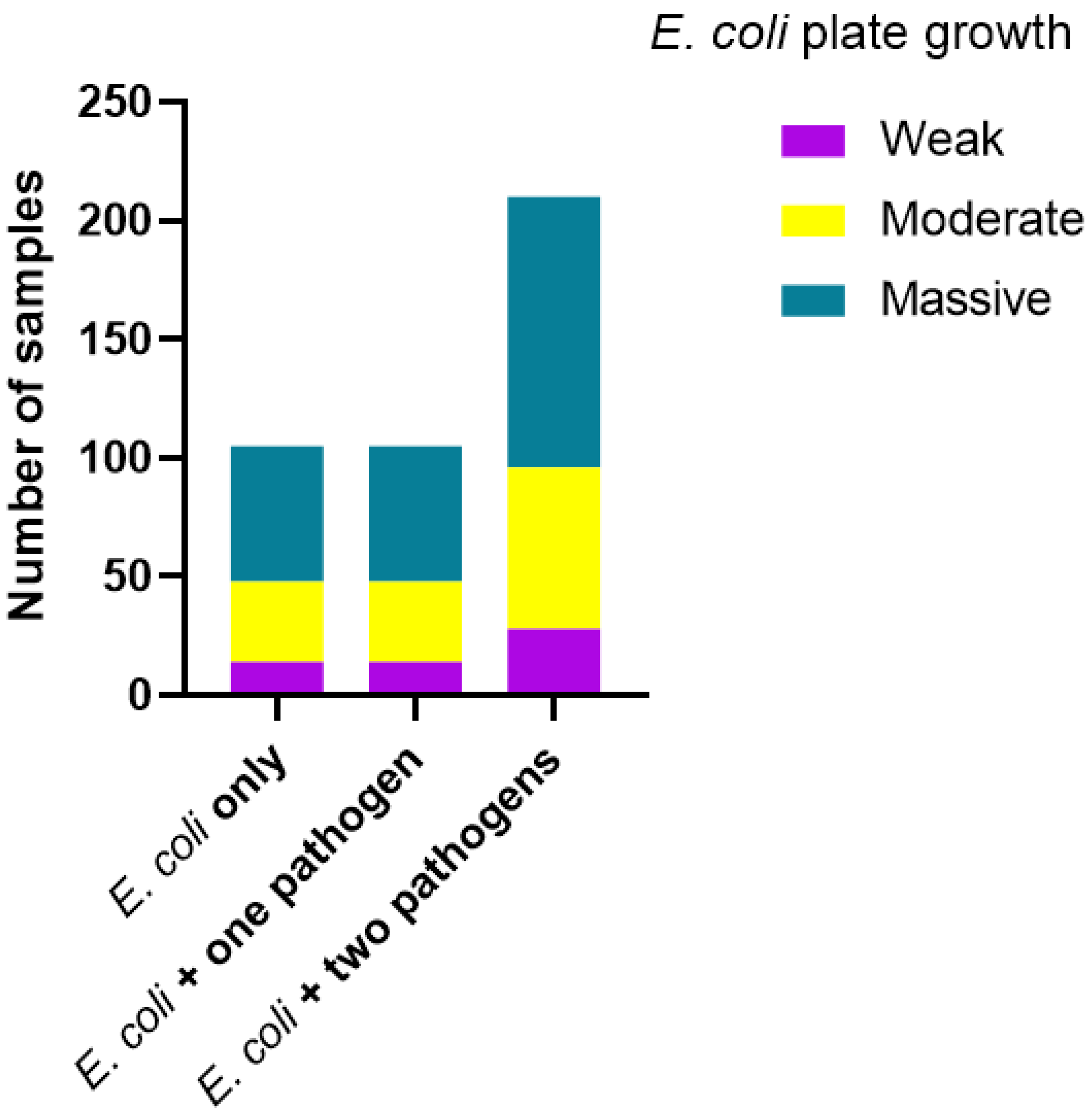
3.3. MLVA Types, Diarrhetic Scores and Quantity of E. coli
3.4. E. coli Genotypes, Sequencetypes, and Serotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ETEC | Enterotoxigenic Escherichia coli |
| EHEC | Enterohemorrhagic Escherichia coli |
| EPEC | Enteropathogenic Escherichia coli |
| ExPEC | Extraintestinal Escherichia coli |
| DAEC | Diffusely adhering Escherichia coli |
| APEC | Avian pathogenic Escherichia coli |
References
- Gross, J.J. Production Diseases in Farm Animals: Pathophysiology, Prophylaxis and Health Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Cho, Y.I.; Han, J.I.; Wang, C.; Cooper, V.; Schwartz, K.; Engelken, T.; Yoon, K.J. Case-Control Study of Microbiological Etiology Associated with Calf Diarrhoea. Vet. Microbiol. 2013, 166, 375–385. [Google Scholar] [CrossRef]
- Cho, Y.I.; Yoon, K.J. An Overview of Calf Diarrhoea—Infectious Etiology, Diagnosis, and Intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef]
- Umpierrez, A.; Acquistapace, S.; Fernandez, S.; Oliver, M.; Acuna, P.; Reolon, E.; Zunino, P. Prevalence of Escherichia coli Adhesion-Related Genes in Neonatal Calf Diarrhoea in Uruguay. J. Infect. Dev. Ctries. 2016, 10, 472–477. [Google Scholar] [CrossRef]
- Coskun, M.R.; Sahin, M. Prevalence of Neonatal Calf Diarrhoea Caused by Escherichia coli and Investigation of Virulence Factors, Serotypes, and Antibiotic Susceptibility. Pol. J. Vet. Sci. 2023, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Al-Alo, K.Z.K.; Nikbakht Brujeni, G.; Lotfollahzadeh, S.; Moosakhani, F.; Gharabaghi, A. Correlation between Neonatal Calf Diarrhoea and the Level of Maternally Derived Antibodies. Iran. J. Vet. Res. 2018, 19, 3–8. [Google Scholar] [PubMed]
- Mohammed, S.A.E.; Marouf, S.A.E.; Erfana, A.M.; El-Jakee, J.; Hessain, A.M.; Dawoud, T.M.; Kabli, S.A.; Moussa, I.M. Risk Factors Associated with E. coli Causing Neonatal Calf Diarrhoea. Saudi J. Biol. Sci. 2019, 26, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Ngeleka, M.; Godson, D.; Vanier, G.; Desmarais, G.; Wojnarowicz, C.; Sayi, S.; Huang, Y.; Movasseghi, R.; Fairbrother, J.M. Frequency of Escherichia coli Virotypes in Calf Diarrhoea and Intestinal Morphologic Changes Associated with These Virotypes or Other Diarrhoeagenic Pathogens. J. Vet. Diagn. Investig. 2019, 31, 611–615. [Google Scholar] [CrossRef]
- Khawaskar, D.P.; Sinha, D.K.; Lalrinzuala, M.V.; Athira, V.; Kumar, M.; Chhakchhuak, L.; Mohanapriya, K.; Sophia, I.; Abhishek; Kumar, O.R.V.; et al. Pathotyping and Antimicrobial Susceptibility Testing of Escherichia coli Isolates from Neonatal Calves. Vet. Res. Commun. 2022, 46, 353–362. [Google Scholar] [CrossRef]
- Chekole, W.S.; Adamu, H.; Sternberg-Lewrein, S.; Magnusson, U.; Tessema, T.S. Occurrence of Escherichia coli Pathotypes in Diarrheic Calves in a Low-Income Setting. Pathogens 2022, 12, 42. [Google Scholar] [CrossRef]
- Fresno, A.H.; Alencar, A.L.F.; Liu, G.; Wridt, M.W.; Andersen, F.B.; Pedersen, H.S.; Martin, H.L.; Nielsen, S.S.; Aabo, S.; Olsen, J.E.; et al. Effect of Feeding Dairy Calves with Milk Fermented with Selected Probiotic Strains on Occurrence of Diarrhoea, Carriage of Pathogenic and Zoonotic Microorganisms and Growth Performance. Vet. Microbiol. 2023, 286, 109885. [Google Scholar] [CrossRef]
- Pansri, P.; Svensmark, B.; Liu, G.; Thamsborg, S.M.; Kudirkiene, E.; Nielsen, H.V.; Goecke, N.B.; Olsen, J.E. Evaluation of a Novel Multiplex Qpcr Method for Rapid Detection and Quantification of Pathogens Associated with Calf Diarrhoea. J. Appl. Microbiol. 2022, 133, 2516–2527. [Google Scholar] [CrossRef]
- Olsen, J.E.; Svensmark, B.; Agerskov, L.; Albrechtsen, M.; Olsen, R.H. Prevalence and Infection Characteristics of Common Pathogens Associated with Calf Diarrhoea in Danish Dairy Calves. Vet. Microbiol. 2025, 307, 110575. [Google Scholar] [CrossRef]
- Chen, J.; Griffiths, M.W. Pcr Differentiation of Escherichia coli from Other Gram-Negative Bacteria Using Primers Derived from the Nucleotide Sequences Flanking the Gene Encoding the Universal Stress Protein. Lett. Appl. Microbiol. 1998, 27, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Camelena, F.; Birgy, A.; Smail, Y.; Courroux, C.; Mariani-Kurkdjian, P.; Le Hello, S.; Bonacorsi, S.; Bidet, P. Rapid and Simple Universal Escherichia coli Genotyping Method Based on Multiple-Locus Variable-Number Tandem-Repeat Analysis Using Single-Tube Multiplex Pcr and Standard Gel Electrophoresis. Appl. Environ. Microbiol. 2019, 85, e02812-18. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using Spades De Novo Assembler. Curr. Protoc. Bioinformatics 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile Genome Assembly Evaluation with Quast-Lg. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy in Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Ponten, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Geurtsen, J.; de Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and Pathotypes of the Many Faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef]
- Pakbin, B.; Bruck, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Tarrien, R.J.; Mobley, H.L. Enzymatically Active and Inactive Phosphodiesterases and Diguanylate Cyclases Are Involved in Regulation of Motility or Sessility in Escherichia coli Cft073. mBio 2012, 3, e00307-12. [Google Scholar] [CrossRef] [PubMed]
- Krogh, H.V. Infection with Enterotoxigenic Escherichia coli in Calves and Protection of the Calves by Vaccination of the Dams. Ann. Rech. Vet. 1983, 14, 522–525. [Google Scholar] [PubMed]
- Krogh, H.V. Occurrence of Enterotoxigenic Escherichia coli in Calves with Acute Neonatal Diarrhoea. Nord. Vet. Med. 1983, 35, 346–352. [Google Scholar] [PubMed]
- Gulliksen, S.M.; Jor, E.; Lie, K.I.; Hamnes, I.S.; Loken, T.; Akerstedt, J.; Osteras, O. Enteropathogens and Risk Factors for Diarrhoea in Norwegian Dairy Calves. J. Dairy. Sci. 2009, 92, 5057–5066. [Google Scholar] [CrossRef]
- Umpierrez, A.; Ernst, D.; Fernandez, M.; Oliver, M.; Casaux, M.L.; Caffarena, R.D.; Schild, C.; Giannitti, F.; Fraga, M.; Zunino, P. Virulence Genes of Escherichia coli in Diarrheic and Healthy Calves. Rev. Argent. Microbiol. 2021, 53, 34–38. [Google Scholar] [CrossRef]
- Bendali, F.; Bichet, H.; Schelcher, F.; Sanaa, M. Pattern of diarrhoea in newborn beef calves in south-west France. Vet. Res. 1999, 30, 61–74. [Google Scholar]
- Shahrani, M.; Dehkordi, F.S.; Momtaz, H. Characterization of Escherichia coli virulence genes, pathotypes and antibiotic resistance properties in diar-rheic calves in Iran. Biol. Res. 2014, 47, 28. [Google Scholar] [CrossRef]
- Petersen, A.M. Gastrointestinal Dysbiosis and Escherichia coli Pathobionts in Inflammatory Bowel Diseases. APMIS 2022, 130 (Suppl. S144), 1–38. [Google Scholar] [CrossRef]
- Morgan, B.L.; Depenbrock, S.; Martinez-Lopez, B. Identifying Associations in Minimum Inhibitory Concentration Values of Escherichia coli Samples Obtained From Weaned Dairy Heifers in California Using Bayesian Network Analysis. Front. Vet. Sci. 2022, 9, 771841. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Sischo, W.M.; Jones, L.P.; Moore, D.A.; Ahmed, S.; Short, D.M.; Besser, T.E. Recent Emergence of Escherichia coli with Cephalosporin Resistance Conferred by blaCTX-M on Washington State Dairy Farms. Appl. Environ. Microbiol. 2015, 81, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Hornitzky, M.A.; Mercieca, K.; Bettelheim, K.A.; Djordjevic, S.P. Bovine Feces from Animals with Gastrointestinal Infections Are a Source of Serologically Diverse Atypical Enteropathogenic Escherichia coli and Shiga Toxin-Producing E. coli Strains That Commonly Possess Intimin. Appl. Environ. Microbiol. 2005, 71, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Habets, A.; Crombe, F.; Nakamura, K.; Guerin, V.; De Rauw, K.; Pierard, D.; Saulmont, M.; Hayashi, T.; Mainil, J.G.; Thiry, D. Genetic Characterization of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 from Diarrhoeic and Septicaemic Calves and Relatedness to Human Shigatoxigenic E. coli O80:H2. J. Appl. Microbiol. 2021, 130, 258–264. [Google Scholar] [CrossRef]
- He, W.Y.; Zhang, X.X.; Gao, G.L.; Gao, M.Y.; Zhong, F.G.; Lv, L.C.; Cai, Z.P.; Si, X.F.; Yang, J.; Liu, J.H. Clonal Spread of Escherichia coli O101: H9-St10 and O101: H9-St167 Strains Carrying Fosa3 and Bla (Ctx-M-14) among Diarrhoeal Calves in a Chinese Farm, with Australian Chroicocephalus as the Possible Origin of E. coli O101: H9-St10. Zool. Res. 2021, 42, 461–468. [Google Scholar] [CrossRef]
- Yadegari, Z.; Nikbakht Brujeni, G.; Ghorbanpour, R.; Moosakhani, F.; Lotfollahzadeh, S. Molecular Characterization of Enterotoxigenic Escherichia coli Isolated from Neonatal Calves Diarrhoea. Vet. Res. Forum 2019, 10, 73–78. [Google Scholar] [CrossRef]
- Mwenifumbo, M.; Cookson, A.L.; Zhao, S.; Fayaz, A.; Browne, A.S.; Benschop, J.; Burgess, S.A. The Characterisation of Antimicrobial Resistant Escherichia coli from Dairy Calves. J. Med. Microbiol. 2023, 72, 001742. [Google Scholar] [CrossRef]
- Haley, B.J.; Kim, S.W.; Salaheen, S.; Hovingh, E.; Van Kessel, J.A.S. Virulome and Genome Analyses Identify Associations between Antimicrobial Resistance Genes and Virulence Factors in Highly Drug-Resistant Escherichia coli Isolated from Veal Calves. PLoS ONE 2022, 17, e0265445. [Google Scholar] [CrossRef]
- Soria-Bustos, J.; Saitz, W.; Medrano, A.; Lara-Ochoa, C.; Bennis, Z.; Monteiro-Neto, V.; Dos Santos, C.I.; Rodrigues, J.; Hernandes, R.T.; Yanez, J.A.; et al. Role of the Yehd Fimbriae in the Virulence-Associated Properties of Enteroaggregative Escherichia coli. Environ. Microbiol. 2022, 24, 1035–1051. [Google Scholar] [CrossRef]
- Gonyar, L.A.; Sauder, A.B.; Mortensen, L.; Willsey, G.G.; Kendall, M.M. The Yad and Yeh Fimbrial Loci Influence Gene Expression and Virulence in Enterohemorrhagic Escherichia coli O157:H7. mSphere 2024, 9, e0012424. [Google Scholar] [CrossRef]
- Le Bouguenec, C.; Servin, A.L. Diffusely Adherent Escherichia coli Strains Expressing Afa/Dr Adhesins (Afa/Dr Daec): Hitherto Unrecognized Pathogens. FEMS Microbiol. Lett. 2006, 256, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Zaraket, H.; Suzuki, H. Molecular Studies on Diarrhoea-Associated Escherichia coli Isolated from Humans and Animals in Egypt. Vet. Microbiol. 2013, 167, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Beinke, C.; Laarmann, S.; Wachter, C.; Karch, H.; Greune, L.; Schmidt, M.A. Diffusely Adhering Escherichia coli Strains Induce Attaching and Effacing Phenotypes and Secrete Homologs of Esp Proteins. Infect. Immun. 1998, 66, 528–539. [Google Scholar] [CrossRef]
- Zhu, D.-D.; Li, X.-R.; Ma, T.-F.; Chen, J.-Q.; Ge, C.-H.; Yang, S.-H.; Zhang, W.; Chen, J.; Zhang, J.-J.; Qi, M.-M.; et al. Multidrug-Resistant Extraintestinal Pathogenic Escherichia coli exhibits high virulence in calf herds: A case report. Microbiol. Res. 2025, 16, 59. [Google Scholar] [CrossRef]
- Yang, H.; Mirsepasi-Lauridsen, H.C.; Struve, C.; Allaire, J.M.; Sivignon, A.; Vogl, W.; Bosman, E.S.; Ma, C.; Fotovati, A.; Reid, G.S.; et al. Ulcerative Colitis-Associated E. coli Pathobionts Potentiate Colitis in Susceptible Hosts. Gut Microbes 2020, 12, 1847976. [Google Scholar] [CrossRef]
- Mirsepasi-Lauridsen, H.C.; Halkjaer, S.I.; Mortensen, E.M.; Lydolph, M.C.; Nordgaard-Lassen, I.; Krogfelt, K.A.; Petersen, A.M. Extraintestinal Pathogenic Escherichia coli Are Associated with Intestinal Inflammation in Patients with Ulcerative Colitis. Sci. Rep. 2016, 6, 31152. [Google Scholar] [CrossRef]
- Petersen, A.M.; Nielsen, E.M.; Litrup, E.; Brynskov, J.; Mirsepasi, H.; Krogfelt, K.A. A Phylogenetic Group of Escherichia coli Associated with Active Left-Sided Inflammatory Bowel Disease. BMC Microbiol. 2009, 9, 171. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-Derived Nitrate Boosts Growth of E. coli in the Inflamed Gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Moazeni, S.; Askari Badouei, M.; Hashemitabar, G.; Rezatofighi, S.E.; Mahmoodi, F. Detection and Characterization of Potentially Hybrid Enteroaggregative Escherichia coli (Eaec) Strains Isolated from Urinary Tract Infection. Braz. J. Microbiol. 2024, 55, 1–9. [Google Scholar] [CrossRef]
- Dutta, S.; Pazhani, G.P.; Nataro, J.P.; Ramamurthy, T. Heterogenic Virulence in a Diarrhoeagenic Escherichia coli: Evidence for an Epec Expressing Heat-Labile Toxin of Etec. Int. J. Med. Microbiol. 2015, 305, 47–54. [Google Scholar] [CrossRef]
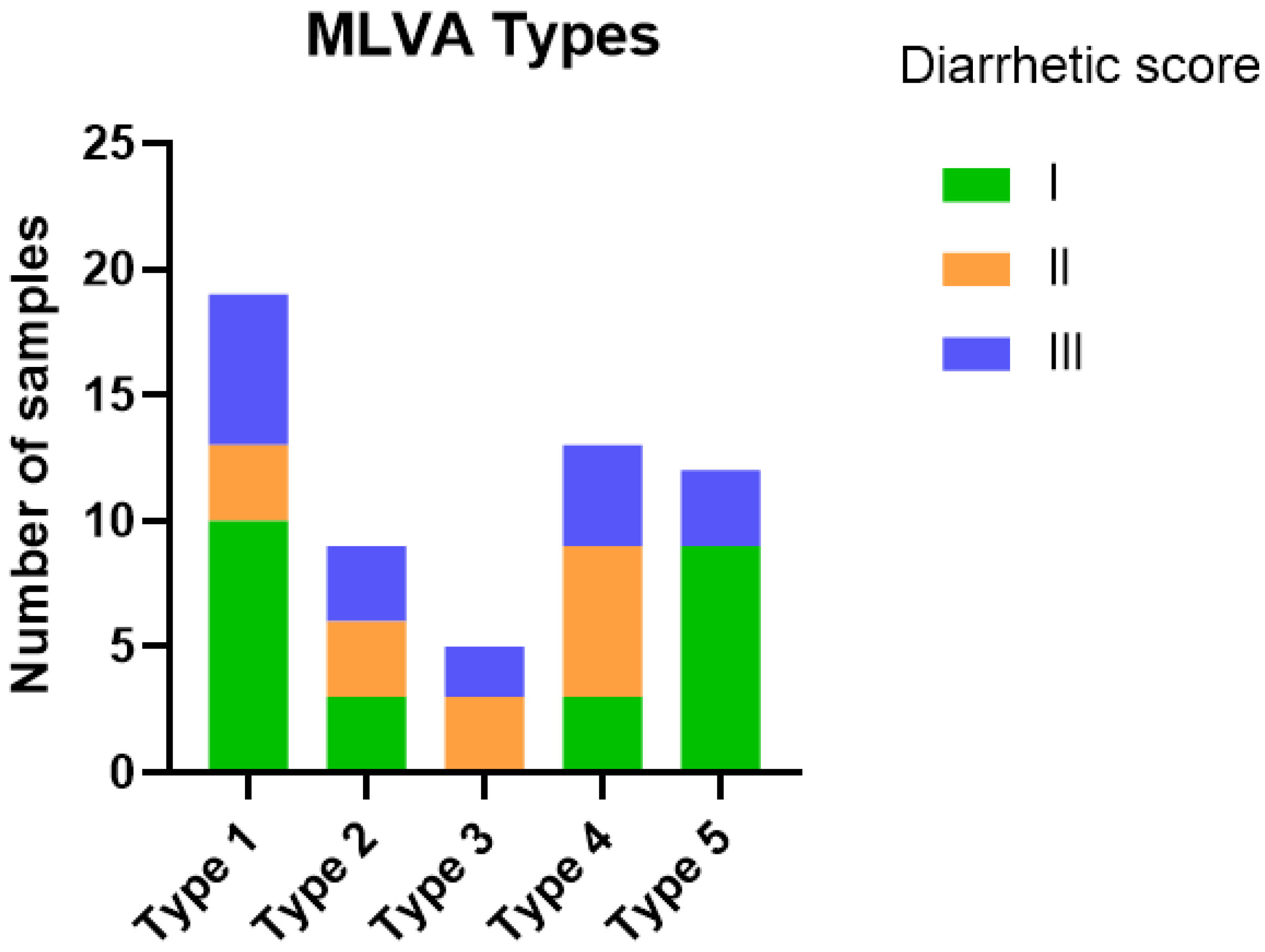
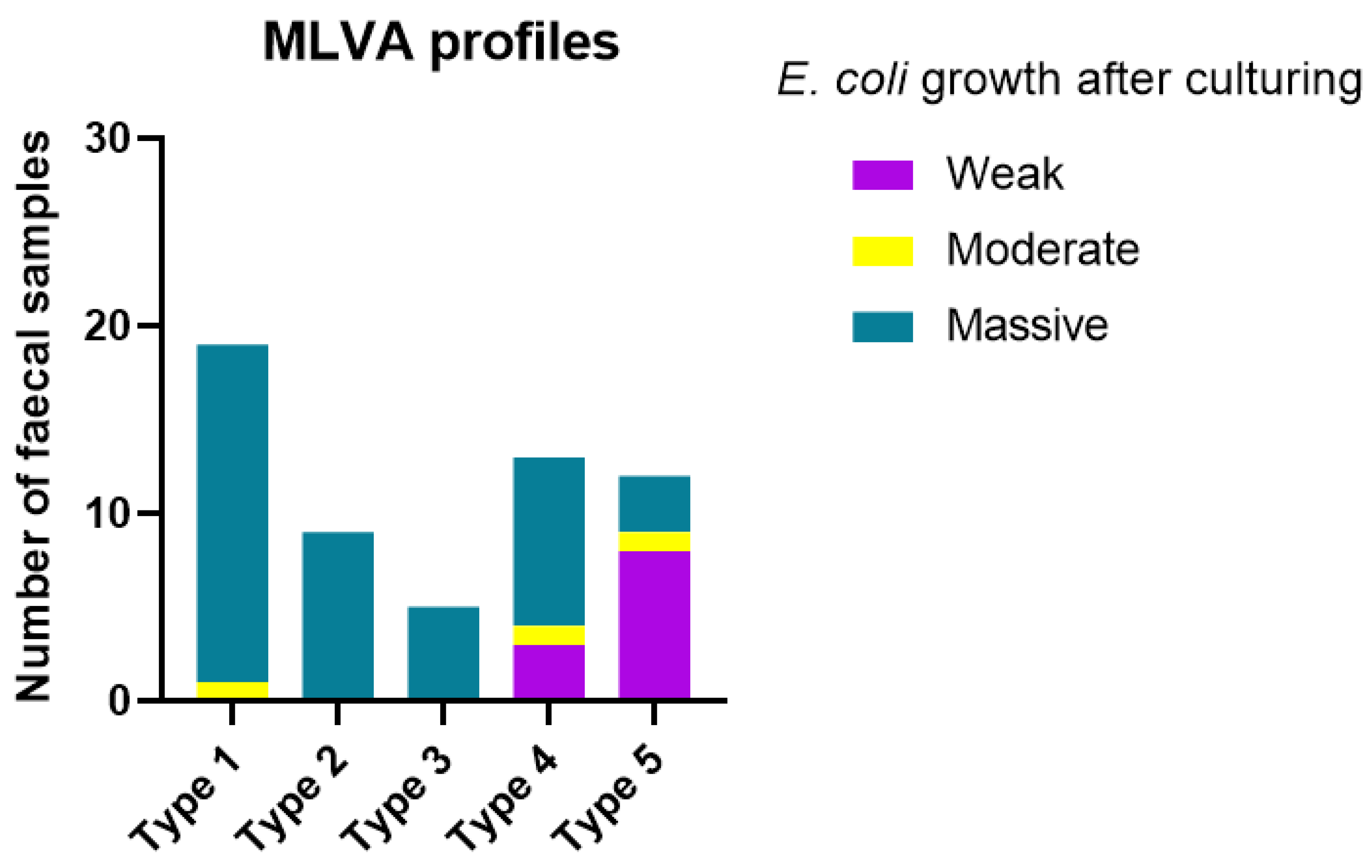
| MLVA Type | N (% of All Samples) | No Other Pathogens Detected (n = 20) | With Other Pathogen (s) (n = 38) | Clinical Isolates (33) |
|---|---|---|---|---|
| 1 | 28 (31%) | 4 | 15 | 9 |
| 2 | 13 (14%) | 4 | 5 | 4 |
| 3 | 13 (14%) | 2 | 3 | 8 |
| 4 | 25 (27%) | 9 | 4 | 12 |
| 5 | 12 (13%) | 1 | 11 | 0 |
| With no Other Pathogens Detected (n = 30) | With Other Pathogen(s) Detected (n = 69) | Unknown Presence of Other Pathogen(s) (Clinical Cases) (n = 62) | |
|---|---|---|---|
| ETEC | 0 | 2 | 6 |
| F5 positive, tox | 0 | 2 | 0 |
| EHEC (+DAEC/ExPEC) | 0 | 2 | 8 |
| EPEC | 0 | 1 | 0 |
| DAEC | 2 | 3 | 3 |
| DAEC/EHEC | 1 | 1 | 2 |
| DAEC/ExPEC | 6 | 21 | 14 |
| ExPEC | 4 | 5 | 7 |
| NG | 17 | 32 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alencar, A.L.F.; Jibril, A.H.; Svensmark, B.; Agerskov, L.; Martin, H.L.; Stegger, M.; Saidenberg, A.B.; Liu, G.; Hounmanou, Y.M.G.; Juel, A.S.; et al. Diversity of Escherichia coli from Faecal Samples of Danish Calves with Diarrhoea. Vet. Sci. 2025, 12, 987. https://doi.org/10.3390/vetsci12100987
Alencar ALF, Jibril AH, Svensmark B, Agerskov L, Martin HL, Stegger M, Saidenberg AB, Liu G, Hounmanou YMG, Juel AS, et al. Diversity of Escherichia coli from Faecal Samples of Danish Calves with Diarrhoea. Veterinary Sciences. 2025; 12(10):987. https://doi.org/10.3390/vetsci12100987
Chicago/Turabian StyleAlencar, Anna Luiza Farias, Abdurrahman Hassan Jibril, Birgitta Svensmark, Lene Agerskov, Henrik Læssøe Martin, Marc Stegger, André Becker Saidenberg, Gang Liu, Yaovi Mahuton Gildas Hounmanou, Annette Sønderholm Juel, and et al. 2025. "Diversity of Escherichia coli from Faecal Samples of Danish Calves with Diarrhoea" Veterinary Sciences 12, no. 10: 987. https://doi.org/10.3390/vetsci12100987
APA StyleAlencar, A. L. F., Jibril, A. H., Svensmark, B., Agerskov, L., Martin, H. L., Stegger, M., Saidenberg, A. B., Liu, G., Hounmanou, Y. M. G., Juel, A. S., Olsen, J. E., & Olsen, R. H. (2025). Diversity of Escherichia coli from Faecal Samples of Danish Calves with Diarrhoea. Veterinary Sciences, 12(10), 987. https://doi.org/10.3390/vetsci12100987





