Presence of Resistant Enterobacteriaceae in Poultry and Synanthropic Birds of an Urban Context of Social Farming in Southern Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Bacterial Isolation and Identification
2.3. Antibiotic Susceptibility Testing
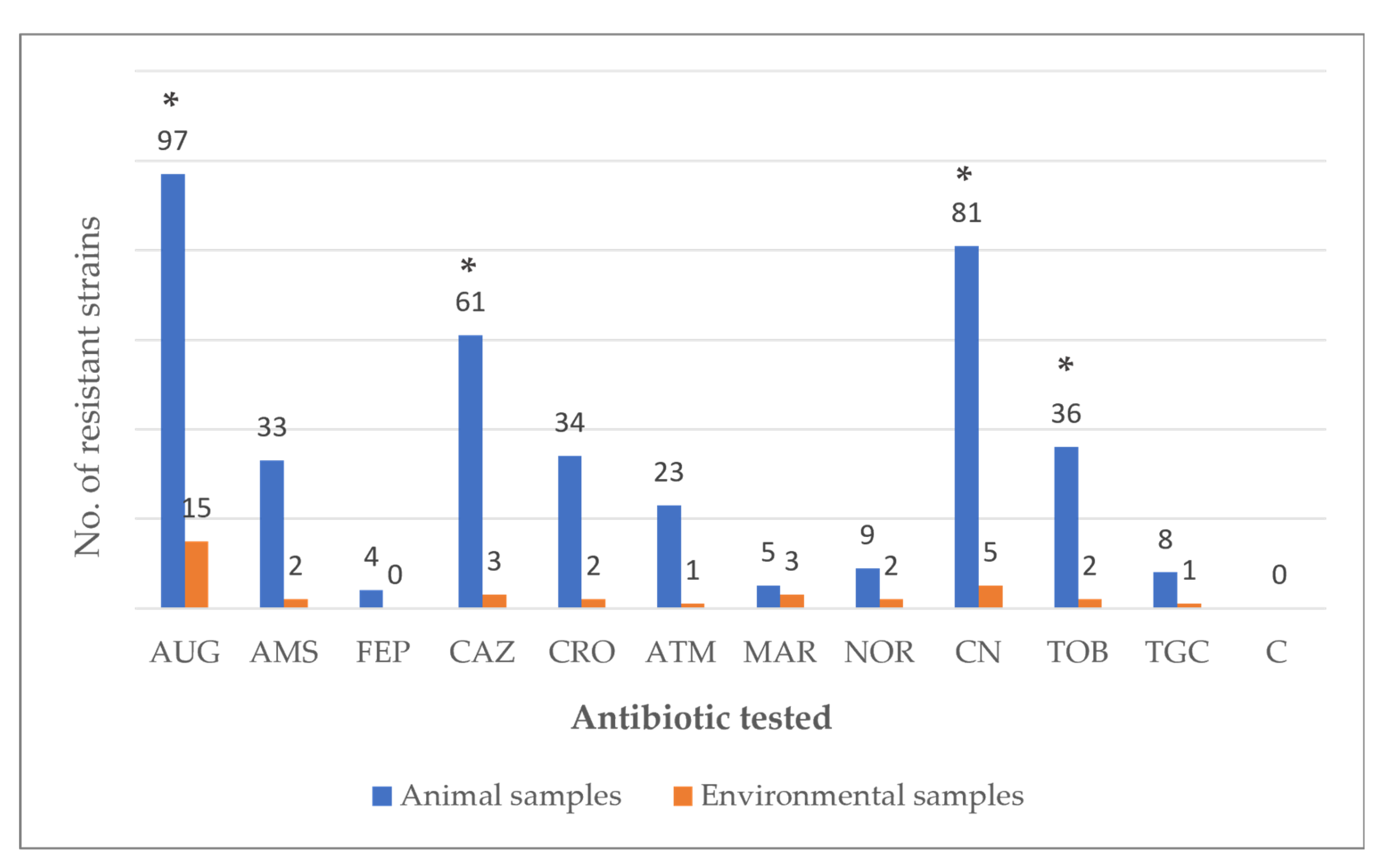
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cano-Verdugo, G.; Flores-García, B.D.; Núñez-Rocha, G.M.; Ávila-Ortíz, M.N.; Nakagoshi-Cepeda, M.A.A. Impact of urban farming on health: A systematic review. J. Public Health 2024, 25, e500–e509. [Google Scholar] [CrossRef] [PubMed]
- Galardi, M.; Filugelli, L.; Moruzzo, R.; Riccioli, F.; Mutinelli, F.; Espinosa Diaz, S.; Contalbrigo, L. Challenges and Perspectives of Social Farming in North-Eastern Italy: The Farmers’ View. Sustainability 2022, 14, 8390. [Google Scholar] [CrossRef]
- Paffarini, C.; Torquati, B.; Sannipoli, M.; Fabbri, A.; Cecchini, L. Social farming and educational needs: How kindergarten farms could fill a gap. Agric. Econ. 2024, 12, 17. [Google Scholar] [CrossRef]
- Göttling, J.; Heckel, J.O.; Hotzel, H.; Fruth, A.; Pfeifer, Y.; Henning, K.; Kopp, P.; Mertens-Scholz, K.; Rietschel, W.; Pfeffer, M. Zoonotic bacteria in clinically healthy goats in petting zoo settings of zoological gardens in Germany. Zoonoses Public Health 2022, 69, 333–343. [Google Scholar] [CrossRef]
- Weese, J.S.; McCarthy, L.; Mossop, M.; Martin, H.; Lefebvre, S. Observation of Practices at Petting Zoos and the Potential Impact on Zoonotic Disease Transmission. Clin. Infect. Dis. 2007, 45, 10–15. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Neumann, N.F.; Munns, K.; Tymensen, L.; Jokinen, C.; McAllister, T.A. Zoonotic Fecal Pathogens and Antimicrobial Resistance in Canadian Petting Zoos. Microorganisms 2018, 6, 70. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Callaway, T.; McAllister, T. Farm Fairs and Petting Zoos: A Review of Animal Contact as a Source of Zoonotic Enteric Disease. Foodborne Pathog. Dis. 2017, 14, 59–73. [Google Scholar] [CrossRef]
- Isler, M.; Wissmann, R.; Morach, M.; Zurfluh, K.; Stephan, R.; Nüesch-Inderbinen, M. Animal petting zoos as sources of Shiga toxin-producing Escherichia coli, Salmonella and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Zoonoses Public Health 2021, 68, 79–87. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Florez-Cuadrado, D.; Moreno, M.A.; Ugarte-Ruíz, M.; Domínguez, L. Antimicrobial resistance in the food chain in the European Union. Adv. Food Nutr. Res. 2018, 86, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Shnaiderman-Torban, A.; Steinman, A.; Meidan, G.; Paitan, Y.; Abu Amad, W.; Navon-Venezia, S. Petting Zoo Animals as an Emerging Reservoir of Extended-Spectrum β-Lactamase and AmpC-Producing Enterobacteriaceae. Front. Microbiol. 2019, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Kunert-Filho, H.C.; Simoni, C.; de Moraes, L.B.; Furian, T.Q.; Borges, K.A.; Breunig, J.G.; Medeiros, L.P.; Kobayashi, R.K.T.; de Brito, K.C.T.; et al. Antimicrobial susceptibility and detection of virulence-associated genes of Escherichia coli and Salmonella spp. isolated from domestic pigeons (Columba livia) in Brazil. Folia Microbiol. 2020, 65, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.A.; Cardozo, M.V.; Beraldo, L.G.; Oliveira, E.S.; Maluta, R.P.; Barboza, K.B.; Werther, K.; Ávila, F.A. Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol. 2017, 55, 344–348. [Google Scholar] [CrossRef]
- Gargiulo, A.; Russo, T.P.; Schettini, R.; Mallardo, K.; Calabria, M.; Menna, L.F.; Raia, P.; Pagnini, U.; Caputo, V.; Fioretti, A.; et al. Occurrence of enteropathogenic bacteria in urban pigeons (Columba livia) in Italy. Vector Borne Zoonotic Dis. 2014, 14, 251–255. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Disk Diffusion Method for Antimicrobial Susceptibility Testing. Version 13.0. January 2025. Available online: https://www.eucast.org (accessed on 1 September 2025).
- WHO’s List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org (accessed on 1 September 2025).
- Khatri, B.; Basnyat, S.; Karki, A.; Poudel, A.; Shrestha, B. Etiology and antimicrobial susceptibility pattern of bacterial pathogens from urinary tract infection. Nepal. Med. Coll. J. 2012, 14, 129–132. [Google Scholar]
- Adorján, A.; Thuma, Á.; Könyves, L.; Tóth, I. First isolation of atypical enteropathogenic Escherichia coli from geese (Anser anser domestica) and first description of atypical EPEC from turkeys and pigeons in Hungary. BMC Vet. Res. 2021, 17, 263. [Google Scholar] [CrossRef]
- Neog, N.; Phukan, U.; Puzari, M.; Sharma, M.; Chetia, P. Klebsiella oxytoca and Emerging Nosocomial Infections. Curr. Microbiol. 2021, 78, 1115–1123. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella Pneumoniae: An Increasing Threat to Public Health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Navidinia, M. The Clinical Importance of Emerging ESKAPE Pathogens in Nosocomial Infections. Arch. Adv. Biosci. 2016, 7, 43–57. [Google Scholar] [CrossRef]
- Russo, T.P.; Minichino, A.; Gargiulo, A.; Varriale, L.; Borrelli, L.; Pace, A.; Santaniello, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and Phenotypic Antimicrobial Resistance among ESKAPE Bacteria and Enterobacterales Strains in Wild Birds. Antibiotics 2022, 11, 1825. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Ochieng, D.J.; Levy, S.B. Commensals: Underappreciated reservoir of antibiotic resistance probing the role of commensals in propagating antibiotic resistance should help preserve the efficacy of these critical drugs. Microbe Mag. 2009, 4, 231–238. [Google Scholar]
- EFSA (European Food Safety Authority); ECDC (European Centre for DiseasePrevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018, 16, 5182. [Google Scholar] [CrossRef] [PubMed]
- Ohene Larbi, R.; Ofori, L.A.; Sylverken, A.A.; Ayim-Akonor, M.; Obiri-Danso, K. Antimicrobial resistance of Escherichia coli from broilers, pigs, and cattle in the Greater Kumasi Metropolis, Ghana. Int. J. Microbiol. 2021, 2021, 5158185. [Google Scholar] [CrossRef]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 8, 1181–1197. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.; Okolocha, E.; Harden, L.; Hull, D.; Hendriksen, R.S.; Thakur, S. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook 2020, 27, 8. [Google Scholar] [CrossRef]
- Oteo, J.; Campos, J.; Lázaro, E.; Cuevas, O.; García-Cobos, S.; Pérez-Vázquez, M.; de Abajo, F.J.; Spanish Members of EARSS. Increased amoxicillin-clavulanic acid resistance in Escherichia coli blood isolates, Spain. Emerg. Infect. Dis. 2008, 14, 1259–1262. [Google Scholar] [CrossRef]
- Tang, B.; Wang, J.; Zheng, X.; Chang, J.; Ma, J.; Wang, J.; Ji, X.; Yang, H.; Ding, B. Antimicrobial resistance surveillance of Escherichia coli from chickens in the Qinghai Plateau of China. Front. Microbiol. 2022, 13, 885132. [Google Scholar] [CrossRef]
- Borges, C.A.; Maluta, R.P.; Beraldo, L.G.; Cardozo, M.V.; Guastalli, E.A.L.; Kariyawasam, S.; DebRoy, C.; Ávila, F.A. Captive and free-living urban pigeons (Columba livia) from Brazil as carriers of multidrug-resistant pathogenic Escherichia coli. Vet. J. 2017, 219, 65–67. [Google Scholar] [CrossRef]
- Freire, S.; Grilo, T.; Poirel, L.; Aires-de-Sousa, M. Urban Pigeons (Columba livia) as a Source of Broad-Spectrum β-Lactamase- Producing Escherichia coli in Lisbon, Portugal. Antibiotics 2022, 11, 1368. [Google Scholar] [CrossRef]
- Mukerji, S.; Gunasekera, S.; Dunlop, J.N.; Stegger, M.; Jordan, D.; Laird, T.; Abraham, R.J.; Barton, M.; O’Dea, M.; Abraham, S. Implications of Foraging and Interspecies Interactions of Birds for Carriage of Escherichia coli Strains Resistant to Critically Important Antimicrobials. Appl. Environ. Microbiol. 2020, 86, e01610-20. [Google Scholar] [CrossRef]
| Animal Species | No. of Sampled Animals |
|---|---|
| Anser anser | 8 |
| Anser cygnoides | 4 |
| Cairina moschata | 16 |
| Columba livia | 20 |
| Gallus gallus domesticus | 28 |
| Subtotal | 76 |
| Environmental Surfaces | No. of sampled surfaces |
| Benches | 4 |
| Drinking trough | 2 |
| Feeders | 2 |
| Fences | 2 |
| Logs | 4 |
| Tables | 2 |
| Subtotal | 16 |
| Grand Total | 92 |
| Animal Species | Citrobacter spp. | Enterobacter spp. | E. coli | K. oxytoca | K. pneumoniae |
|---|---|---|---|---|---|
| A. anser | - | - | 25% (2/8) | - | - |
| A. cygnoides | - | - | 50% (2/4) | - | 25% (1/4) |
| C. livia | 10% (2/20) | 25% (5/20) | 85% (17/20) | - | 45% (9/20) |
| C. moschata | - | - | 68.8% (11/16) | 37.5% (6/16) | 43.8% (7/16) |
| G. gallus domesticus | - | - | 85.7% (24/28) | - | 39.3% (11/28) |
| Subtotal | 2.6% (2/76) | 6.6% (5/76) | 73.7% (56/76) | 7.9% (6/76) | 36.8% (28/76) |
| Environmental surfaces | |||||
| Benches | - | - | 50% (2/4) | - | 25% (1/4) |
| Drinking trough | 50% (1/2) | - | - | - | - |
| Feeders | - | 100% (2/2) | 50% (1/2) | - | - |
| Fences | - | - | 100% (2/2) | 50% (1/2) | - |
| Logs | - | - | 75% (3/4) | 50% (2/4) | 50% (2/4) |
| Tables | - | - | 50% (1/2) | - | 50% (1/2) |
| Subtotal | 6.3% (1/16) | 12.5% (2/16) | 56.3% (9/16) | 18.8% (3/16) | 25% (4/16) |
| Grand Total | 3.3% (3/92) | 7.6% (7/92) | 70.7% (65/92) | 9.8% (9/92) | 34.8% (32/92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, A.; Longobardi, M.; Russo, T.P.; Borrelli, L.; Fioretti, A.; Dipineto, L.; Santaniello, A. Presence of Resistant Enterobacteriaceae in Poultry and Synanthropic Birds of an Urban Context of Social Farming in Southern Italy. Vet. Sci. 2025, 12, 961. https://doi.org/10.3390/vetsci12100961
Pace A, Longobardi M, Russo TP, Borrelli L, Fioretti A, Dipineto L, Santaniello A. Presence of Resistant Enterobacteriaceae in Poultry and Synanthropic Birds of an Urban Context of Social Farming in Southern Italy. Veterinary Sciences. 2025; 12(10):961. https://doi.org/10.3390/vetsci12100961
Chicago/Turabian StylePace, Antonino, Mattia Longobardi, Tamara Pasqualina Russo, Luca Borrelli, Alessandro Fioretti, Ludovico Dipineto, and Antonio Santaniello. 2025. "Presence of Resistant Enterobacteriaceae in Poultry and Synanthropic Birds of an Urban Context of Social Farming in Southern Italy" Veterinary Sciences 12, no. 10: 961. https://doi.org/10.3390/vetsci12100961
APA StylePace, A., Longobardi, M., Russo, T. P., Borrelli, L., Fioretti, A., Dipineto, L., & Santaniello, A. (2025). Presence of Resistant Enterobacteriaceae in Poultry and Synanthropic Birds of an Urban Context of Social Farming in Southern Italy. Veterinary Sciences, 12(10), 961. https://doi.org/10.3390/vetsci12100961







