Bactericidal and Anti-Inflammatory Effects of Ashitaba-Extract Ameliorate the Gingivitis and Halitosis in Dogs with Porphyromonas gulae-Infected Periodontal Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bactericidal Effects of Ashitaba-Extract on P. gulae
2.2. Evaluation of Hydrogen Sulfide and Methyl Mercaptan Generation by P. gulae
2.3. Ashitaba Extract Suppresses Pro-Inflammatory Cytokines in P. gulae- and LPS-Stimulated Macrophages
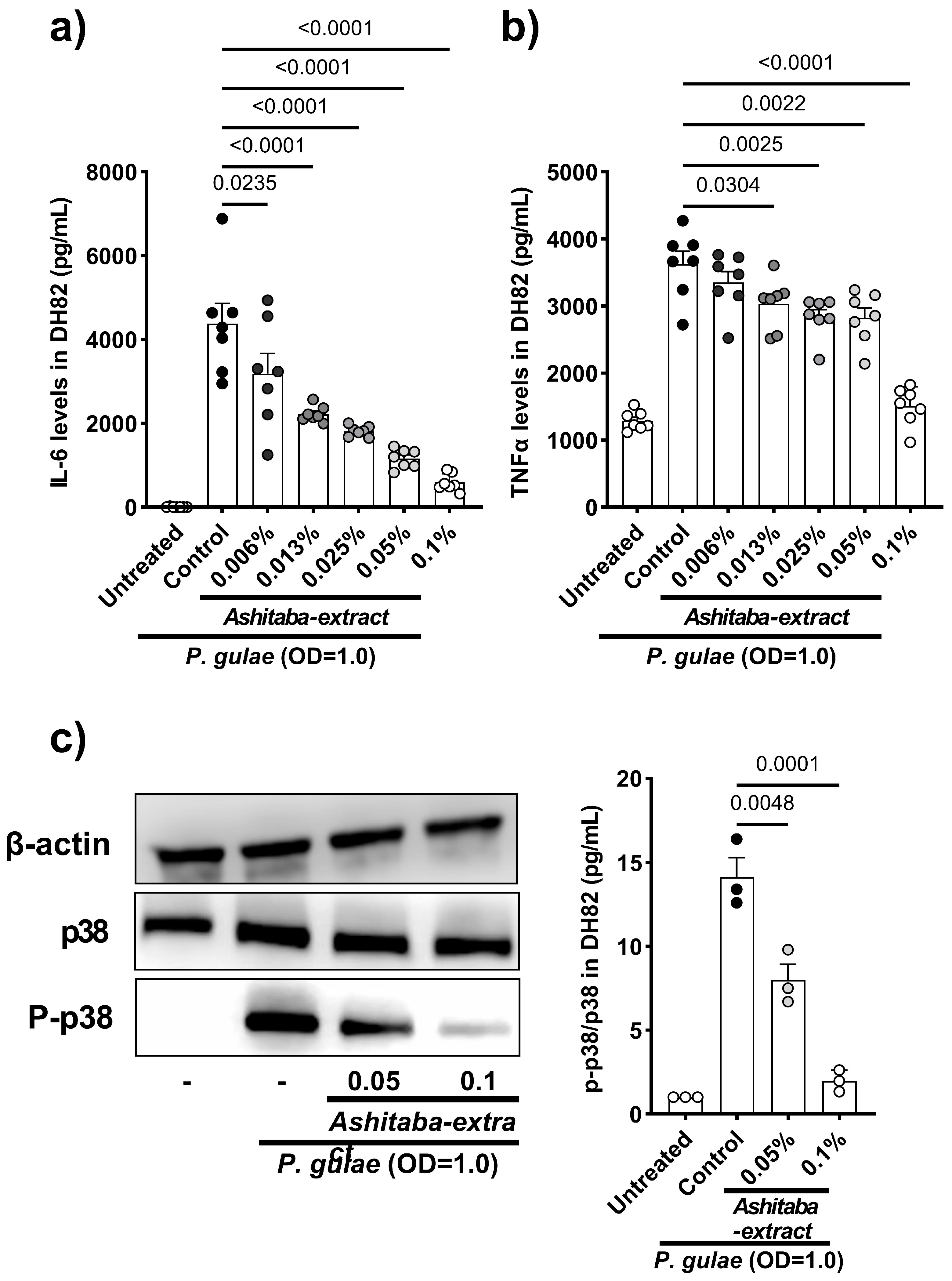
2.4. Effects of Ashitaba Extract on P. gulae-Induced p38 Phosphorylation in DH82 Cells
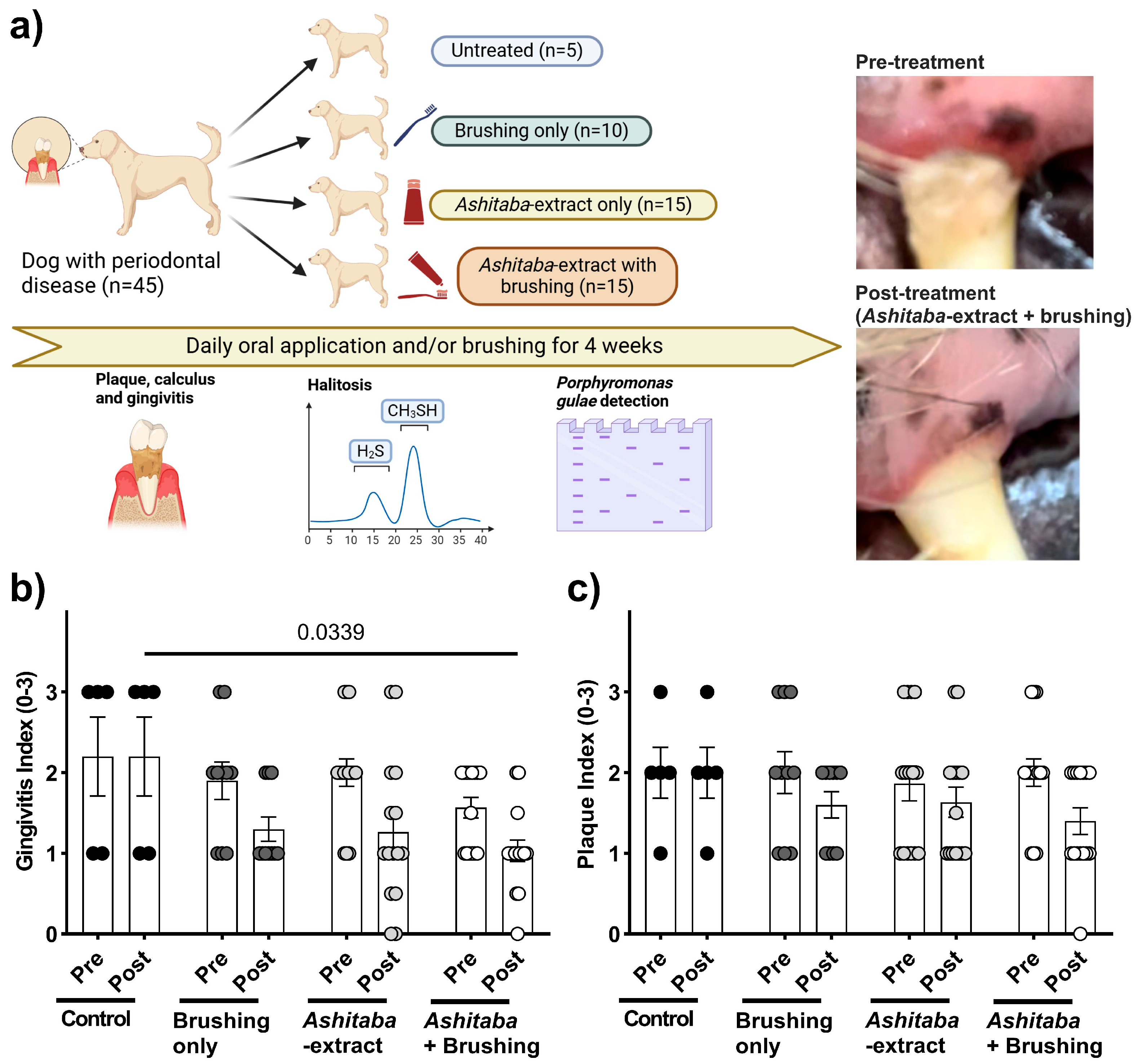
2.5. Daily Oral Ashitaba-Extract Treatments in Dogs with P. gulae-Positive PD
2.6. Statistical Analyses
3. Results
3.1. Ashitaba Extract Inhibits the Viability and Biofilm Formation of P. gulae
3.2. Ashitaba Extract Reduced P. gulae-Derived Hydrogen Sulfide and Methyl Mercaptan
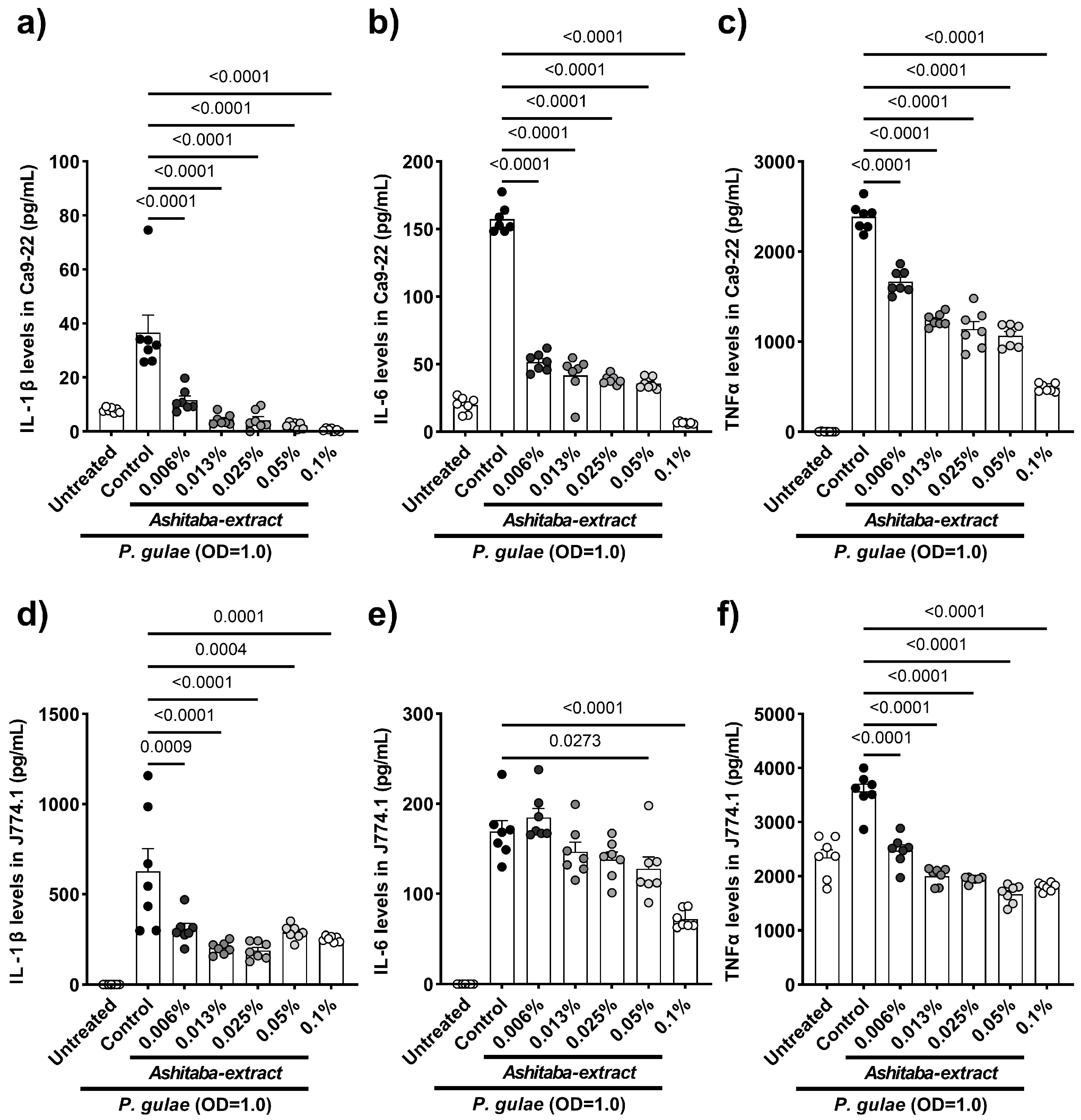
3.3. P. gulae-Stimulated Cytokine Production Was Inhibited by Ashitaba-Extract
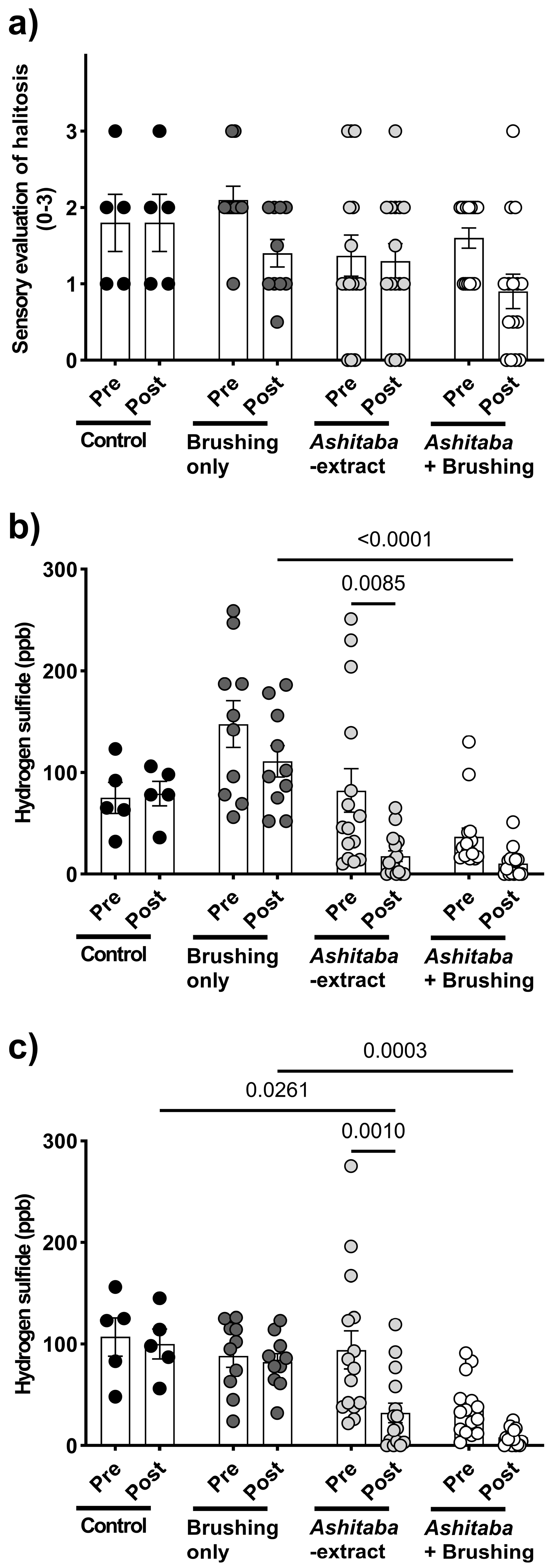
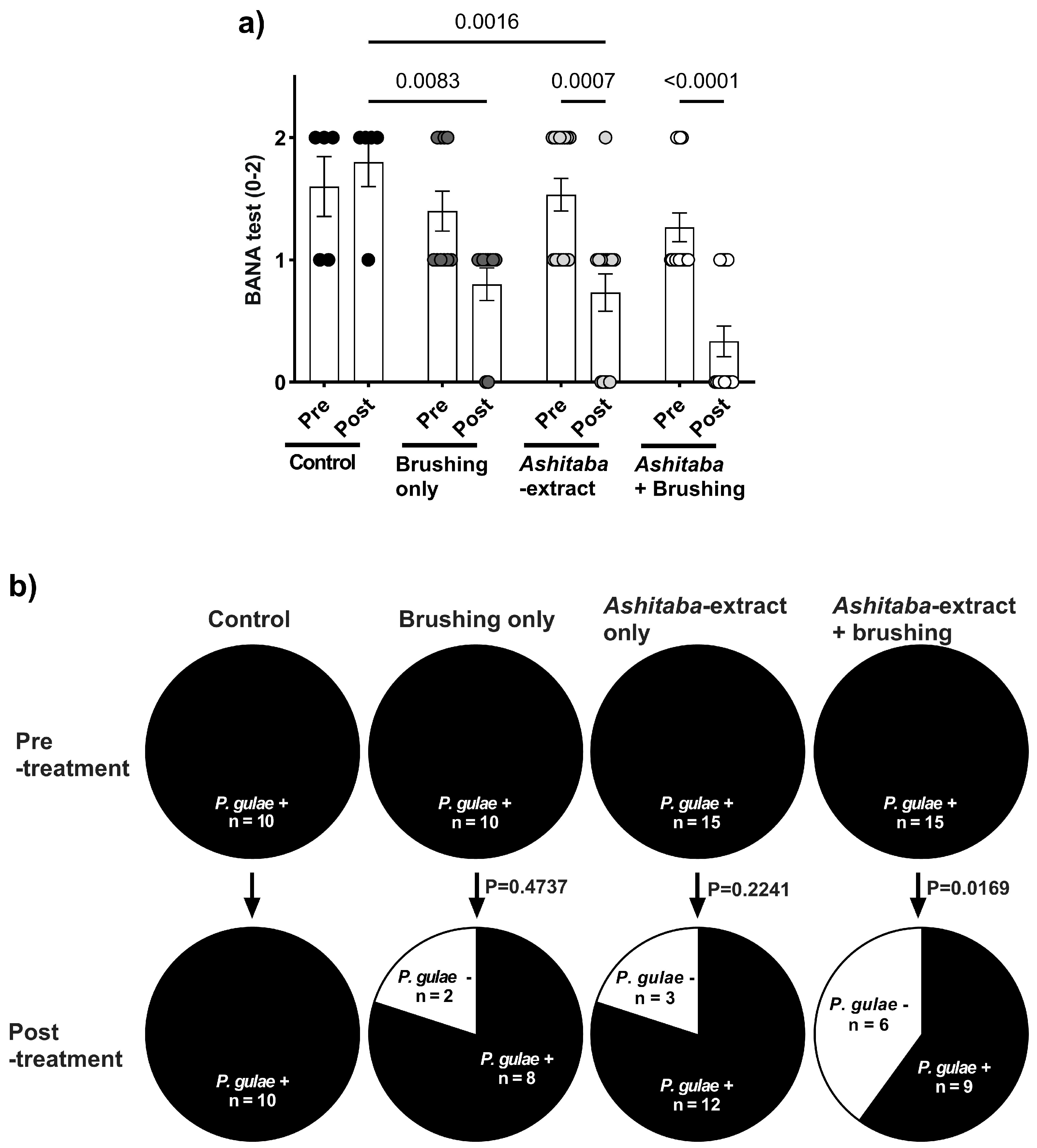
3.4. Ashitaba-Extract Gel (0.05%) Alleviates PD Symptoms in P. gulae-Infected Dogs, With or Without Brushing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Periodontal disease |
| P. gulae | Porphyromonas gulae |
| RPMI | Roswell Park Memorial Institute |
| ELISA | Enzyme-linked immunosorbent assay |
| BANA | N-benzoyl-DL-arginine-naphthylamide |
| SEM | Standard error of the mean |
| ANOVA | Analysis of variance |
| VSC | Volatile sulfide compounds |
| MAPKs | Mitogen-activated protein kinases |
References
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal disease in research beagle dogs—An epidemiological study. J. Small Anim. Pract. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- Niemiec, B.; Gawor, J.; Nemec, A.; Clarke, D.; McLeod, K.; Tutt, C.; Gioso, M.; Steagall, P.V.; Chandler, M.; Morgenegg, G.; et al. World Small Animal Veterinary Association Global Dental Guidelines. J. Small Anim. Pract. 2020, 61, 395–403. [Google Scholar] [CrossRef]
- Cunha, E.; Tavares, L.; Oliveira, M. Revisiting Periodontal Disease in Dogs: How to Manage This New Old Problem? Antibiotics 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Isogai, H.; Isogai, E.; Okamoto, H.; Shirakawa, H.; Nakamura, F.; Matsumoto, T.; Watanabe, T.; Miura, H.; Aoi, Y.; Kagota, W.; et al. Epidemiological study on periodontal diseases and some other dental disorders in dogs. Nihon Juigaku Zasshi 1989, 51, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Martins, T.; Dias, I.; Guedes-Pinto, H.; Bastos, E.; Viegas, C. Canine periodontitis: The dog as an important model for periodontal studies. Vet. J. 2012, 191, 299–305. [Google Scholar] [CrossRef]
- Pavlica, Z.; Petelin, M.; Juntes, P.; Erzen, D.; Crossley, D.A.; Skaleric, U. Periodontal disease burden and pathological changes in organs of dogs. J. Vet. Dent. 2008, 25, 97–105. [Google Scholar] [CrossRef]
- Pereira Dos Santos, J.D.; Cunha, E.; Nunes, T.; Tavares, L.; Oliveira, M. Relation between periodontal disease and systemic diseases in dogs. Res. Vet. Sci. 2019, 125, 136–140. [Google Scholar] [CrossRef]
- Ohira, C.; Kaneki, M.; Shirao, D.; Kurauchi, N.; Fukuyama, T. Oral treatment with catechin isolated from Japanese green tea significantly inhibits the growth of periodontal pathogen Porphyromonas gulae and ameliorates the gingivitis and halitosis caused by periodontal disease in cats and dogs. Int. Immunopharmacol. 2024, 146, 113805. [Google Scholar] [CrossRef]
- Shirahata, S.; Katayama, Y.; Kaneki, M.; Uchiyama, J.; Fukuyama, T. The Effect of Subacute Oral Folic Acid Treatment on Growth of Porphyromonas gulae in Dogs. J. Vet. Dent. 2024, 41, 281–287. [Google Scholar] [CrossRef]
- Ohkura, N.; Atsumi, G.; Ohnishi, K.; Baba, K.; Taniguchi, M. Possible antithrombotic effects of Angelica keiskei (Ashitaba). Pharmazie 2018, 73, 315–317. [Google Scholar] [CrossRef]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Inamori, Y.; Baba, K.; Tsujibo, H.; Taniguchi, M.; Nakata, K.; Kozawa, M. Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated from the root of Angelica keiskei KOIDZUMI. Chem. Pharm. Bull. 1991, 39, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Takata, M.; Takayasu, J.; Hasegawa, T.; Tokuda, H.; Nishino, A.; Nishino, H.; Iwashima, A. Anti-tumor-promotion by principles obtained from Angelica keiskei. Planta Med. 1991, 57, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. A Review of the Medicinal Uses and Pharmacology of Ashitaba. Planta Med. 2016, 82, 1236–1245. [Google Scholar] [CrossRef]
- Maronpot, R.R. Toxicological assessment of Ashitaba Chalcone. Food Chem. Toxicol. 2015, 77, 111–119. [Google Scholar] [CrossRef]
- Kato, Y.; Shirai, M.; Murakami, M.; Mizusawa, T.; Hagimoto, A.; Wada, K.; Nomura, R.; Nakano, K.; Ooshima, T.; Asai, F. Molecular detection of human periodontal pathogens in oral swab specimens from dogs in Japan. J. Vet. Dent. 2011, 28, 84–89. [Google Scholar] [CrossRef]
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The bacteriome of the oral cavity in healthy dogs and dogs with periodontal disease. Am. J. Vet. Res. 2021, 83, 50–58. [Google Scholar] [CrossRef]
- Carroll, M.Q.; Oba, P.M.; Sieja, K.M.; Alexander, C.; Lye, L.; de Godoy, M.R.C.; He, F.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; et al. Effects of novel dental chews on oral health outcomes and halitosis in adult dogs. J. Anim. Sci. 2020, 98, skaa274. [Google Scholar] [CrossRef]
- Rawlings, J.M.; Culham, N. Halitosis in dogs and the effect of periodontal therapy. J. Nutr. 1998, 128, 2715S–2716S. [Google Scholar] [CrossRef]
- Loesche, W.J.; Giordano, J.; Hujoel, P.P. The utility of the BANA test for monitoring anaerobic infections due to spirochetes (Treponema denticola) in periodontal disease. J. Dent. Res. 1990, 69, 1696–1702. [Google Scholar] [CrossRef]
- Gawor, J.P.; Ziemann, D.; Nicolas, C.S. A water additive with pomegranate can reduce dental plaque and calculus accumulation in dogs. Front. Vet. Sci. 2023, 10, 1241197. [Google Scholar] [CrossRef]
- Sordillo, A.; Casella, L.; Turcotte, R.; Sheth, R.U. A Novel Postbiotic Reduces Canine Halitosis. Animals 2025, 15, 1596. [Google Scholar] [CrossRef]
- Wallis, C.; Holcombe, L.J. A review of the frequency and impact of periodontal disease in dogs. J. Small Anim. Pract. 2020, 61, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.; Brinckmann, C.; Feuereisen, M.M.; Schieber, A. Effects of structural differences on the antibacterial activity of biflavonoids from fruits of the Brazilian peppertree (Schinus terebinthifolius Raddi). Food Res. Int. 2020, 133, 109134. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Inaba, H.; Yasuda, H.; Shirai, M.; Kato, Y.; Murakami, M.; Iwashita, N.; Shirahata, S.; Yoshida, S.; Matayoshi, S.; et al. Inhibition of Porphyromonas gulae and periodontal disease in dogs by a combination of clindamycin and interferon alpha. Sci. Rep. 2020, 10, 3113. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hayashi, K.; Kijima, S.; Nonaka, C.; Yamazoe, K. Tooth brushing inhibits oral bacteria in dogs. J. Vet. Med. Sci. 2015, 77, 1323–1325. [Google Scholar] [CrossRef]
- Croft, J.M.; Patel, K.V.; Inui, T.; Ruparell, A.; Staunton, R.; Holcombe, L.J. Effectiveness of oral care interventions on malodour in dogs. BMC Vet. Res. 2022, 18, 164. [Google Scholar] [CrossRef]
- Inaba, H.; Yoshida, S.; Nomura, R.; Kato, Y.; Asai, F.; Nakano, K.; Matsumoto-Nakano, M. Porphyromonas gulae lipopolysaccharide elicits inflammatory responses through toll-like receptor 2 and 4 in human gingivalis epithelial cells. Cell Microbiol. 2020, 22, e13254. [Google Scholar] [CrossRef]
- Kato, S.; Sugimura, N.; Nakashima, K.; Nishihara, T.; Kowashi, Y. Actinobacillus actinomycetemcomitans induces apoptosis in human monocytic THP-1 cells. J. Med. Microbiol. 2005, 54, 293–298. [Google Scholar] [CrossRef]
- Tamai, R.; Deng, X.; Kiyoura, Y. Porphyromonas gingivalis with either Tannerella forsythia or Treponema denticola induces synergistic IL-6 production by murine macrophage-like J774.1 cells. Anaerobe 2009, 15, 87–90. [Google Scholar] [CrossRef]
- Peyret-Lacombe, A.; Brunel, G.; Watts, M.; Charveron, M.; Duplan, H. TLR2 sensing of F. nucleatum and S. sanguinis distinctly triggered gingival innate response. Cytokine 2009, 46, 201–210. [Google Scholar] [CrossRef]
- Ohno, T.; Yamamoto, G.; Hayashi, J.I.; Nishida, E.; Goto, H.; Sasaki, Y.; Kikuchi, T.; Fukuda, M.; Hasegawa, Y.; Mogi, M.; et al. Angiopoietin-like protein 2 regulates Porphyromonas gingivalis lipopolysaccharide-induced inflammatory response in human gingival epithelial cells. PLoS ONE 2017, 12, e0184825. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, T.W.; Kim, H.J.; Nam, D.; Jung, S.H.; Lee, E.H.; Lee, H.J.; Shin, E.M.; Jang, H.J.; Ahn, K.S.; et al. Anti-Inflammatory activity of Angelica keiskei through suppression of mitogen-activated protein kinases and nuclear factor-kappaB activation pathways. J. Med. Food 2010, 13, 691–699. [Google Scholar] [CrossRef]

| Group | Breeds | Age | Sex |
|---|---|---|---|
| Untreated group (n = 5) | Toy Poodle | 12 years | Male (castration) |
| Shiba | 7 years | Female (spay) | |
| Miniature Dachshund | 8 years | Female (spay) | |
| Miniature Dachshund | <10 years | Female (spay) | |
| Toy Poodle | 6 years | Male (castration) | |
| Brushing-only group (n = 10) | Toy Poodle | 8 years | Male (castration) |
| Toy Poodle | 6 years | Female (spay) | |
| Toy Poodle | 14 years | Female (spay) | |
| Miniature Dachshund | unknown | Female (spay) | |
| Chihuahua | 5 years | Female (spay) | |
| Miniature Dachshund | 7 years | Female (spay) | |
| Chihuahua | 12 years | Male (castration) | |
| Chihuahua | 8 years | Female (spay) | |
| Beagle | 10 years | Female (spay) | |
| Italian Greyhound | 6 years | Male (castration) | |
| Ashitaba-extract group (n = 15) | Yorkshire terrier | 10 years | Female (spay) |
| Toy Poodle | 5 years | Male (castration) | |
| Miniature Dachshund | 6 years | Female (spay) | |
| Maltese × Toy Poodle | 5 years | Male (castration) | |
| Miniature Dachshund | unknown | Female (spay) | |
| Miniature Dachshund | 5 years | Male (castration) | |
| Toy Poodle | 16 years | Male (castration) | |
| Miniature Dachshund | 11 years | Female (spay) | |
| Miniature Dachshund | <10 years | Female (spay) | |
| Toy Poodle | 4 years | Female (spay) | |
| Chihuahua | 6 years | Male (castration) | |
| Pomeranian | 5 years | Female (spay) | |
| Italian Greyhound | 9 years | Female (spay) | |
| Pomeranian | 11 years | Male (castration) | |
| Ashitaba-extract + Brushing group (n = 15) | Yorkshire terrier | 11 years | Female (spay) |
| Miniature Dachshund | 9 years | Female (spay) | |
| Miniature Dachshund | 8 years | Female (spay) | |
| Toy Poodle | 5 years | Male (castration) | |
| Toy Poodle | 6 years | Female (spay) | |
| Chihuahua | 13 years | Female (spay) | |
| Toy Poodle | unknown | Male (castration) | |
| Chihuahua | 11 years | Female (spay) | |
| Chihuahua | <10 years | Female (spay) | |
| Toy Poodle | 4 years | Female (spay) | |
| Jack Russell | unknown | Male (castration) | |
| Pomeranian | 5 years | Female (spay) | |
| Italian Greyhound | 8 years | Female (spay) | |
| Pomeranian | 7 years | Male (castration) | |
| Jack Russell | 8 years | Male (castration) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, T.; Shirahata, S.; Komuro, M.; Kaneki, M.; Ohira, C.; Fukuyama, T. Bactericidal and Anti-Inflammatory Effects of Ashitaba-Extract Ameliorate the Gingivitis and Halitosis in Dogs with Porphyromonas gulae-Infected Periodontal Disease. Vet. Sci. 2025, 12, 981. https://doi.org/10.3390/vetsci12100981
Miyamoto T, Shirahata S, Komuro M, Kaneki M, Ohira C, Fukuyama T. Bactericidal and Anti-Inflammatory Effects of Ashitaba-Extract Ameliorate the Gingivitis and Halitosis in Dogs with Porphyromonas gulae-Infected Periodontal Disease. Veterinary Sciences. 2025; 12(10):981. https://doi.org/10.3390/vetsci12100981
Chicago/Turabian StyleMiyamoto, Takayoshi, So Shirahata, Mariko Komuro, Mao Kaneki, Chiharu Ohira, and Tomoki Fukuyama. 2025. "Bactericidal and Anti-Inflammatory Effects of Ashitaba-Extract Ameliorate the Gingivitis and Halitosis in Dogs with Porphyromonas gulae-Infected Periodontal Disease" Veterinary Sciences 12, no. 10: 981. https://doi.org/10.3390/vetsci12100981
APA StyleMiyamoto, T., Shirahata, S., Komuro, M., Kaneki, M., Ohira, C., & Fukuyama, T. (2025). Bactericidal and Anti-Inflammatory Effects of Ashitaba-Extract Ameliorate the Gingivitis and Halitosis in Dogs with Porphyromonas gulae-Infected Periodontal Disease. Veterinary Sciences, 12(10), 981. https://doi.org/10.3390/vetsci12100981







