Remarkable Inhibition Efficacy of a Compound Plant Essential Oil Disinfectant Against Bacteria, Viruses, and Mycoplasmas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibacterial Activity
2.2.1. Neutralizing Agent Identification
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.2.3. Determination of Optimal Disinfection Concentration
2.2.4. Determination of Optimal Disinfection Time
2.3. Anti-IBV Activity
2.3.1. Toxicity Testing for Embryonated Chicken Eggs
2.3.2. Determination of Optimal Disinfection Concentration
2.3.3. Determination of Optimal Disinfection Time
2.4. Anti-Mycoplasma Activity
2.4.1. Resuscitation Culture of Mycoplasma and Determination of CCU
2.4.2. Determination of the MIC of Mycoplasma
2.5. Statistical Analysis
3. Results
3.1. Antibacterial Activity
3.1.1. Neutralizing Agent Identification
3.1.2. Results of MIC Determination for Bacteria
3.1.3. Optimal Disinfection Concentration for Bacteria
3.1.4. Optimal Disinfection Time for Bacteria
3.2. Anti-IBV Activity
3.2.1. Toxicity Testing for Embryonated Chicken Eggs
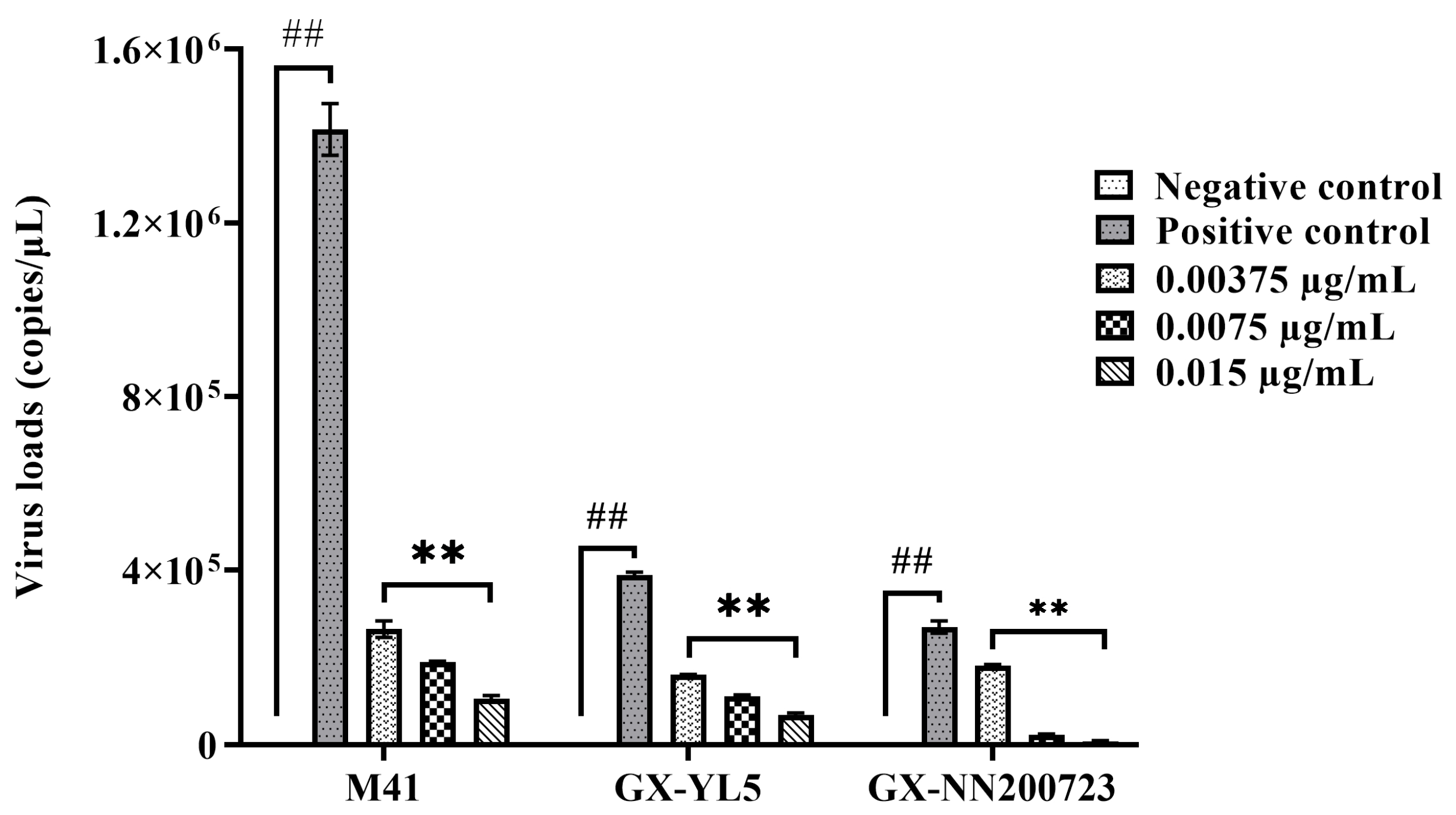
3.2.2. Optimal Disinfection Concentration for IBV
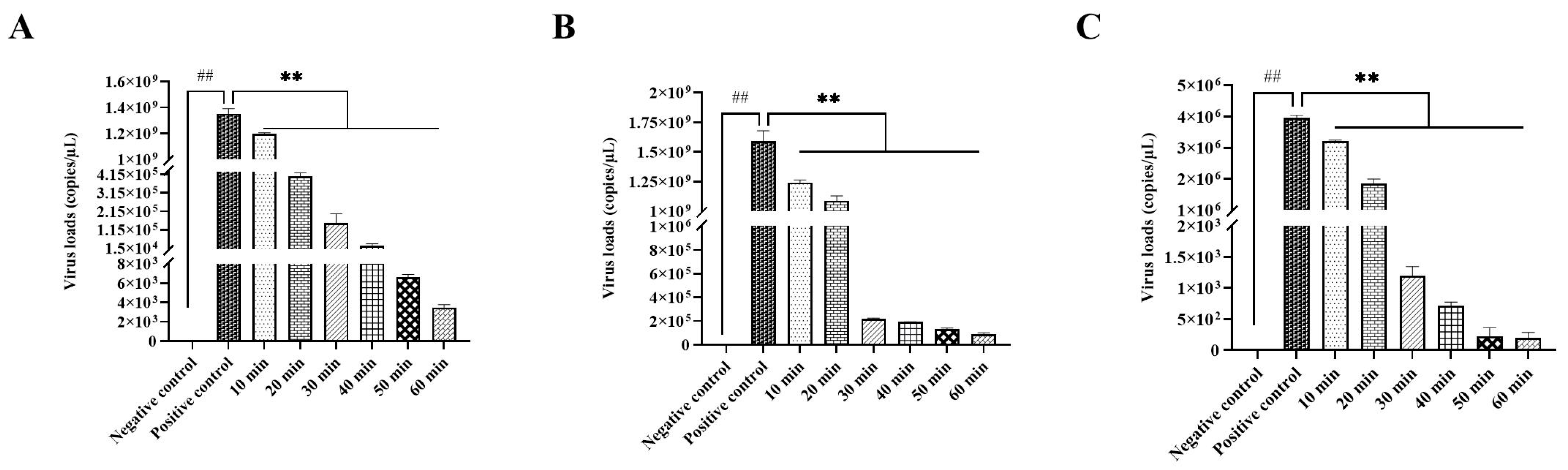
3.2.3. Optimal Disinfection Time for IBV
3.3. Anti-Mycoplasma Activity
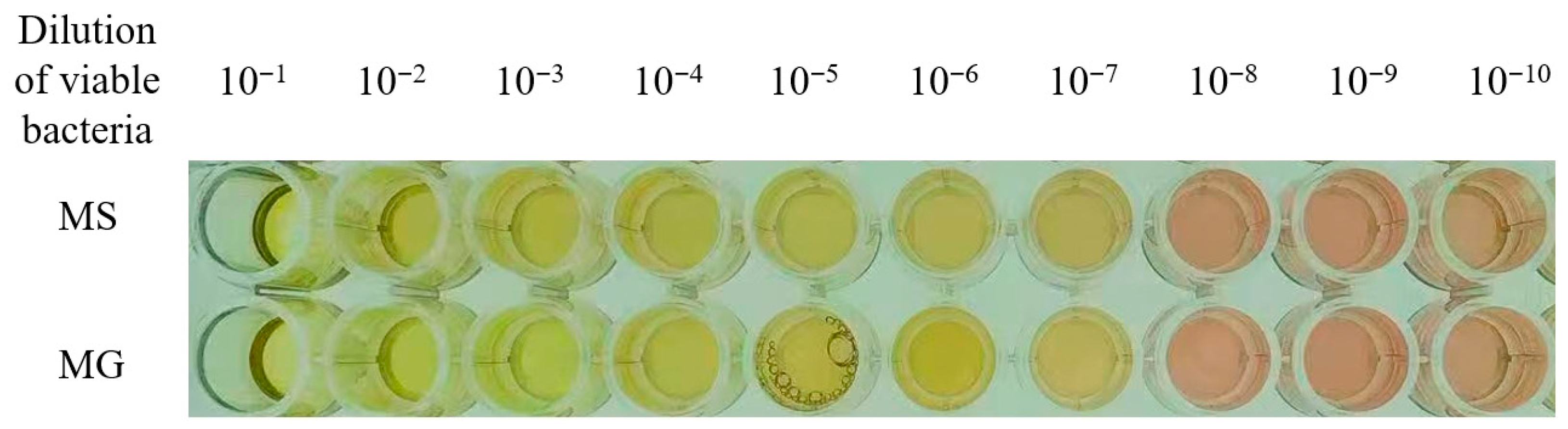
3.3.1. Results of Color-Changing Units (CCU)
3.3.2. Results of MIC Determination for Mycoplasmas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chmielowiec-Korzeniowska, A.; Trawińska, B.; Tymczyna, L.; Bis-Wencel, H.; Matuszewski, L. Microbial contamination of the air in livestock buildings as a threat to human and animal health—A review. Ann. Anim. Sci. 2021, 21, 417–431. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Qadrie, Z.; Ashraf, H.; Dar, M.A.; Qadir, A. The Growing threat of antibiotic resistance: Mechanisms, couses, csuses, consequences, and solutions. Int. J. Cogn. Neurosci. Psychol. 2025, 3, 28–36. [Google Scholar]
- Cars, O.; Nordberg, P. Antibiotic resistance—The faceless threat. Int. J. Risk Saf. Med. 2005, 17, 103–110. [Google Scholar] [CrossRef]
- Yang, L.; Bajinka, O.; Jarju, P.O.; Tan, Y.R.; Taal, A.M.; Ozdemir, G. The varying effects of antibiotics on gut microbiota. AMB Express 2021, 11, 116. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, C.; Anjankar, A.; Agrawal, J. Self-Medication With Antibiotics: An Element Increasing Resistance. Cureus 2022, 14, e30844. [Google Scholar] [CrossRef]
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.L. A history of antimicrobial drugs in animals: Evolution and revolution. J. Vet. Pharmacol. Ther. 2021, 44, 137–171. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y.J. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Chima, I.U.; Unamba-Opara, I.C.; Ugwu, C.C.; Udebuani, A.C.; Okoli, C.G. Biosecurity and Disinfection Controls of Poultry Microbial Pathogen Infections in Nigeria. J. World’s Poult. Res. 2012, 2, 5–17. [Google Scholar]
- Perry, K.; Caveney, L. Chemical Disinfectants. In Veterinary Infection Prevention and Control; Caveney, L., Jones, B., Ellis, K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 129–143. [Google Scholar]
- Wales, A.D.; Gosling, R.J.; Bare, H.L.; Davies, R.H. Disinfectant testing for veterinary and agricultural applications: A review. Zoonoses Public Health 2021, 68, 361–375. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Academic Press: Cambridge, MA, USA, 2017; pp. 379–419. [Google Scholar]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; GIl, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Tan, Y.X.; Huang, Y.Q.; Qian, Y.J.; Zhang, G.Y.; Si, H.B. Effect of compounding Cinnamomum cassia essential oil and Litsea cubeba essential oil on biofilm of Salmonella pullorum. Feed Res. 2024, 47, 99. [Google Scholar]
- Issa, N.A. Evaluation the Antimicrobial Activity of Essential Oils against Veterinary Pathogens, Multidrug-resistant Bacteria and Dermatophytes. Pak. Vet. J. 2024, 44, 260–265. [Google Scholar]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Amadesi, A.; Rinaldi, L.; Stojanovic, D.; Simin, N.; Orcic, D.; Pusic, I.; Krnjajic, S.; Ratajac, R. Ovicidal Potential of Five Different Essential Oils to Control Gastrointestinal Nematodes of Sheep. Pak. Vet. J. 2021, 41, 353–358. [Google Scholar]
- Gendy, H.; Masoud, S.; Elnahriry, S.; Attia, T.; Mansour, A.; Elihusseiny, E. Enhancement of the efficacy of Tylvalosin by administration with Eucalyptus oil or Bromhexine against Mycoplasma gallisepticum-infected broiler chickens. Alex. J. Vet. Sci. 2024, 81, 74. [Google Scholar] [CrossRef]
- Yingngam, B. Chemistry of Essential Oils. In ACS Symposium Series; Balakrishnan, P., Gopi, S., Eds.; American Chemical Society: Washington, DC, USA, 2022; pp. 189–223. [Google Scholar]
- Khaledi, M.; Afkhami, H.; Atani, Z.R.; Sepehrnia, S.; Atani, F.R.; Ahmadi, M.H. Novel Perspective for Treatment of Mycoplasma Infections: A Promising Future. Int. J. Pept. Res. Ther. 2021, 28, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, H.; Li, K. Litsea cubeba essential oil: Extraction, chemical composition, antioxidant and antimicrobial properties, and applications in the food industry. J. Food Sci. 2024, 89, 4583–4603. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Kowalczewski, P.; Bakay, L.; Kačániová, M. Role of Litsea cubeba Essential Oil in Agricultural Products Safety: Antioxidant and Antimicrobial Applications. Plants 2022, 11, 1504. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Li, C.; Xu, Z.; Chen, W.; Zhou, C.; Wang, C.; Wang, M.; Liang, J.; Wei, P. The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens. Antibiotics 2022, 11, 1579. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.-Y.; Wei, P.; Chen, Q.-Y.; Wei, Z.-J.; Mo, M.-L. Serotype and genotype diversity of infectious bronchitis viruses isolated during 1985–2008 in Guangxi, China. Arch. Virol. 2011, 157, 467–474. [Google Scholar] [CrossRef]
- Yong, L.; Lü, D.; Fan, W.; Lin, L.T.; Tang, J.W.; Zhang, Y.; Lin, Z.X.; Wei, P.; Mo, M.L. Isolation and Identification of Taiwan Genotype Avian Infectious Bronchitis Virus Recombinant Strain and Analysis of Its S1 Gene Sequence and Serotype. China Poult. 2022, 44, 29–35. (In Chinese) [Google Scholar]
- Ministry of Health Legal System and Supervision Department. Disinfection Technical Specification; Ministry of Health of the People’s Republic of China: Beijing, China, 2002. (In Chinese)
- Mo, M.-L.; Chen, Q.-Y.; Hou, J.-L.; Fan, W.-S.; Li, M.; Wei, P.; Wei, T.-C. Development of a real-time PCR assay for detection of infectious bronchitis virus. Vet. Sci. 2011, 41, 193–198. (In Chinese) [Google Scholar]
- Wesey, S.; Daniel, D.C. Public Health Implications of Bacterial Zoonotic Diseases. In The One Health Model as Applied to Zoonotic Diseases; Samples, O.M., McCommon, G.W., Terrill, T.H., Eds.; Wiley: Hoboken, NJ, USA, 2025; pp. 107–114. [Google Scholar]
- Yemiş, F.; Harmanci, N.Y. Classification, Uses and Environmental Implications of Disinfectants. Pak. J. Anal. Environ. Chem. 2020, 21, 179–192. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, S.; Zhu, Z.; Chen, X.; Wang, J. Current progress, opportunities and challenges of developing green disinfectants for the remediation of disinfectant emerging contaminants. Sustain. Chem. Pharm. 2024, 42, 101775. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2011, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, C.; Zhang, Y.; Tong, W.; Du, L.; Liu, F. Proteomic analysis of Aspergillus flavus reveals the antifungal action of Perilla frutescens essential oil by interfering with energy metabolism and defense function. LWT 2022, 154, 112660. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, F.; Li, Q.; Huang, J.L.; Ju, J. Antifungal mechanism of essential oil against foodborne fungi and its application in the preservation of baked food. Crit. Rev. Food Sci. Nutr. 2024, 64, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Kolypetri, S.; Kostoglou, D.; Nikolaou, A.; Kourkoutas, Y.; Giaouris, E. Chemical Composition, Antibacterial and Antibiofilm Actions of Oregano (Origanum vulgare subsp. hirtum) Essential Oil against Salmonella Typhimurium and Listeria monocytogenes. Foods 2023, 12, 2893. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Q.; Yang, G.Q. Chemical Composition and Antimicrobial Activity of Essential Oil of Camphora glanduliferum ‘Honganzhang’. Horticulturae 2025, 11, 67. [Google Scholar] [CrossRef]
- Mei, C.C.; Wang, X.; Chen, Y.C.; Wang, Y.D.; Yao, F.; Li, Z.C.; Gu, Q.; Song, D.F. Antibacterial activity and mechanism of Litsea cubeba essential oil against food contamination by Escherichia coli and Salmonella enterica. J. Food Saf. 2020, 40, e12809. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Zhang, G.Z. Key Aspects of Coronavirus Avian Infectious Bronchitis Virus. Pathogens 2023, 12, 698. [Google Scholar] [CrossRef]
- Romeo, A.; Iacovelli, F.; Scagnolari, C.; Scordio, M.; Frasca, F.; Condò, R.; Ammendola, S.; Gaziano, R.; Anselmi, M.; Divizia, M.; et al. Potential Use of Tea Tree Oil as a Disinfectant Agent against Coronaviruses: A Combined Experimental and Simulation Study. Molecules 2022, 27, 3786. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.Y.; Zhang, B.-S.; Ren, L.-N.; Lu, Y.-P.; Tang, J.-W.; Lv, D.; Yong, L.; Lin, L.-T.; Lin, Z.-X.; et al. In vivo antiviral effect of plant essential oils against avian infectious bronchitis virus. BMC Vet. Res. 2022, 18, 90. [Google Scholar] [CrossRef]
- Parija, S.C. Introduction to Viruses. In Textbook of Microbiology and Immunology; Springer Nature: Singapore, 2023; pp. 687–713. [Google Scholar]
- Feberwee, A.; Ferguson-Noel, N.; Catania, S.; Bottinelli, M.; Wawagema, N.; Gyuranecz, M.; Gautier-Bouchardon, A.V.; Lysnyansky, I.; Wiegel, J.; Möller Palau-Ribes, F.; et al. Mycoplasma Gallisepticum and Mycoplasma Synoviae in Commercial Poultry: Current Control Strategies and Future Challenges. Avian Pathol. 2025, 54, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Luo, Y.; Li, C.; Yin, H.; Zhou, Q.; Cui, S.; Xu, M.; Zhang, H.; Qin, A.; Li, W. Transmission investigation of Mycoplasma synoviae in Chinese indigenous chickens. Front. Vet. Sci. 2025, 12, 1555604. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, T.G. Basic Bacteriology. In Veterinary Microbiology; McVey, D.S., Kennedy, M., Chengappa, M.M., Wilkes, R., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 11–28. [Google Scholar]
- Abd El-Hamid, M.I.; Awad, N.F.S.; Hashem, Y.M.; Abdel-Rahman, M.A.; Abdelaziz, A.M.; Modhammed, I.A.A.; Abo-Shama, U.H. In vitro evaluation of various antimicrobials against field mycoplasma gallisepticum and mycoplasma synoviae isolates in Egypt. Poult. Sci. 2019, 98, 6281–6288. [Google Scholar] [CrossRef] [PubMed]

| Experimental Groups | Results |
|---|---|
| Broth | Clear |
| Broth + bacterial suspension | Turbid |
| Disinfectant + Bacterial suspension | Clear |
| Neutralizing agent + Bacterial suspension | Turbid |
| (Disinfectant + Bacterial suspension) + Neutralizing agent | Clear |
| (Disinfectant + Neutralizing agent) + Bacterial suspension | Turbid |
| Bacteria Strains | Negative Control | Positive Control 0.000000 µg/mL | Concentrations of PEO Disinfectant | |||||
|---|---|---|---|---|---|---|---|---|
| 0.001875 µg/mL | 0.00375 µg/mL | 0.0075 µg/mL | 0.015 µg/mL | 0.03 µg/mL | 0.06 µg/mL | |||
| Escherichia coli (8099) | − | + | + | + | + | − | − | − |
| Escherichia coli (isolate) | − | + | + | − | − | − | − | − |
| Staphylococcus aureus (ATCC 6538) | − | + | + | − | − | − | − | − |
| Salmonella spp. (isolate) | − | + | + | + | + | + | − | − |
| Bacterial Strains | Negative Control | Positive Control 0.000000 µg/mL | Bactericidal Rates of PEO Disinfectant at Different Concentrations | df (Between Groups/ Within Groups) | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.001875 µg/mL | 0.00375 µg/mL | 0.0075 µg/mL | 0.015 µg/mL | 0.03 µg/mL | 0.06 µg/mL | ||||||
| Escherichia coli (8099) | − | 00.00% | 61.26% | 85.86% | 94.59% | 97.38% | 98.78% | 99.30% | 5/48 | 569.1 | <0.001 |
| Escherichia coli (isolate) | − | 00.00% | 77.23% | 89.30% | 94.97% | 98.83% | 99.20% | 99.63% | 5/48 | 621.5 | <0.001 |
| Staphylococcus aureus (ATCC 6538) | − | 00.00% | 61.26% | 95.03% | 95.72% | 96.58% | 98.63% | 99.83% | 5/48 | 712.3 | <0.001 |
| Salmonella spp. (isolate) | − | 00.00% | 69.22% | 78.97% | 88.97% | 89.74% | 97.86% | 98.47% | 5/48 | 598.2 | <0.001 |
| Bacterial Strains | Negative Control | Positive Control 0 min | Bactericidal Rates at Different Exposure Times | df (Between Groups/ Within Groups) | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | ||||||
| Escherichia coli (8099) | − | 00.00% | 84.11% | 91.56% | 97.55% | 97.90% | 98.78% | 99.48% | 5/48 | 426.4 | <0.001 |
| Escherichia coli (isolates) | − | 00.00% | 88.04% | 91.72% | 97.04% | 97.30% | 98.75% | 99.22% | 5/48 | 458.7 | <0.001 |
| Staphylococcus aureus (ATCC 6538) | − | 00.00% | 86.64% | 91.72% | 95.21% | 96.40% | 97.09% | 98.63% | 5/48 | 492.1 | <0.001 |
| Salmonella spp. (isolate) | − | 00.00% | 78.42% | 85.97% | 96.25% | 97.16% | 97.94% | 98.31% | 5/48 | 475.3 | <0.001 |
| Parallelism Degree | Different Concentrations of PEOs Disinfectant | ||||
|---|---|---|---|---|---|
| 0.00375 µg/mL | 0.0075 µg/mL | 0.015 µg/mL | 0.03 µg/mL | 0.06 µg/mL | |
| 1 | − | − | − | + | + |
| 2 | − | − | − | − | + |
| 3 | − | − | − | + | + |
| 4 | − | − | − | + | + |
| 5 | − | − | − | + | + |
| Mycoplasma Strains | Negative Control | Dilution of Viable Bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | 10−9 | 10−10 | ||
| MG | − | + | + | + | + | + | + | + | − | − | − |
| MS | − | + | + | + | + | + | + | + | − | − | − |
| Mycoplas-ma Strains | Negative Control | Positive Control | Concentrations of PEOs Disinfectant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000000 µg /mL | 0.48 µg /mL | 0.24 µg /mL | 0.12 µg /mL | 0.06 µg /mL | 0.03 µg /mL | 0.015 µg /mL | 0.0075 µg /mL | 0.00375 µg /mL | 0.001875 µg /mL | 0.000938 µg /mL | ||
| MG | − | + | − | − | − | − | − | − | − | − | + | + |
| MS | − | + | − | − | − | − | − | − | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, M.; Zhang, T.-N.; Lu, C.; Zhou, J.-X.; Yang, R.-W.; Dong, X.-M.; Zhang, C.-Y.; Wang, Q.; Zhao, W.-Q.; Zhang, Y.; et al. Remarkable Inhibition Efficacy of a Compound Plant Essential Oil Disinfectant Against Bacteria, Viruses, and Mycoplasmas. Vet. Sci. 2025, 12, 978. https://doi.org/10.3390/vetsci12100978
Guan M, Zhang T-N, Lu C, Zhou J-X, Yang R-W, Dong X-M, Zhang C-Y, Wang Q, Zhao W-Q, Zhang Y, et al. Remarkable Inhibition Efficacy of a Compound Plant Essential Oil Disinfectant Against Bacteria, Viruses, and Mycoplasmas. Veterinary Sciences. 2025; 12(10):978. https://doi.org/10.3390/vetsci12100978
Chicago/Turabian StyleGuan, Ming, Tao-Ni Zhang, Cheng Lu, Jin-Xin Zhou, Ri-Wang Yang, Xuan-Ming Dong, Cheng-Yu Zhang, Qi Wang, Wen-Qing Zhao, Yu Zhang, and et al. 2025. "Remarkable Inhibition Efficacy of a Compound Plant Essential Oil Disinfectant Against Bacteria, Viruses, and Mycoplasmas" Veterinary Sciences 12, no. 10: 978. https://doi.org/10.3390/vetsci12100978
APA StyleGuan, M., Zhang, T.-N., Lu, C., Zhou, J.-X., Yang, R.-W., Dong, X.-M., Zhang, C.-Y., Wang, Q., Zhao, W.-Q., Zhang, Y., Wei, T.-C., Huang, J.-N., Huang, T., & Mo, M.-L. (2025). Remarkable Inhibition Efficacy of a Compound Plant Essential Oil Disinfectant Against Bacteria, Viruses, and Mycoplasmas. Veterinary Sciences, 12(10), 978. https://doi.org/10.3390/vetsci12100978





