Erector Spinae Plane Block for Perioperative Analgesia in a Rabbit
Abstract
Simple Summary
Abstract
1. Introduction
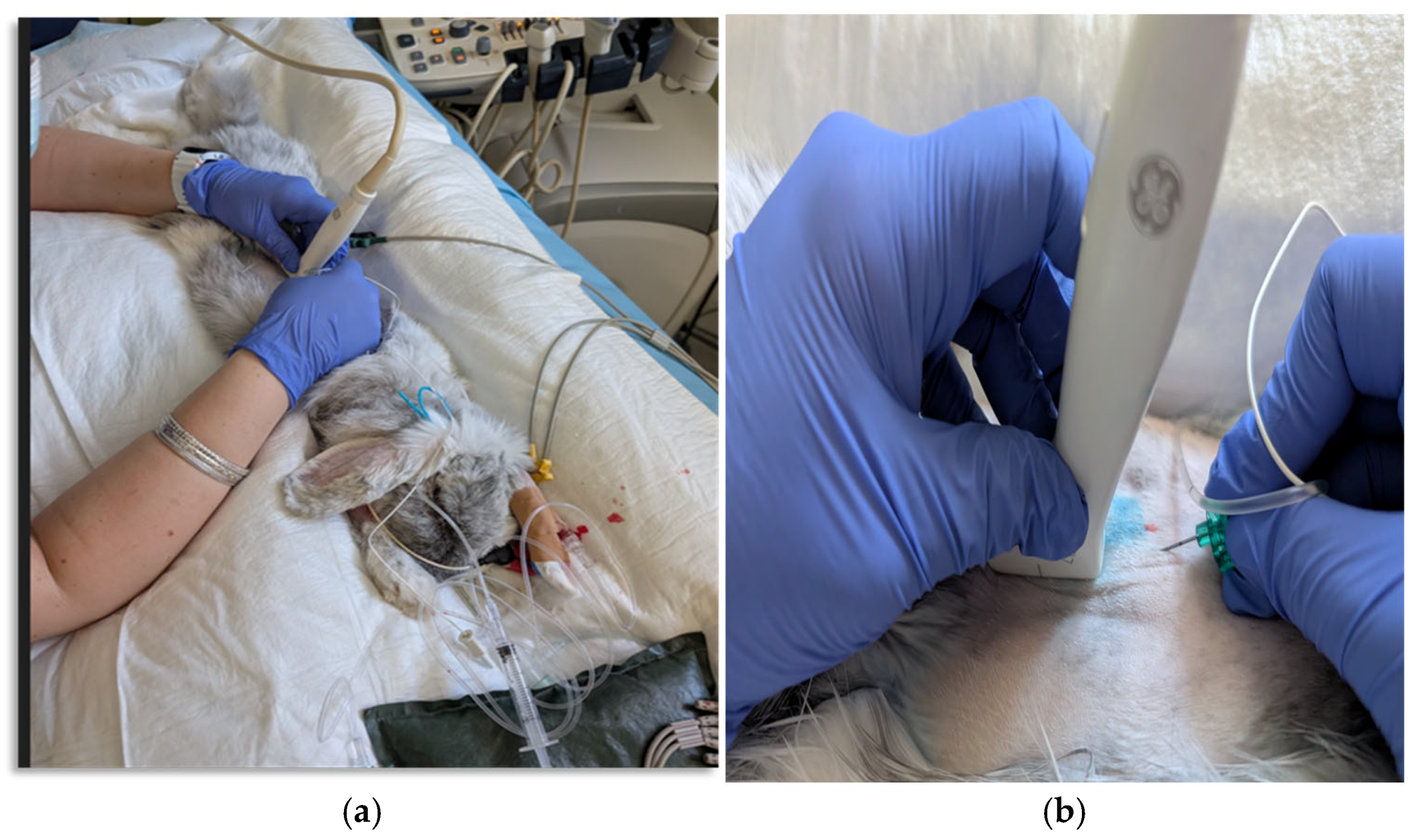
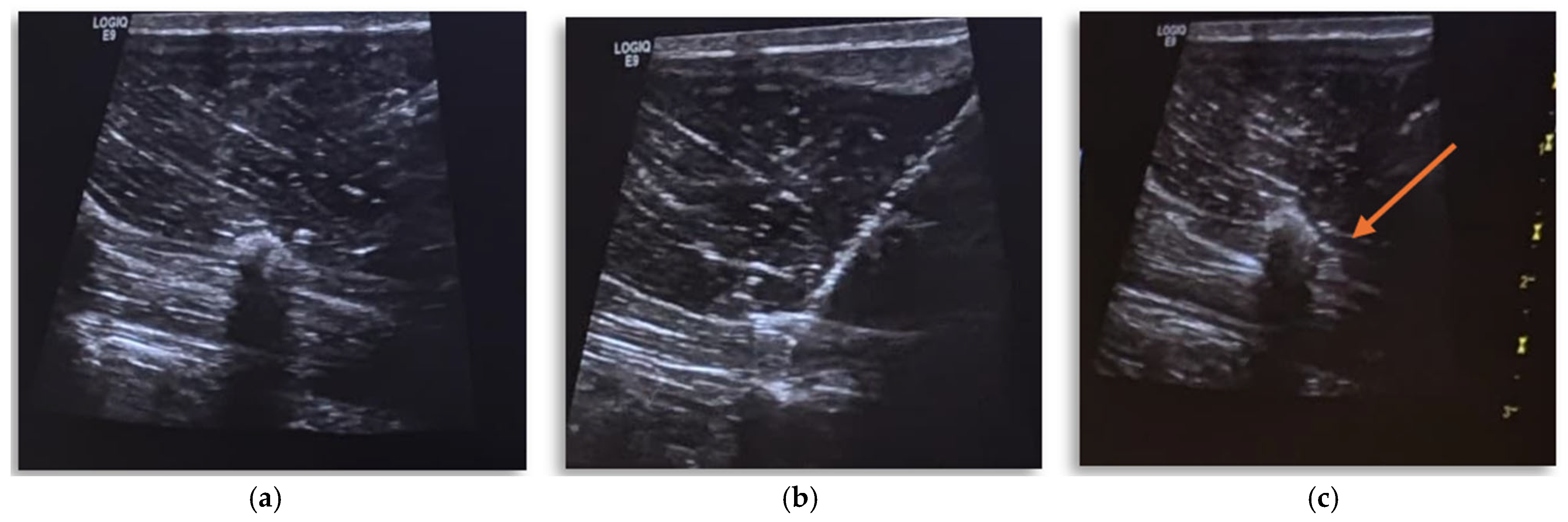
2. Case Presentation
Anesthetic Management
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lascelles, B.D.X.; Kirkby Shaw, K. An extended release local anaesthetic: Potential for future use in veterinary surgical patients? Vet. Med. Sci. 2016, 2, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fozzard, H.; Lee, P.; Lipkind, G. Mechanism of Local Anesthetic Drug Action on Voltage-Gated Sodium Channels. Curr. Pharm. Des. 2005, 11, 2671–2686. [Google Scholar] [CrossRef]
- Degani, M.; Briganti, A.; Dupont, J.; Tutunaru, A.; Picavet, P.P.; Bolen, G.; Sandersen, C. Perioperative analgesic efficacy of lumbar erector spinae plane block in dogs undergoing hemilaminectomy: A randomized blinded clinical trial. Vet. Anaesth. Analg. 2024, 51, 181–189. [Google Scholar] [CrossRef]
- Degani, M.; Paolini, A.; Bianchi, A.; Tamburro, R.; Di Matteo, L.; Sandersen, C.; Briganti, A. Comparative study between lateral versus latero-ventral quadratus lumborum block for perioperative analgesia in canine laparoscopic ovariectomy. Vet. Anaesth. Analg. 2024, 51, 738–745. [Google Scholar] [CrossRef]
- Ferré, B.M.I.; Drozdzynska, M.; Vettorato, E. Ultrasound-guided bilateral erector spinae plane block in dogs undergoing sternotomies anaesthetised with propofol-dexmedetomidine continuous infusion. Vet. Res. Commun. 2022, 46, 1331–1337. [Google Scholar] [CrossRef]
- Paolini, A.; Bianchi, A.; Bucci, R.; Parrillo, S.; Di Giosia, A.; Ristori, C.; Carluccio, A.; Tamburro, R.; Vignoli, M.; Collivignarelli, F.; et al. Use of a quadratus lumborum block in queens undergoing ovariectomy: A randomised controlled trial. J. Feline Med. Surg. 2024, 26, 1098612X241275277. [Google Scholar] [CrossRef]
- Di Franco, C.; Cozzani, C.; Vannozzi, I.; Briganti, A. Low-Volume (0.3 mL/kg) Ropivacaine 0.5% for a Quadratus Lumborum Block in Cats Undergoing Ovariectomy: A Randomized Study. Vet. Sci. 2025, 12, 524. [Google Scholar] [CrossRef]
- d’Ovidio, D.; Adami, C. Locoregional Anesthesia in Exotic Pets. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 301–314. [Google Scholar] [CrossRef]
- Tutunaru, A.C.; Morata, D.A.; Pollet, V. The Successful Use of an Ultrasound-Guided Mid-Femur Sciatic Nerve Block in a Juvenile Emu (Dromaius novaehollandiae) under General Anaesthesia. Animals 2024, 14, 1178. [Google Scholar] [CrossRef] [PubMed]
- Vettorato, E.; Schmidt, K.J.; Horgan, M.D.; Chiavaccini, L.; Portela, D.A. Quadratus lumborum block as part of multimodal analgesia in a rabbit undergoing liver lobectomy. Vet. Anaesth. Analg. 2023, 50, S1467298723001447. [Google Scholar] [CrossRef] [PubMed]
- Trujanovic, R.; Otero, P.E.; Larenza-Menzies, M.P. Ultrasound- and nerve stimulation-guided femoral and sciatic nerve block in a duck (Anas platyrhynchos) undergoing surgical fixation of a tibiotarsal fracture. Vet. Anaesth. Analg. 2021, 48, 277–278. [Google Scholar] [CrossRef]
- Herron, A.; Bianchi, C.; Viscasillas, J.; Sanchis, S.; Foster, A.; Medina, R. The Erector Spinae Plane block for intraoperative analgesia in dogs undergoing hemilaminectomy: A retrospective study. In BSAVA Congress Proceedings 2019; British Small Animal Veterinary Association: Gloucestershire, UK; p. 504.
- Portela, D.A.; Castro, D.; Romano, M.; Gallastegui, A.; Garcia-Pereira, F.; Otero, P.E. Ultrasound-guided erector spinae plane block in canine cadavers: Relevant anatomy and injectate distribution. Vet. Anaesth. Analg. 2020, 47, 229–237. [Google Scholar] [CrossRef]
- Delgado, O.B.D.; Louro, L.F.; Rocchigiani, G.; Verin, R.; Humphreys, W.; Senior, M.; Campagna, I. Ultrasound-guided erector spinae plane block in horses: A cadaver study. Vet. Anaesth. Analg. 2021, 48, 577–584. [Google Scholar] [CrossRef]
- Medina-Serra, R.; Foster, A.; Plested, M.; Sanchis, S.; Gil-Cano, F.; Viscasillas, J. Lumbar erector spinae plane block: An anatomical and dye distribution evaluation of two ultrasound-guided approaches in canine cadavers. Vet. Anaesth. Analg. 2021, 48, 125–133. [Google Scholar] [CrossRef]
- Serpieri, M.; Bonaffini, G.; Ottino, C.; Quaranta, G.; Mauthe von Degerfeld, M. Effects of Intratesticular Lidocaine in Pet Rabbits Undergoing Orchiectomy. Animals 2024, 14, 551. [Google Scholar] [CrossRef]
- Serpieri, M.; Ottino, C.; Bonaffini, G.; Banchi, P.; Quaranta, G.; Mauthe von Degerfeld, M. Comparison between Carprofen and Meloxicam for Post-Neutering Pain Management in Pet Rabbits. Vet. Sci. 2024, 11, 257. [Google Scholar] [CrossRef]
- Ozawa, S.; Cenani, A.; Sanchez-Migallon Guzman Lv, D. Treatment of Pain in Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2023, 26, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Benito, J.; Finck, C.; Moreira, J.; Garbin, M. Ultrasound-guided transversus abdominis plane block in rabbits: An anatomical and computed tomography study. Vet. Anaesth. Analg. 2025, 52, 124.e8. [Google Scholar] [CrossRef]
- Serino, F.; Pennasilico, L.; Galosi, M.; Palumbo Piccionello, A.; Tambella, A.M.; Di Bella, C. Transversus Abdominis Plane (TAP) Block in Rabbit Cadavers: Anatomical Description and Measurements of Injectate Spread Using One- and Two-Point Approaches. Animals 2024, 14, 684. [Google Scholar] [CrossRef]
- Torres Cantó, L.; Felisberto, R.; Economou, A.; Flaherty, D.; Moreno Aguado, B.; Tayari, H. Ultrasound-Guided Dorsolateral Approach for Quadratus Lumborum Block in Rabbits (Oryctolagus cuniculus): A Prospective, Randomized, Blinded, Cadaveric Study Comparing Four Different Injectate Volumes. Animals 2023, 13, 2559. [Google Scholar] [CrossRef]
- Miller, A.L.; Clarkson, J.M.; Quigley, C.; Neville, V.; Krall, C.; Geijer-Simpson, A.; Flecknell, P.A.; Leach, M.C. Evaluating Pain and Analgesia Effectiveness Following Routine Castration in Rabbits Using Behavior and Facial Expressions. Front. Vet. Sci. 2022, 9, 782486. [Google Scholar] [CrossRef]
- Marco-Martorell, M.; Duffy, N.; Martinez, M.; Maddox, T.; Robson, K. Agreement of Pain Assessment Using the Short Form of the Canine Glasgow Composite Measure Pain Scale between Veterinary Students, Veterinary Nurses, Veterinary Surgeons, and ECVAA-Diplomates. Animals 2024, 14, 2310. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; de Lima, M.T.; Trindade, P.H.E.; Luna, S.P.L. The impact of using pain scales by untrained students on the decision to provide analgesia to multiple species. Vet. Anaesth. Analg. 2024, 51, 548–557. [Google Scholar] [CrossRef]
- Alza Salvatierra, D.N.; Herrera Linares, M.E.; Motta, L.; Martinez, M. Ultrasound-guided erector spinae interfascial plane block for spinal surgery in three cats. J. Feline Med. Surg. Open Rep. 2021, 7, 20551169211043814. [Google Scholar] [CrossRef]
- Chin, K.J.; El-Boghdadly, K. Mechanisms of action of the erector spinae plane (ESP) block: A narrative review. Can. J. Anesth/J. Can. Anesth. 2021, 68, 387–408. [Google Scholar] [CrossRef]
- Dautzenberg, K.H.; Zegers, M.J.; Bleeker, C.P.; Tan, E.C.; Vissers, K.C.; van Geffen, G.J.; van der Wal, S.E. Unpredictable Injectate Spread of the Erector Spinae Plane Block in Human Cadavers. Anesth. Analg. 2019, 129, e163–e166. [Google Scholar] [CrossRef] [PubMed]
- Harbell, M.W.; Langley, N.R.; Seamans, D.P.; Koyyalamudi, V.; Kraus, M.B.; Carey, F.J.; Craner, R. Evaluating two approaches to the erector spinae plane block: An anatomical study. Reg. Anesth. Pain Med. 2023, 48, 495–500. [Google Scholar] [CrossRef]
- Ferreira, T.H.; James, M.S.; Schroeder, C.A.; Hershberger-Braker, K.L.; Teixeira, L.B.; Schroeder, K.M. Description of an ultrasound-guided erector spinae plane block and the spread of dye in dog cadavers. Vet. Anaesth. Analg. 2019, 46, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Pentsou, K.; Huuskonen, V. Thoracolumbar retrolaminar block in seven dogs undergoing spinal surgery. Ir. Vet. J. 2022, 75, 17. [Google Scholar] [CrossRef] [PubMed]
- Machi, A.; Joshi, G.P. Interfascial plane blocks. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 303–315. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scialanca, S.; Bersanetti, G.; Parrillo, S.; Paolini, A. Erector Spinae Plane Block for Perioperative Analgesia in a Rabbit. Vet. Sci. 2025, 12, 984. https://doi.org/10.3390/vetsci12100984
Scialanca S, Bersanetti G, Parrillo S, Paolini A. Erector Spinae Plane Block for Perioperative Analgesia in a Rabbit. Veterinary Sciences. 2025; 12(10):984. https://doi.org/10.3390/vetsci12100984
Chicago/Turabian StyleScialanca, Silvia, Giulia Bersanetti, Salvatore Parrillo, and Andrea Paolini. 2025. "Erector Spinae Plane Block for Perioperative Analgesia in a Rabbit" Veterinary Sciences 12, no. 10: 984. https://doi.org/10.3390/vetsci12100984
APA StyleScialanca, S., Bersanetti, G., Parrillo, S., & Paolini, A. (2025). Erector Spinae Plane Block for Perioperative Analgesia in a Rabbit. Veterinary Sciences, 12(10), 984. https://doi.org/10.3390/vetsci12100984





