Beyond TFRC: The Pivotal Role of mGluR2 in Feline Calicivirus Entry and Replication
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Virus
2.2. Reagents and Antibodies
2.3. Plasmid Constructs
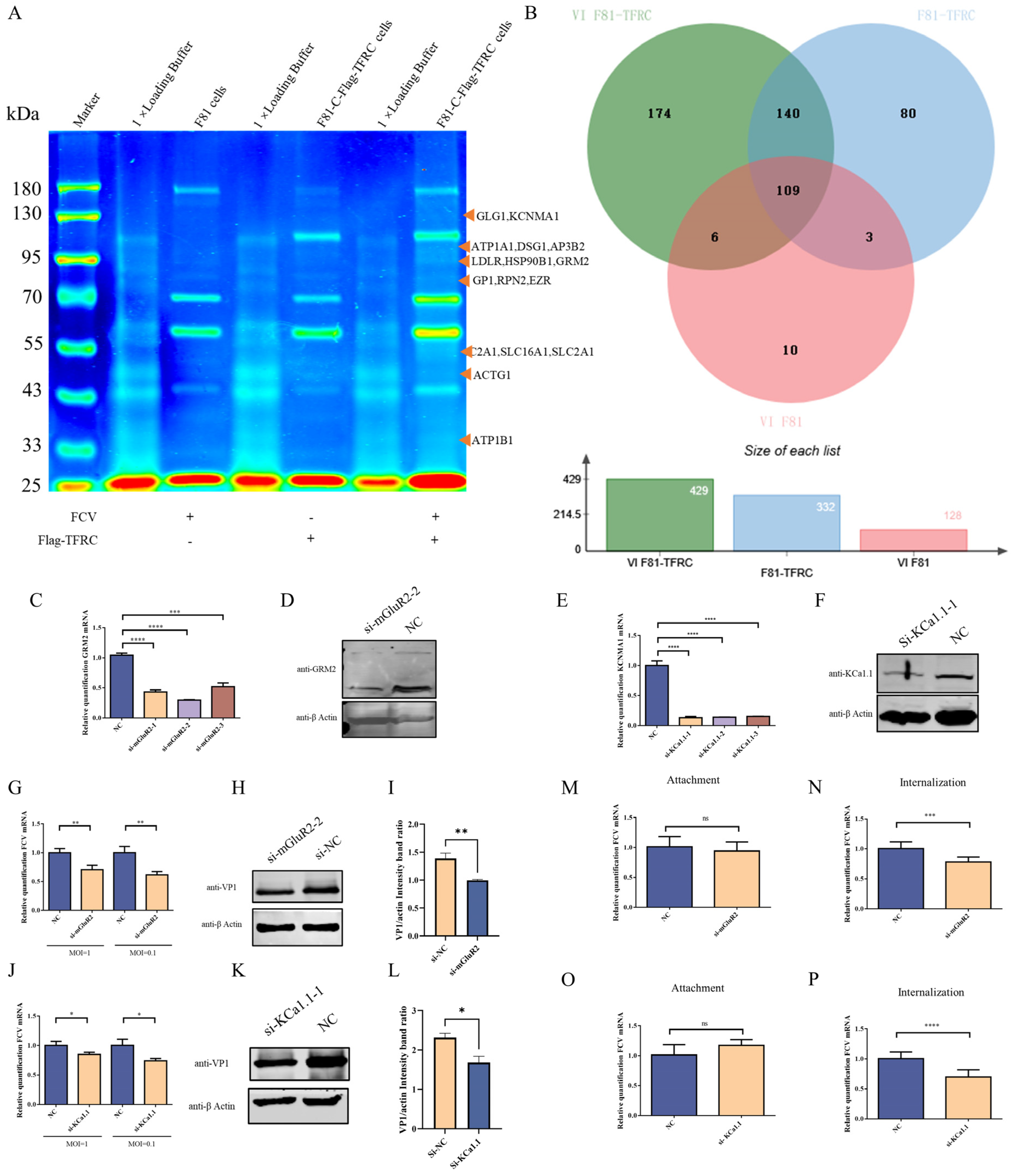
2.4. The Potential TFRC-Binding Proteins Identified by Co-IP Followed by LC-MS Assays
2.5. Small-Interfering RNA (siRNA) Transfection
2.6. Quantitative Reverse Transcription PCR (qRT-PCR)
2.7. Western Blotting (WB)
2.8. Viral Infection
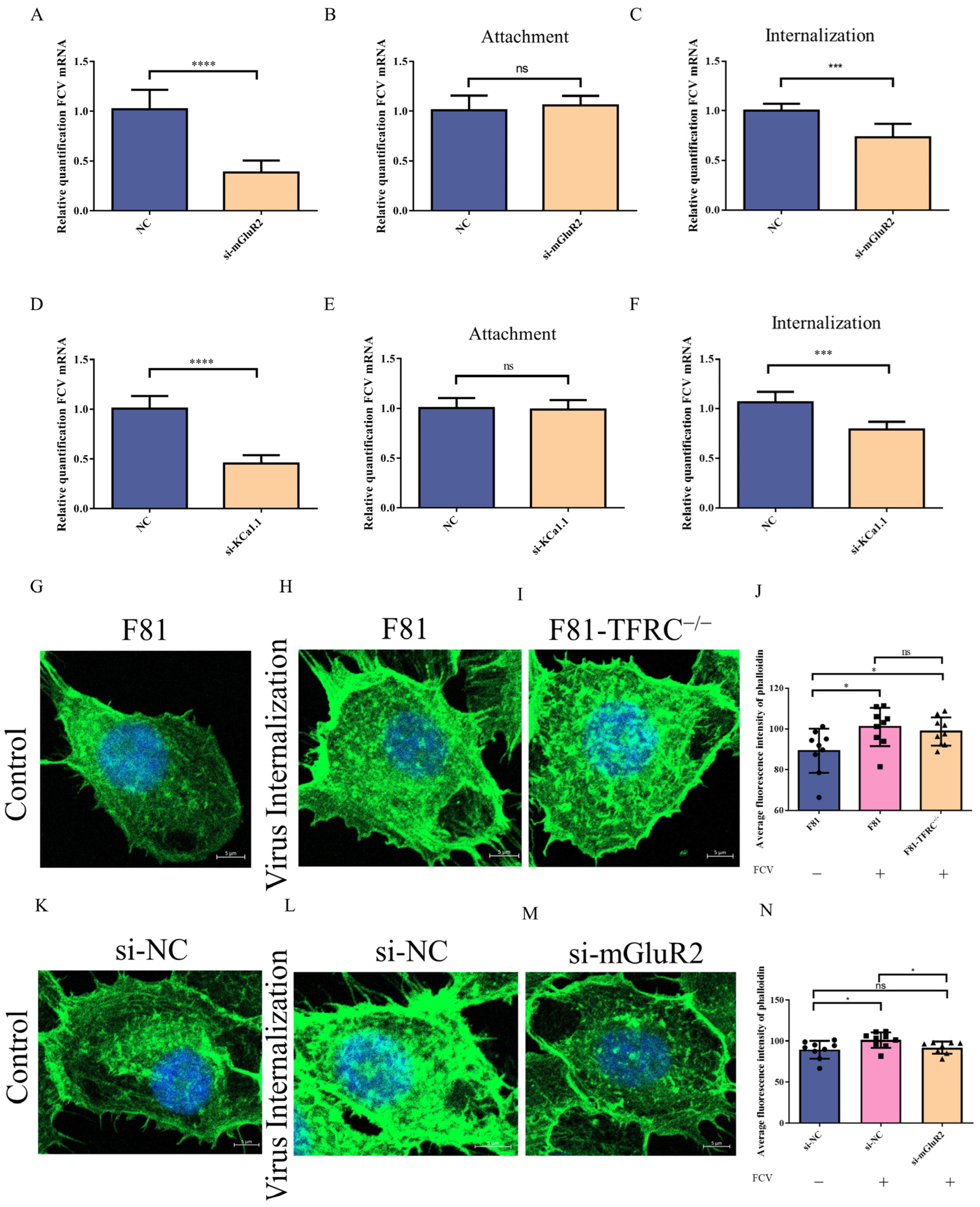
2.9. Measurement of Attachment and Internalization Rates
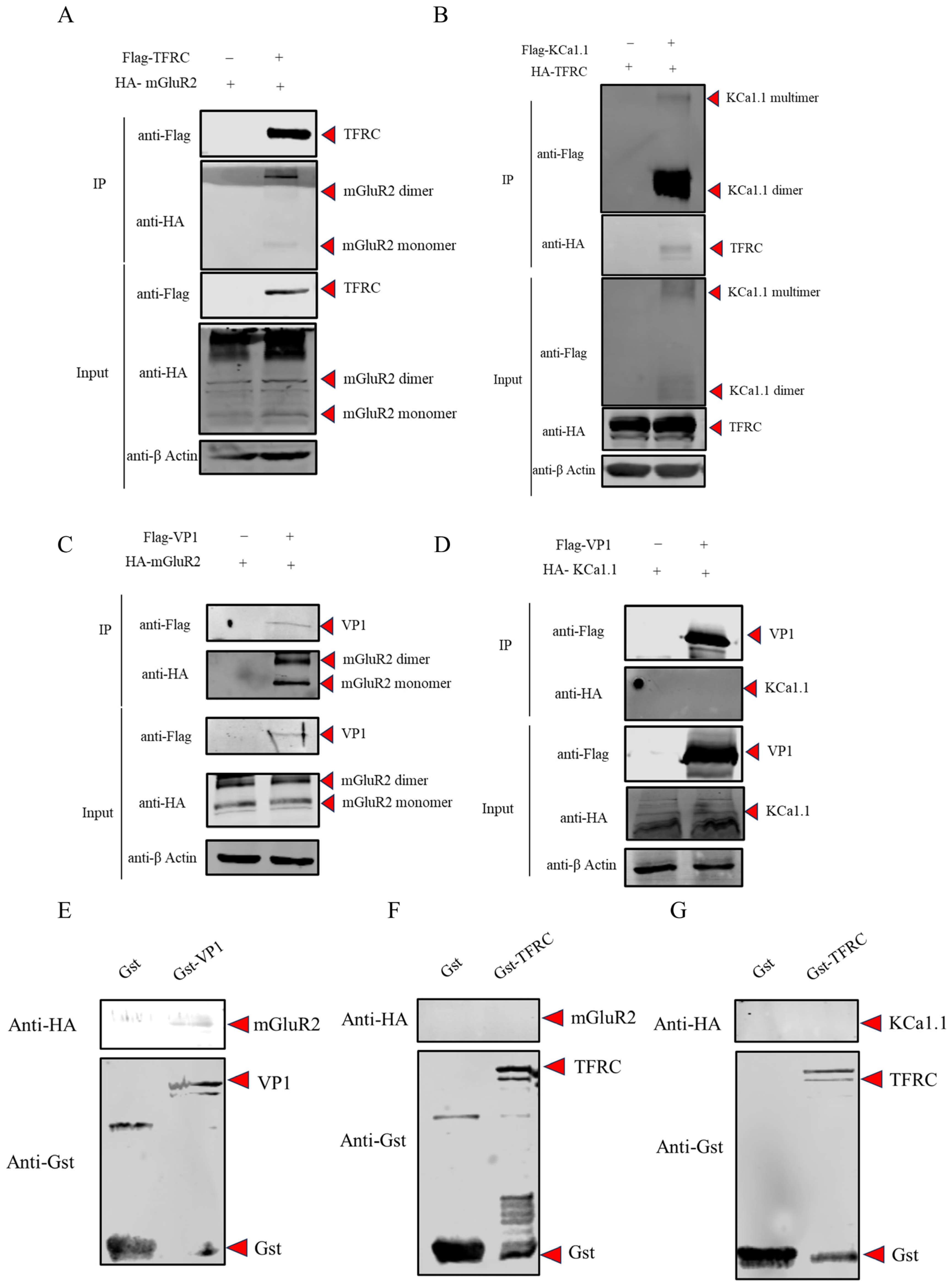
2.10. Co-Immunoprecipitation (Co-IP) Analysis
2.11. Glutathion-S-Transferase (GST) Pull-Down Assay
2.12. F-Actin Polymerization Assay
2.13. Statistics
3. Results
3.1. Identification of Host Proteins Interacting with TFRC
3.2. mGluR2 and KCa1.1 Modulate the Replication of FCV by Regulating Its Internalization into Host Cells
3.3. The Interaction Analysis Among mGluR2, KCa1.1, TFRC and FCV VP1
3.4. mGluR2 Mediates FCV Internalization Without TFRC Involved
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Abouelkhair, M.A.; Kennedy, M. Caliciviridae. In Veterinary Microbiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 543–551. [Google Scholar]
- Cheng, C.; Cai, X.; Li, J.; Zhang, X.; Xie, Y.; Zhang, J. In Vitro Culture of Human Norovirus in the Last 20 Years. Biomedicines 2024, 12, 2442. [Google Scholar] [CrossRef]
- Desselberger, U. Caliciviridae Other Than Noroviruses. Viruses 2019, 11, 286. [Google Scholar] [CrossRef]
- Haga, K.; Fujimoto, A.; Takai-Todaka, R.; Miki, M.; Doan, Y.H.; Murakami, K.; Yokoyama, M.; Murata, K.; Nakanishi, A.; Katayama, K. Functional receptor molecules CD300lf and CD300ld within the CD300 family enable murine noroviruses to infect cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6248–E6255. [Google Scholar] [CrossRef]
- Marionneau, S.; Ruvoën, N.; Le Moullac-Vaidye, B.; Clement, M.; Cailleau-Thomas, A.; Ruiz-Palacois, G.; Huang, P.; Jiang, X.; Le Pendu, J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 2002, 122, 1967–1977. [Google Scholar] [CrossRef]
- Tamura, M.; Natori, K.; Kobayashi, M.; Miyamura, T.; Takeda, N. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 2004, 78, 3817–3826. [Google Scholar] [CrossRef]
- Taube, S.; Perry, J.W.; Yetming, K.; Patel, S.P.; Auble, H.; Shu, L.; Nawar, H.F.; Lee, C.H.; Connell, T.D.; Shayman, J.A.; et al. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J. Virol. 2009, 83, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.D.; Brown, T.D. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Virol. 2006, 80, 7500–7509. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ledgerwood, E.D.; Hinchman, M.M.; Dick, R.; Parker, J.S.L. Conserved Surface Residues on the Feline Calicivirus Capsid Are Essential for Interaction with Its Receptor Feline Junctional Adhesion Molecule A (fJAM-A). J. Virol. 2018, 92, e00035-18. [Google Scholar] [CrossRef]
- Makino, A.; Shimojima, M.; Miyazawa, T.; Kato, K.; Tohya, Y.; Akashi, H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2006, 80, 4482–4490. [Google Scholar] [CrossRef]
- Wang, X.; Wen, Z.; Cao, H.; Luo, J.; Shuai, L.; Wang, C.; Ge, J.; Wang, X.; Bu, Z.; Wang, J. Transferrin Receptor Protein 1 Cooperates with mGluR2 To Mediate the Internalization of Rabies Virus and SARS-CoV-2. J. Virol. 2023, 97, e0161122. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Wang, J.; Yu, X.; Wang, Y.; Wang, J.; He, X.; Li, C.; Deng, G.; Shi, J.; Kong, H.; et al. Influenza virus uses mGluR2 as an endocytic receptor to enter cells. Nat. Microbiol. 2024, 9, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Loebrich, S. The role of F-actin in modulating Clathrin-mediated endocytosis: Lessons from neurons in health and neuropsychiatric disorder. Commun. Integr. Biol. 2014, 7, e28740. [Google Scholar] [CrossRef]
- Yu, X.; Ni, Z.; Wang, Y.; Wang, J.; Deng, G.; Shi, J.; Kong, H.; Jiang, Y.; Tian, G.; Li, C.; et al. Claudin-11 plays a pivotal role in the clathrin-mediated endocytosis of influenza A virus. Sci. China Life Sci. 2025, 68, 1463–1477. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Liu, C.; Li, T.; Wang, X.; Li, X.; Bao, M.; Li, J.; Huang, L.; Zhang, Z.; et al. CD1d facilitates African swine fever virus entry into the host cells via clathrin-mediated endocytosis. Emerg. Microbes Infect. 2023, 12, 2220575. [Google Scholar] [CrossRef]
- Shi, R.; Hou, L.; Wei, L.; Liu, J. Involvement of adaptor proteins in clathrin-mediated endocytosis of virus entry. Microb. Pathog. 2021, 161, 105278. [Google Scholar] [CrossRef]
- Ehrlich, M.; Boll, W.; Van Oijen, A.; Hariharan, R.; Chandran, K.; Nibert, M.L.; Kirchhausen, T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 2004, 118, 591–605. [Google Scholar] [CrossRef]
- Johannsdottir, H.K.; Mancini, R.; Kartenbeck, J.; Amato, L.; Helenius, A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009, 83, 440–453. [Google Scholar] [CrossRef]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, I.S.; Chen, M.M.; King, J.R.; Kaushansky, K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood 2008, 112, 2222–2231. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Huang, H.C.; Chen, C.C.; Chang, W.C.; Tao, M.H.; Huang, C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J. Virol. 2012, 86, 9443–9453. [Google Scholar] [CrossRef] [PubMed]
- Jatuyosporn, T.; Laohawutthichai, P.; Supungul, P.; Sotelo-Mundo, R.R.; Ochoa-Leyva, A.; Tassanakajon, A.; Krusong, K. Role of Clathrin Assembly Protein-2 Beta Subunit during White Spot Syndrome Virus Infection in Black Tiger Shrimp Penaeus monodon. Sci. Rep. 2019, 9, 13489. [Google Scholar] [CrossRef]
- Farquhar, M.J.; Hu, K.; Harris, H.J.; Davis, C.; Brimacombe, C.L.; Fletcher, S.J.; Baumert, T.F.; Rappoport, J.Z.; Balfe, P.; McKeating, J.A. Hepatitis C virus induces CD81 and claudin-1 endocytosis. J. Virol. 2012, 86, 4305–4316. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Liu, R.; Shuai, L.; Wang, X.; Luo, J.; Wang, C.; Chen, W.; Wang, X.; Ge, J.; et al. Metabotropic glutamate receptor subtype 2 is a cellular receptor for rabies virus. PLoS Pathog. 2018, 14, e1007189. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.C.; Dunn, H.; Ferguson, S.S. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 2012, 165, 1717–1736. [Google Scholar] [CrossRef]
- Wolfe, B.L.; Trejo, J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 2007, 8, 462–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, R.; Kang, H.; Yang, Y.; Feng, K.; Liu, Z.; Gao, S.; Jiang, Q.; Qu, L.; Liu, J. Beyond TFRC: The Pivotal Role of mGluR2 in Feline Calicivirus Entry and Replication. Vet. Sci. 2025, 12, 980. https://doi.org/10.3390/vetsci12100980
Qi R, Kang H, Yang Y, Feng K, Liu Z, Gao S, Jiang Q, Qu L, Liu J. Beyond TFRC: The Pivotal Role of mGluR2 in Feline Calicivirus Entry and Replication. Veterinary Sciences. 2025; 12(10):980. https://doi.org/10.3390/vetsci12100980
Chicago/Turabian StyleQi, Ruibin, Hongtao Kang, Yupeng Yang, Kexin Feng, Zhe Liu, Silu Gao, Qian Jiang, Liandong Qu, and Jiasen Liu. 2025. "Beyond TFRC: The Pivotal Role of mGluR2 in Feline Calicivirus Entry and Replication" Veterinary Sciences 12, no. 10: 980. https://doi.org/10.3390/vetsci12100980
APA StyleQi, R., Kang, H., Yang, Y., Feng, K., Liu, Z., Gao, S., Jiang, Q., Qu, L., & Liu, J. (2025). Beyond TFRC: The Pivotal Role of mGluR2 in Feline Calicivirus Entry and Replication. Veterinary Sciences, 12(10), 980. https://doi.org/10.3390/vetsci12100980






