Effects of a Dietary Blend of Essential Oils, Capsaicin, and Yeast Metabolites on Performance, Physiological, Metabolism, and Immune Response of Heat-Stressed Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Experimental Design
2.2. Data Collection
2.2.1. Temperature–Humidity Index
2.2.2. Rectal Temperature Data Collection
2.2.3. Growth Performance and Body Composition Measurements
2.2.4. Blood Sampling Analysis
2.2.5. Statistical Analysis
3. Results
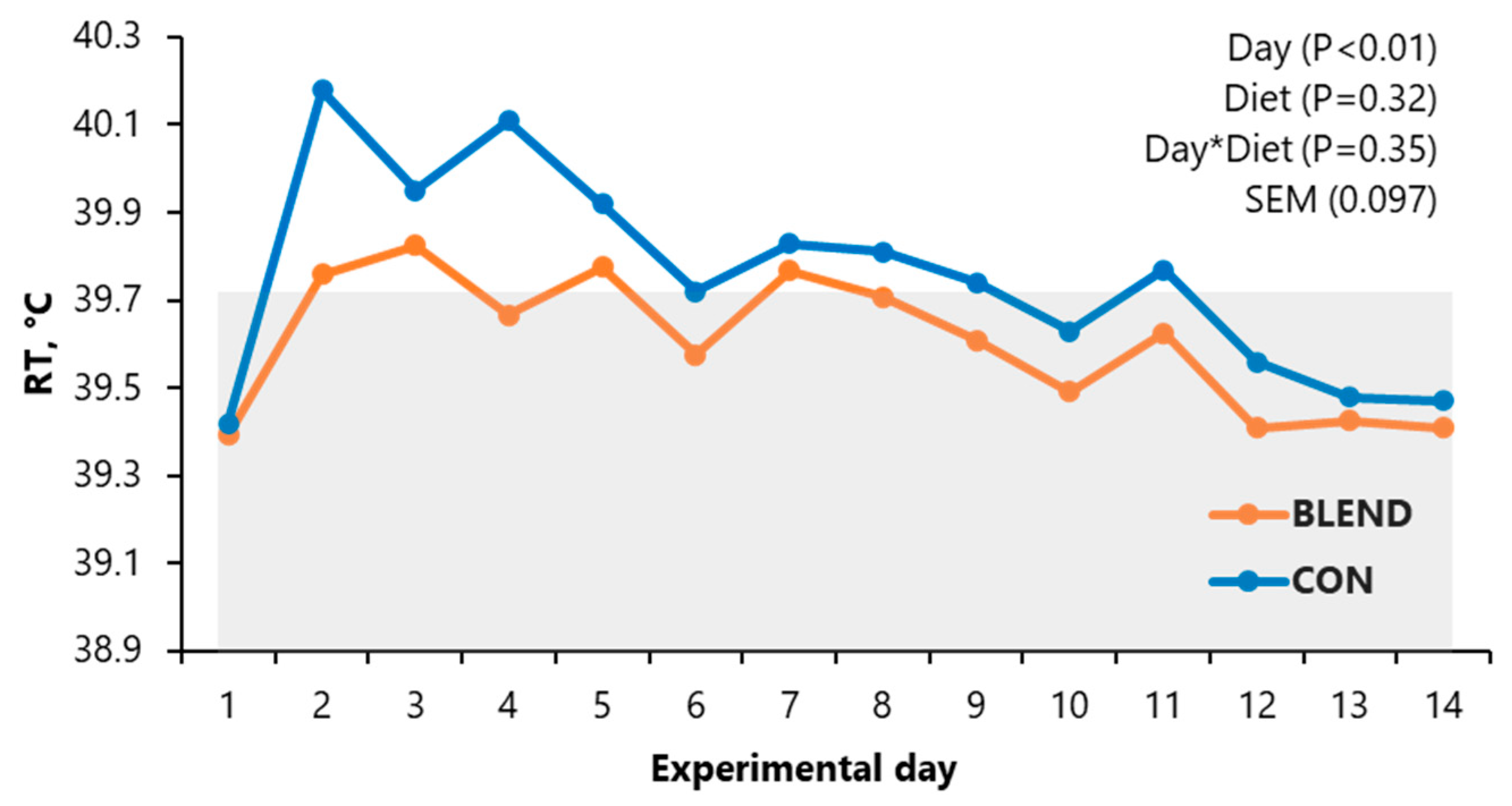
3.1. Rectal Temperature
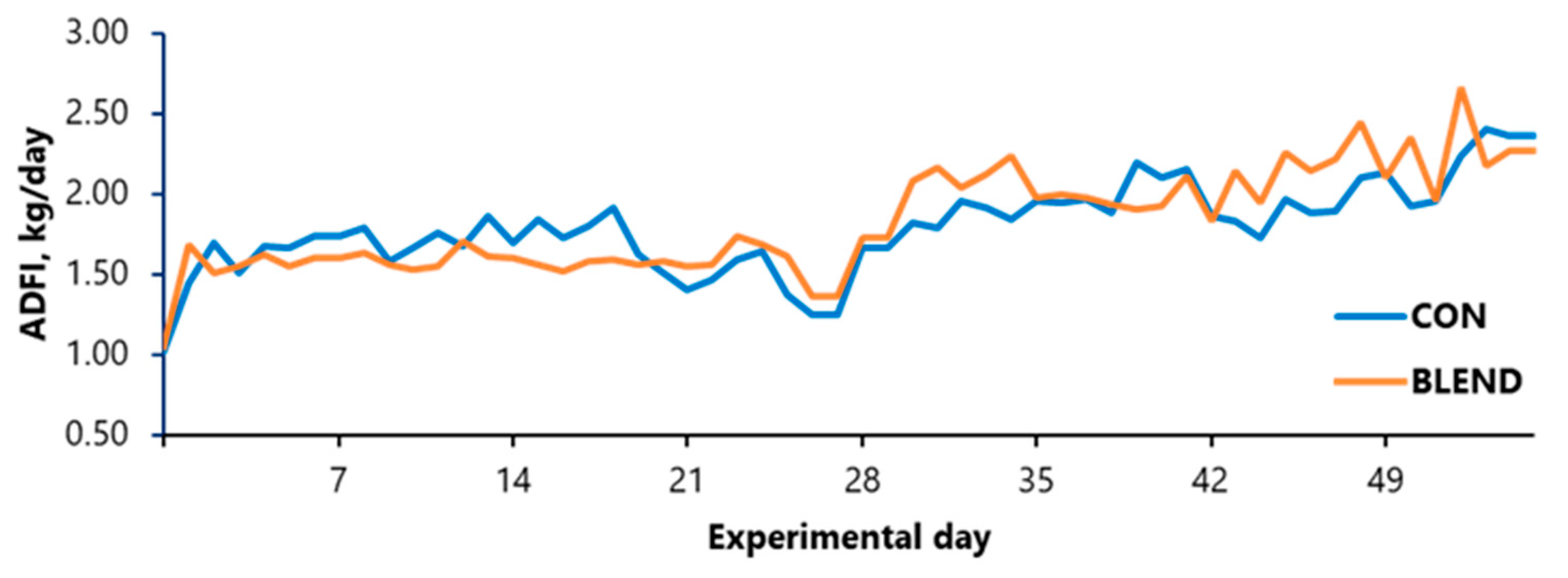
3.2. Growth Performance and Body Composition
3.3. Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADFI | Average Daily-feed Intake |
| ADG | Average Daily Gain |
| AIPF | Automatic and Intelligent Precision Feeders |
| APP | Acute-phase Proteins |
| BW | Body Weight |
| CON | Control |
| DXA | Dual-energy X-ray Absorptiometry |
| G:F | Gain-to-feed Ratio |
| HS | Heat Stress |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin B |
| LHD | Lactate Dehydrogenase |
| RT | Rectal Temperature |
| THI | Temperature–humidity Index |
| TNZ | Thermoneutral Zone |
References
- Adaptation to Hot Climate and Strategies to Alleviate Heat Stress in Livestock Production|Animal|Cambridge Core. Available online: https://www.cambridge.org/core/journals/animal/article/abs/adaptation-to-hot-climate-and-strategies-to-alleviate-heat-stress-in-livestock-production/45CDE339A49147C69DBF0D3EB438EB75 (accessed on 29 June 2025).
- Diurnal Heat Stress Reduces Pig Intestinal Integrity and Increases Endotoxin Translocation|Translational Animal Science|Oxford Academic. Available online: https://academic.oup.com/tas/article/2/1/1/4824980 (accessed on 29 June 2025).
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Barrier Function and Alters Intestinal Metabolism in Growing Pigs1. J. Anim. Sci. 2012, 90, 257–259. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic Heat Stress Induces Acute Phase Responses and Serum Metabolome Changes in Finishing Pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef]
- Pastorelli, H.; van Milgen, J.; Lovatto, P.; Montagne, L. Meta-Analysis of Feed Intake and Growth Responses of Growing Pigs after a Sanitary Challenge. Animal 2012, 6, 952–961. [Google Scholar] [CrossRef]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The Effects of Heat Stress and Plane of Nutrition on Metabolism in Growing Pigs1. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Jr, R.P.R. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Kouba, M.; Hermier, D.; Le Dividich, J. Influence of a High Ambient Temperature on Lipid Metabolism in the Growing Pig. J. Anim. Sci. 2001, 79, 81–87. [Google Scholar] [CrossRef]
- Liu, F.; Celi, P.; Cottrell, J.J.; Chauhan, S.S.; Leury, B.J.; Dunshea, F.R. Effects of a Short-Term Supranutritional Selenium Supplementation on Redox Balance, Physiology and Insulin-Related Metabolism in Heat-Stressed Pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 276–285. [Google Scholar] [CrossRef]
- Bellego, L.L.; van Milgen, J.; Noblet, J. Effect of High Ambient Temperature on Protein and Lipid Deposition and Energy Utilization in Growing Pigs. Anim. Sci. 2002, 75, 85–96. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of Essential Oil Supplementation of a Low-Energy Diet on Performance, Intestinal Morphology and Microflora, Immune Properties and Antioxidant Activities in Weaned Pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Li, T.S.; Kim, I.H. Effects of Essential Oils Supplementation in Different Nutrient Densities on Growth Performance, Nutrient Digestibility, Blood Characteristics and Fecal Microbial Shedding in Weaning Pigs. Anim. Feed Sci. Technol. 2016, 214, 77–85. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I. Effects of Feeding Diets Containing Essential Oils and Betaine to Heat-Stressed Growing-Finishing Pigs. Arch. Anim. Nutr. 2018, 72, 368–378. [Google Scholar] [CrossRef]
- Vohra, A.; Syal, P.; Madan, A. Probiotic Yeasts in Livestock Sector. Anim. Feed Sci. Technol. 2016, 219, 31–47. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and Yeast Derivatives in Feed Additives and Ingredients: Sources, Characteristics, Animal Responses, and Quantification Methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Dávila-Ramírez, J.L.; Carvajal-Nolazco, M.R.; López-Millanes, M.J.; González-Ríos, H.; Celaya-Michel, H.; Sosa-Castañeda, J.; Barrales-Heredia, S.M.; Moreno-Salazar, S.F.; Barrera-Silva, M.A. Effect of Yeast Culture (Saccharomyces cerevisiae) Supplementation on Growth Performance, Blood Metabolites, Carcass Traits, Quality, and Sensorial Traits of Meat from Pigs under Heat Stress. Anim. Feed Sci. Technol. 2020, 267, 114573. [Google Scholar] [CrossRef]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of Plant Extracts and Formic Acid on the Intestinal Equilibrium of Early-Weaned Pigs1. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef]
- Szolcsányi, J. Effect of Capsaicin on Thermoregulation: An Update with New Aspects. Temperature 2015, 2, 277–296. [Google Scholar] [CrossRef]
- Moraes, D.C.A.; Nagi, J.G.; Fritzen, J.; Vitagliano, L.A.; Oliveira, E.R.; Oba, A.; Silva, C.A. Effect of Capsaicin on the Feed Intake and Immunoglobin Concentration of Sows, and Performance of Piglets. Trop. Anim. Health Prod. 2022, 54, 241. [Google Scholar] [CrossRef]
- Liu, Y.; Che, T.M.; Song, M.; Lee, J.J.; Almeida, J.A.S.; Bravo, D.; Van Alstine, W.G.; Pettigrew, J.E. Dietary Plant Extracts Improve Immune Responses and Growth Efficiency of Pigs Experimentally Infected with Porcine Reproductive and Respiratory Syndrome Virus1. J. Anim. Sci. 2013, 91, 5668–5679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Pettigrew, J.E. Anti-Inflammatory Effects of Several Plant Extracts on Porcine Alveolar Macrophages in Vitro1. J. Anim. Sci. 2012, 90, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Pomar, J.; López, V.; Pomar, C. Agent-Based Simulation Framework for Virtual Prototyping of Advanced Livestock Precision Feeding Systems. Comput. Electron. Agric. 2011, 78, 88–97. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Ravagnolo, O.; Misztal, I. Genetic Component of Heat Stress in Dairy Cattle, Parameter Estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.; Rivest, J. The Effect of Body Position and Data Analysis on the Estimation of Body Composition of Pigs by Dual Energy X-Ray Absorptiometry (DEXA). In Proceedings of the 46th Annual Conference of the Canadian Society of Animal Science, Lethbridge, AB, Canada, 7–11 July 1996; p. 26. [Google Scholar]
- Ross, J.W.; Hale, B.J.; Gabler, N.K.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological Consequences of Heat Stress in Pigs. Anim. Prod. Sci. 2015, 55, 1381–1390. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Studio University Edition, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2020. Available online: https://welcome.oda.sas.com/ (accessed on 29 June 2025).
- da Fonseca de Oliveira, A.C.; Vanelli, K.; Sotomaior, C.S.; Weber, S.H.; Costa, L.B. Impacts on Performance of Growing-Finishing Pigs under Heat Stress Conditions: A Meta-Analysis. Vet. Res. Commun. 2019, 43, 37–43. [Google Scholar] [CrossRef]
- Quiniou, N.; Noblet, J.; van Milgen, J.; Dubois, S. Modelling Heat Production and Energy Balance in Group-Housed Growing Pigs Exposed to Low or High Ambient Temperatures. Br. J. Nutr. 2001, 85, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.K.; Roychoudhury, R.; Saharia, J.; Borah, M.C.; Dutta, D.J.; Bhuyan, R.; Kalita, D. Impact of Seasonal Thermal Stress on Physiological and Blood Biochemical Parameters in Pigs under Different Dietary Energy Levels. Trop. Anim. Health Prod. 2018, 50, 1025–1032. [Google Scholar] [CrossRef]
- Serviento, A.M.; Labussière, E.; Castex, M.; Renaudeau, D. Effect of Heat Stress and Feeding Management on Growth Performance and Physiological Responses of Finishing Pigs. J. Anim. Sci. 2020, 98, skaa387. [Google Scholar] [CrossRef]
- José Karpeggiane de Oliveira, M.; Diego Brandão Melo, A.; Alves Marçal, D.; Alves da Cunha Valini, G.; Alisson Silva, C.; Mari Veira, A.; Zem Fraga, A.; Righetti Arnaut, P.; Henrique Reis Furtado Campos, P.; Sousa dos Santos, L.; et al. Effects of Lowering Dietary Protein Content without or with Increased Protein-Bound and Feed-Grade Amino Acids Supply on Growth Performance, Body Composition, Metabolism, and Acute-Phase Protein of Finishing Pigs under Daily Cyclic Heat Stress. J. Anim. Sci. 2023, 101, skac387. [Google Scholar] [CrossRef]
- Renaudeau, D.; Anais, C.; Tel, L.; Gourdine, J.L. Effect of Temperature on Thermal Acclimation in Growing Pigs Estimated Using a Nonlinear Function1. J. Anim. Sci. 2010, 88, 3715–3724. [Google Scholar] [CrossRef]
- Quiniou, N.; Dubois, S.; Noblet, J. Voluntary Feed Intake and Feeding Behaviour of Group-Housed Growing Pigs Are Affected by Ambient Temperature and Body Weight. Livest. Prod. Sci. 2000, 63, 245–253. [Google Scholar] [CrossRef]
- Yan, L.; Meng, Q.W.; Kim, I.H. The Effect of an Herb Extract Mixture on Growth Performance, Nutrient Digestibility, Blood Characteristics and Fecal Noxious Gas Content in Growing Pigs. Livest. Sci. 2011, 141, 143–147. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Świątkiewicz, M.; Grela, E.R. Effect of Dietary Inclusion of a Herbal Extract Mixture and Different Oils on Pig Performance and Meat Quality. Meat Sci. 2015, 108, 61–66. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Kvidera, S.K.; Horst, E.A.; Al-Qaisi, M.; McCarthy, C.S.; Abeyta, M.A.; Lei, S.; Elsasser, T.H.; Kahl, S.; Kiros, T.G.; et al. Effects of Dietary Live Yeast Supplementation on Growth Performance and Biomarkers of Metabolism and Inflammation in Heat-Stressed and Nutrient-Restricted Pigs1. Transl. Anim. Sci. 2021, 5, txab072. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.J.K.; Polycarpo, G.V.; Andretta, I.; Melo, A.D.B.; Marçal, D.A.; Létourneau-Montminy, M.P.; Hauschild, L. Effect of Constant and Cyclic Heat Stress on Growth Performance, Water Intake, and Physiological Responses in Pigs: A Meta-Analysis. Anim. Feed Sci. Technol. 2024, 309, 115904. [Google Scholar] [CrossRef]
- Yunianto, V.D.; Hayashit, K.; Kaiwda, S.; Ohtsuka, A.; Tomita, Y. Effect of Environmental Temperature on Muscle Protein Turnover and Heat Production in Tube-Fed Broiler Chickens. Br. J. Nutr. 1997, 77, 897–909. [Google Scholar] [CrossRef]
- Cervantes, M.; Ibarra, N.; Vásquez, N.; Reyes, F.; Avelar, E.; Espinoza, S.; Morales, A. Serum Concentrations of Free Amino Acids in Growing Pigs Exposed to Diurnal Heat Stress Fluctuations. J. Therm. Biol. 2017, 69, 69–75. [Google Scholar] [CrossRef]
- Heat Stress Increases Insulin Sensitivity in Pigs—Sanz Fernandez—2015—Physiological Reports—Wiley Online Library. Available online: https://physoc.onlinelibrary.wiley.com/doi/full/10.14814/phy2.12478 (accessed on 29 June 2025).
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal Barrier Integrity and Favors Intestinal Glucose Transport in Growing Pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Vásquez, N.; Cervantes, M.; Bernal-Barragán, H.; Rodríguez-Tovar, L.E.; Morales, A. Short- and Long-Term Exposure to Heat Stress Differently Affect Performance, Blood Parameters, and Integrity of Intestinal Epithelia of Growing Pigs. Animals 2022, 12, 2529. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat Stress Effects on Livestock: Molecular, Cellular and Metabolic Aspects, a Review. Anim. Physiol. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.S.; Pomar, C.; Campos, P.H.R.F.; da Silva, W.C.; Gobi, J.d.P.; Veira, A.M.; Fraga, A.Z.; Hauschild, L. Precision Feeding Strategy for Growing Pigs under Heat Stress Conditions1. J. Anim. Sci. 2018, 96, 4789–4801. [Google Scholar] [CrossRef] [PubMed]
- Keshaviah, P.R.; Nolph, K.D.; Moore, H.L.; Prowant, B.; Emerson, P.F.; Meyer, M.; Twardowski, Z.J.; Khanna, R.; Ponferrada, L.; Collins, A. Lean Body Mass Estimation by Creatinine Kinetics. J. Am. Soc. Nephrol. 1994, 4, 1475. [Google Scholar] [CrossRef]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The Metabolic Burden of Creatine Synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Riesberg, L.A.; Weed, S.A.; McDonald, T.L.; Eckerson, J.M.; Drescher, K.M. Beyond Muscles: The Untapped Potential of Creatine. Int. Immunopharmacol. 2016, 37, 31–42. [Google Scholar] [CrossRef]
- James, B.W.; Goodband, R.D.; Unruh, J.A.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S.; O’Quinn, P.R.; Andrews, B.S. Effect of Creatine Monohydrate on Finishing Pig Growth Performance, Carcass Characteristics and Meat Quality. Anim. Feed Sci. Technol. 2002, 96, 135–145. [Google Scholar] [CrossRef]
- Adejumo, D.O.; Egbunike, G.N. Effect of Thermal Stress and Water Deprivation on the Acetylcholinesterase Activity of the Pig Brain and Hypophyses. Int. J. Biometeorol. 1988, 32, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.M.; Lucke, J.N.; Lovell, R.; Lister, D. Porcine malignant hyperthermia. VII: Hepatic metabolism. Br. J. Anaesth. 1980, 52, 11–17. [Google Scholar] [CrossRef]
- Yaspelkis, B.B.; Scroop, G.C.; Wilmore, K.M.; Ivy, J.L. Carbohydrate Metabolism during Exercise in Hot and Thermoneutral Environments. Int. J. Sports Med. 2008, 14, 13–19. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Metabolism: Basic Concepts and Design. In Biochesmistry, 6th ed.; Freeman WH and Co.: New York, NY, USA, 2007. [Google Scholar]
- Kvidera, S.K.; Horst, E.A.; Sanz Fernandez, M.V.; Abuajamieh, M.; Ganesan, S.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; Trout, W.E.; Keating, A.F.; et al. Characterizing Effects of Feed Restriction and Glucagon-like Peptide 2 Administration on Biomarkers of Inflammation and Intestinal Morphology. J. Dairy Sci. 2017, 100, 9402–9417. [Google Scholar] [CrossRef]
- Campos, P.H.R.F.; Noblet, J.; Jaguelin-Peyraud, Y.; Gilbert, H.; Mormède, P.; de Oliveira Donzele, R.F.M.; Donzele, J.L.; Renaudeau, D. Thermoregulatory Responses during Thermal Acclimation in Pigs Divergently Selected for Residual Feed Intake. Int. J. Biometeorol. 2014, 58, 1545–1557. [Google Scholar] [CrossRef]
- dos Santos, L.S.; Campos, P.H.R.F.; da Silva, W.C.; Veira, A.M.; Fraga, A.Z.; Caetano, R.P.; Hauschild, L. Corrigendum to: Performance and Carcass Composition of Pigs from Two Sire Lines Are Affected Differently by Ambient Temperature. Anim. Prod. Sci. 2021, 61, 620. [Google Scholar] [CrossRef]
- Zsila, F. Chaperone-like Activity of the Acute-Phase Component Human Serum A1-Acid Glycoprotein: Inhibition of Thermal- and Chemical-Induced Aggregation of Various Proteins. Bioorganic Med. Chem. Lett. 2010, 20, 1205–1209. [Google Scholar] [CrossRef]
- Machado-Neto, R.; Graves, C.N.; Curtis, S.E. Immunoglobulins in Piglets from Sows Heat-Stressed Prepartum. J. Anim. Sci. 1987, 65, 445–455. [Google Scholar] [CrossRef]
- Kroscher, K.A.; Fausnacht, D.W.; McMillan, R.P.; El-Kadi, S.W.; Wall, E.H.; Bravo, D.M.; Rhoads, R.P. Supplementation with Artificial Sweetener and Capsaicin Alters Metabolic Flexibility and Performance in Heat-Stressed and Feed-Restricted Pigs. J. Anim. Sci. 2022, 100, skac195. [Google Scholar] [CrossRef] [PubMed]
- Labussière, E.; Achard, C.; Dubois, S.; Combes, S.; Castex, M.; Renaudeau, D. Saccharomyces Cerevisiae Boulardii CNCM I-1079 Supplementation in Finishing Male Pigs Helps to Cope with Heat Stress through Feeding Behaviour and Gut Microbiota Modulation. Br. J. Nutr. 2022, 127, 353–368. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Furness, J.B.; Wijesiriwardana, U.A.; Ringuet, M.; Liu, F.; DiGiacomo, K.; Leury, B.J.; Clarke, I.J.; Dunshea, F.R. The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs. Animals 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.E.; Kroscher, K.A.; Zhao, L.D.; Zhang, Z.; Wall, E.H.; Bravo, D.M.; Rhoads, R.P. Dietary Supplementation of Artificial Sweetener and Capsicum Oleoresin as a Strategy to Mitigate the Negative Consequences of Heat Stress on Pig Performance. J. Anim. Sci. 2020, 98, skaa131. [Google Scholar] [CrossRef] [PubMed]
| Diets | ||||
|---|---|---|---|---|
| Phase 1 | Phase 2 | |||
| Items | CON | BLEND | CON | BLEND |
| Ingredients, % | ||||
| Corn | 73.62 | 73.62 | 79.30 | 79.30 |
| Soybean meal | 22.55 | 22.55 | 16.54 | 16.54 |
| Dicalcium phosphate | 1.18 | 1.18 | 1.06 | 1.06 |
| Limestone | 0.73 | 0.73 | 0.71 | 0.71 |
| Salt | 0.25 | 0.25 | 0.22 | 0.22 |
| L-Lysine, 60% | 0.32 | 0.32 | 0.36 | 0.36 |
| DL-Methionine, 99% | 0.03 | 0.03 | 0.02 | 0.02 |
| L-Threonine, 98.5% | 0.01 | 0.01 | 0.30 | 0.30 |
| L-Tryptophan, 98% | - | - | 0.01 | 0.01 |
| L-Valine, 96.5% | - | - | - | - |
| Choline chloride, 60% | 0.06 | 0.06 | 0.06 | 0.06 |
| Premix mineral/vitamin 1 | 0.25 | 0.25 | 0.25 | 0.25 |
| Dextrine | 0.50 | 0.50 | 0.50 | 0.50 |
| Soybean oil | 0.25 | 0.25 | 0.54 | 0.54 |
| Inert | 0.25 | - | 0.25 | - |
| Feed additive 2 | - | 0.25 | - | 0.25 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutritional composition | ||||
| Net Energy, Kcal/kg | 2475 | 2475 | 2525 | 2525 |
| Crude protein, % | 16.56 | 16.56 | 14.27 | 14.27 |
| SID Lys 3, % | 0.90 | 0.90 | 0.78 | 0.78 |
| SID Met, % | 0.26 | 0.26 | 0.23 | 0.23 |
| SID Met + cys, % | 0.51 | 0.51 | 0.45 | 0.45 |
| SID Thp, % | 0.54 | 0.54 | 0.48 | 0.48 |
| SID Trp, % | 0.17 | 0.17 | 0.15 | 0.15 |
| SID Ile, % | 0.61 | 0.61 | 0.51 | 0.51 |
| SID Leu, % | 1.35 | 1.35 | 1.20 | 1.20 |
| SID Val, % | 0.69 | 0.69 | 0.59 | 0.59 |
| SID Arg, % | 0.97 | 0.97 | 0.80 | 0.80 |
| SID Phe, % | 1.28 | 1.28 | 1.08 | 1.08 |
| SID His, % | 0.40 | 0.40 | 0.35 | 0.35 |
| Ca, % | 0.66 | 0.66 | 0.60 | 0.60 |
| Na, % | 0.11 | 0.11 | 0.10 | 0.10 |
| Available p, % | 0.31 | 0.31 | 0.28 | 0.28 |
| Diets 1 | ||||
|---|---|---|---|---|
| Items | CON | BLEND | SEM 2 | p-Value |
| Initial conditions | ||||
| Initial BW, kg | 51.1 | 51.1 | 2.48 | 0.964 |
| Initial body protein, kg | 8.35 | 8.39 | 0.43 | 0.780 |
| Initial body lipid, kg | 8.03 | 7.82 | 0.54 | 0.509 |
| Phase 1 (0 to 28 days)—Performance and final conditions | ||||
| ADFI, kg | 1.62 | 1.53 | 0.11 | 0.505 |
| ADG, kg | 0.70 | 0.65 | 0.04 | 0.097 |
| G:F | 0.44 | 0.43 | 0.02 | 0.890 |
| Protein deposition, g/d | 121.2 | 115.9 | 7.17 | 0.397 |
| Protein deposition, % ADG | 17.5 | 17.9 | 0.52 | 0.430 |
| Lipid deposition, g/d | 120.7 | 96.2 | 20.86 | 0.168 |
| Lipid deposition, % ADG | 16.9 | 14.8 | 2.39 | 0.388 |
| Final BW, kg | 70.6 | 69.2 | 3.71 | 0.119 |
| Final body protein, kg | 11.74 | 11.64 | 0.61 | 0.587 |
| Final body lipid, kg | 11.43 | 10.51 | 1.07 | 0.123 |
| Phase 2 (28 to 56 days)—Performance and final conditions | ||||
| ADFI, kg | 1.92 | 2.09 | 0.11 | 0.260 |
| ADG, kg | 0.77 | 0.78 | 0.02 | 0.862 |
| G:F | 0.41 | 0.38 | 0.02 | 0.394 |
| Protein deposition, g/d | 120.5 | 128.4 | 5.44 | 0.296 |
| Protein deposition, % ADG | 16.1 | 17.0 | 0.48 | 0.175 |
| Lipid deposition, g/d | 175.6 | 146.4 | 16.72 | 0.174 |
| Lipid deposition, % ADG | 23.5 | 19.7 | 2.20 | 0.206 |
| Final BW, kg | 91.5 | 90.2 | 4.11 | 0.287 |
| Final body protein, kg | 15.12 | 15.23 | 0.63 | 0.686 |
| Final body lipid, kg | 16.35 | 14.61 | 1.45 | 0.100 |
| Overall (0 to 56 days) | ||||
| ADFI, kg | 1.76 | 1.79 | 0.11 | 0.763 |
| ADG, kg | 0.72 | 0.70 | 0.03 | 0.338 |
| G:F | 0.42 | 0.40 | 0.02 | 0.533 |
| Protein deposition, g/d | 120.9 | 122.1 | 4.34 | 0.798 |
| Protein deposition, % ADG | 16.8 | 17.5 | 0.41 | 0.125 |
| Lipid deposition, g/d | 148.3 | 121.3 | 17.18 | 0.094 |
| Lipid deposition, % ADG | 20.4 | 17.2 | 1.90 | 0.141 |
| Diets 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Items | CON | BLEND | Mean (Day) | SEM 2 | Day | Diet | Day × Diet |
| Total protein, g/dL | |||||||
| Day 0 | 6.60 | 6.61 | 6.60 a | 0.173 | <0.001 | 0.855 | 0.421 |
| Day 7 | 3.56 | 3.34 | 3.45 c | ||||
| Day 28 | 5.80 | 6.14 | 5.97 b | ||||
| Mean (Diet) | 5.32 | 5.37 | |||||
| Urea, mg/dL | |||||||
| Day 0 | 20.06 | 20.17 | 20.11 a | 0.980 | <0.001 | 0.748 | 0.584 |
| Day 7 | 13.56 | 11.78 | 12.67 c | ||||
| Day 28 | 17.11 | 17.28 | 17.19 b | ||||
| Mean (Diet) | 16.91 | 16.41 | |||||
| Creatinine, mg/dL | |||||||
| Day 0 | 1.70 | 1.92 | 1.81 b | 0.053 | <0.001 | 0.032 | 0.402 |
| Day 7 | 1.23 | 1.25 | 1.24 c | ||||
| Day 28 | 1.95 | 2.12 | 2.04 a | ||||
| Mean (Diet) | 1.63 | 1.76 | |||||
| Glucose, mg/dL | |||||||
| Day 0 | 73.60 | 79.67 | 76.63 b | 2.742 | <0.001 | 0.266 | 0.314 |
| Day 7 | 82.30 | 90.17 | 86.23 a | ||||
| Day 28 | 54.20 | 50.94 | 52.57 c | ||||
| Mean (Diet) | 70.03 | 73.59 | |||||
| LDH, U/L | |||||||
| Day 0 | 1131.15 | 1324.78 | 1227.96 a | 46.182 | <0.001 | 0.075 | 0.218 |
| Day 7 | 691.40 | 719.06 | 705.23 c | ||||
| Day 28 | 994.95 | 1150.78 | 1072.86 b | ||||
| Mean (Diet) | 939.17 | 1064.87 | |||||
| Lactate, mg/dL | |||||||
| Day 0 | 20.90 | 24.94 | 22.92 a | 2.089 | <0.001 | 0.758 | 0.409 |
| Day 7 | 23.15 | 24.81 | 23.98 a | ||||
| Day 28 | 12.70 | 9.69 | 11.19 b | ||||
| Mean (Diet) | 18.92 | 19.81 | |||||
| Triglycerides, mg/dL | |||||||
| Day 0 | 35.80 | 37.28 | 36.54 a | 1.724 | <0.001 | 0.879 | 0.911 |
| Day 7 | 15.05 | 14.94 | 15.00 b | ||||
| Day 28 | 17.85 | 17.50 | 17.68 b | ||||
| Mean (Diet) | 22.90 | 23.24 | |||||
| Diet 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Items | CON | BLEND | Mean (Day) | SEM 2 | Day | Diet | Day × Diet |
| Immunoglobulin A, mg/mL | |||||||
| Day 0 | 1.28 | 1.33 | 1.30 a | 0.070 | <0.001 | 0.679 | 0.987 |
| Day 7 | 0.78 | 0.82 | 0.81 b | ||||
| Day 28 | 1.31 | 1.36 | 1.33 a | ||||
| Mean (Diet) | 1.12 | 1.16 | |||||
| Immunoglobulin G, mg/mL | |||||||
| Day 0 | 13.61 | 12.51 | 13.06 b | 0.886 | <0.001 | 0.605 | 0.595 |
| Day 7 | 8.99 | 7.56 | 8.27 c | ||||
| Day 28 | 15.24 | 15.38 | 15.31 a | ||||
| Mean (Diet) | 12.61 | 11.82 | |||||
| Albumin, mg/mL | |||||||
| Day 0 | 38.88 | 39.63 | 39.25 a | 0.812 | <0.001 | 0.597 | 0.39 |
| Day 7 | 18.88 | 17.89 | 18.38 c | ||||
| Day 28 | 31.23 | 33.20 | 32.21 b | ||||
| Mean (Diet) | 29.66 | 30.24 | |||||
| Haptoglobin, mg/dL | |||||||
| Day 0 | 0.40 | 0.40 | 0.40 a | 0.051 | <0.001 | 0.777 | 0.568 |
| Day 7 | 0.22 | 0.12 | 0.17 b | ||||
| Day 28 | 0.38 | 0.41 | 0.40 a | ||||
| Mean (Diet) | 0.33 | 0.31 | |||||
| α-1 Acid glycoprotein, µg/mL | |||||||
| Day 0 | 35.70 | 37.99 | 36.84 b | 3.552 | <0.001 | 0.964 | 0.889 |
| Day 7 | 33.39 | 31.77 | 32.58 b | ||||
| Day 28 | 49.43 | 49.50 | 49.46 a | ||||
| Mean (Diet) | 39.51 | 39.75 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, L.D.; Marçal, D.A.; França, I.; Silva, C.A.; Veira, A.M.; Oliveira, A.F.; Fraga, A.Z.; de Araujo, R.C.; Maia, A.S.C.; Hauschild, L. Effects of a Dietary Blend of Essential Oils, Capsaicin, and Yeast Metabolites on Performance, Physiological, Metabolism, and Immune Response of Heat-Stressed Pigs. Vet. Sci. 2025, 12, 976. https://doi.org/10.3390/vetsci12100976
Campos LD, Marçal DA, França I, Silva CA, Veira AM, Oliveira AF, Fraga AZ, de Araujo RC, Maia ASC, Hauschild L. Effects of a Dietary Blend of Essential Oils, Capsaicin, and Yeast Metabolites on Performance, Physiological, Metabolism, and Immune Response of Heat-Stressed Pigs. Veterinary Sciences. 2025; 12(10):976. https://doi.org/10.3390/vetsci12100976
Chicago/Turabian StyleCampos, Lorena Duarte, Danilo Alves Marçal, Ismael França, Cleslei Alisson Silva, Alini Mari Veira, Amanda Faria Oliveira, Alícia Zem Fraga, Rafael C. de Araujo, Alex Sandro Campos Maia, and Luciano Hauschild. 2025. "Effects of a Dietary Blend of Essential Oils, Capsaicin, and Yeast Metabolites on Performance, Physiological, Metabolism, and Immune Response of Heat-Stressed Pigs" Veterinary Sciences 12, no. 10: 976. https://doi.org/10.3390/vetsci12100976
APA StyleCampos, L. D., Marçal, D. A., França, I., Silva, C. A., Veira, A. M., Oliveira, A. F., Fraga, A. Z., de Araujo, R. C., Maia, A. S. C., & Hauschild, L. (2025). Effects of a Dietary Blend of Essential Oils, Capsaicin, and Yeast Metabolites on Performance, Physiological, Metabolism, and Immune Response of Heat-Stressed Pigs. Veterinary Sciences, 12(10), 976. https://doi.org/10.3390/vetsci12100976







