Antioxidant Strategies for Age-Related Oxidative Damage in Dogs
Abstract
Simple Summary
Abstract
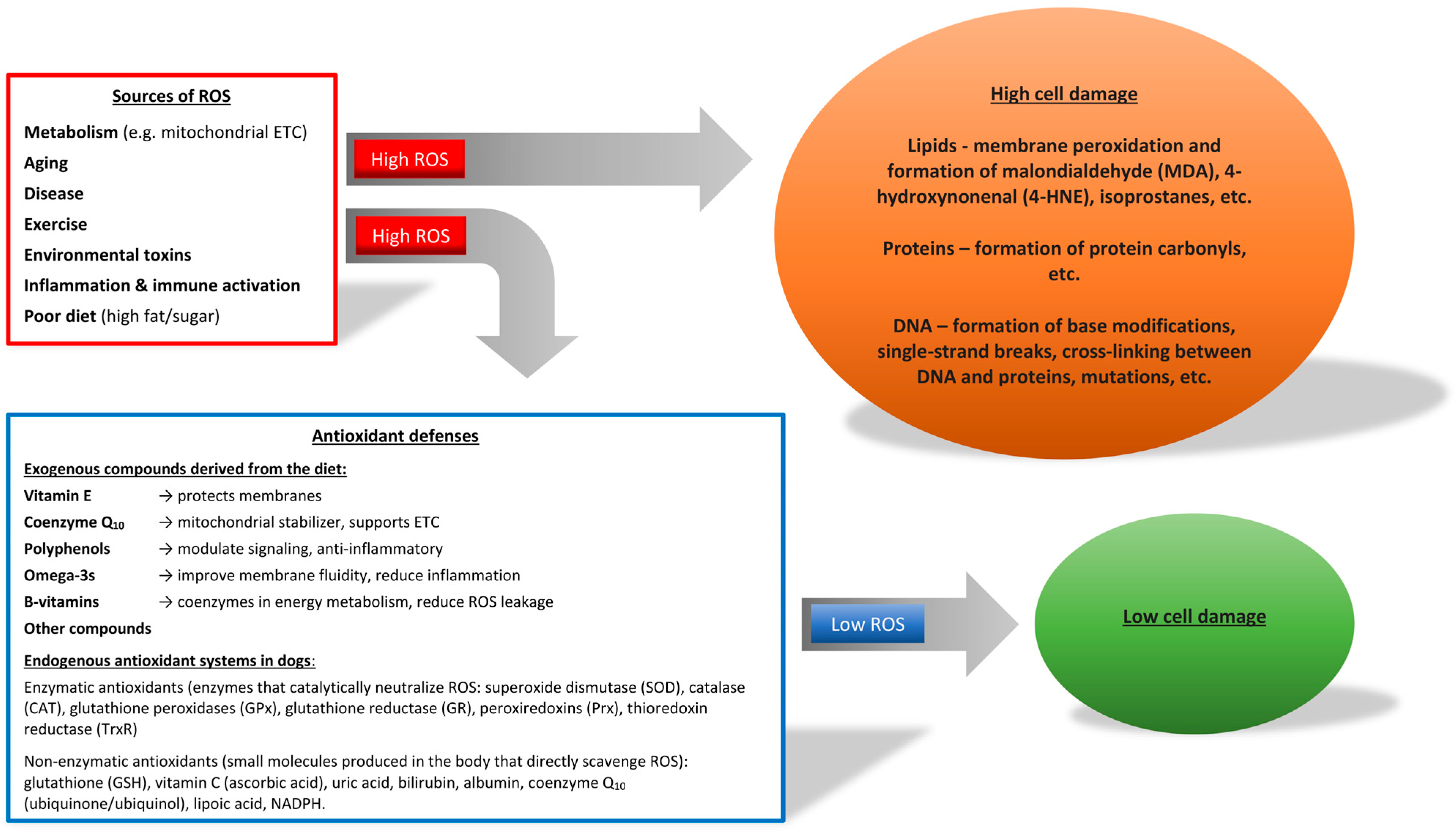
1. Introduction
2. Materials and Methods
3. The Correlation Between Oxidative Stress and Aging in Canids
4. The Impact of Oxidative Stress on Canine Cognitive Dysfunction and the Role of Antioxidants
5. The Impact of Nutrition on Oxidative Stress in Dogs
6. Discussion
Critical Perspective and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| DNA | Deoxyribonucleic acid |
| SOD | Superoxide dismutase |
| CoQ10 | Coenzyme Q10 |
| IGF-1 | Insulin-like growth factor 1 |
| DHA | Docosahexaenoic acid |
| ATP | Adenosine triphosphate |
| ETC | Electron transport chain |
| EPA | Eicosapentaenoic acid |
| MDA | Malondialdehyde |
| MMVD | Myxomatous mitral valve disease |
| AGEs | Advanced glycation end-products |
| AGE-BSA | Advanced glycation end-products bound to bovine serum albumin |
References
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Sies, H. On the History of Oxidative Stress: Concept and Some Aspects of Current Development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Dossena, S.; Marino, A. Cellular Oxidative Stress. Antioxidants 2021, 10, 399. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Bracarense, A.P.F.R.L. Estresse oxidativo em cães. Semin. Ciências Agrárias 2016, 37, 1431–1440. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Demillard, L.J.; Ren, J. Reactive Oxygen Species in Cardiovascular Diseases: An Update. Explor. Med. 2022, 3, 188–204. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Spinelli, S.; Vitale, G.; Trichilo, V.; Loddo, S.; Marino, A. High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 365. [Google Scholar] [CrossRef]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef]
- Marino, A.; Dossena, S.; Tamma, G.; Donnini, S. Oxidative Stress and Membrane Transport Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9625213. [Google Scholar] [CrossRef]
- Vasquez, M.; Zuniga, M.; Rodriguez, A. Oxidative Stress and Pathogenesis in Malaria. Front. Cell. Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Reybier, K.; Marchetti, G.; Pau, M.C.; Virdis, P.; Fozza, C.; Nepveu, F.; Low, P.S.; Turrini, F.M.; Pantaleo, A. Syk Kinase Inhibitors Synergize with Artemisinins by Enhancing Oxidative Stress in Plasmodium Falciparum-Parasitized Erythrocytes. Antioxidants 2020, 9, 753. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Guisado-Corcoll, A.; Herrero-Lorenzo, M.; Solaguren-Beascoa, M.; Martí, E. Non-Coding RNAs as Sensors of Oxidative Stress in Neurodegenerative Diseases. Antioxidants 2020, 9, 1095. [Google Scholar] [CrossRef]
- Lin, T.-K.; Chen, S.-D.; Lin, K.-J.; Chuang, Y.-C. Seizure-Induced Oxidative Stress in Status Epilepticus: Is Antioxidant Beneficial? Antioxidants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Nikolaidis, M.G.; Kyparos, A.; Kouretas, D. Blood Reflects Tissue Oxidative Stress Depending on Biomarker and Tissue Studied. Free Radic. Biol. Med. 2009, 47, 1371–1374. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood Reflects Tissue Oxidative Stress: A Systematic Review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The Etiology of Oxidative Stress in the Various Species of Animals, a Review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Blanca, P.-M.; María Luisa, F.-R.; Guadalupe, M.; Fátima, C.-L. Oxidative Stress in Canine Diseases: A Comprehensive Review. Antioxidants 2024, 13, 1396. [Google Scholar] [CrossRef]
- Tomsič, K.; Nemec Svete, A. A Mini-Review of the Effects of Inhalational and Intravenous Anesthetics on Oxidative Stress in Dogs. Front. Vet. Sci. 2022, 9, 987536. [Google Scholar] [CrossRef] [PubMed]
- Sagols, E.; Priymenko, N. Oxidative Stress in Dog with Heart Failure: The Role of Dietary Fatty Acids and Antioxidants. Vet. Med. Int. 2011, 2011, 180206. [Google Scholar] [CrossRef]
- Tomsič, K.; Domanjko Petrič, A.; Nemec, A.; Pirman, T.; Rezar, V.; Seliškar, A.; Vovk, T.; Nemec Svete, A. Evaluation of Antioxidant Status and Lipid Peroxidation in Dogs with Myxomatous Mitral Valve Degeneration Stage B1. Front. Vet. Sci. 2023, 10, 1203480. [Google Scholar] [CrossRef]
- Nemec Svete, A.; Verk, B.; Čebulj-Kadunc, N.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Inflammation and Its Association with Oxidative Stress in Dogs with Heart Failure. BMC Vet. Res. 2021, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant Status and Biomarkers of Oxidative Stress in Dogs with Congestive Heart Failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.J.; Häggström, J.; Møller, J.E.; Lykkesfeldt, J.; Falk, T.; Olsen, L.H. Markers of Oxidative Stress in Dogs with Myxomatous Mitral Valve Disease Are Influenced by Sex, Neuter Status, and Serum Cholesterol Concentration. J. Vet. Intern. Med. 2017, 31, 295–302. [Google Scholar] [CrossRef]
- Kogika, M.M.; Lustoza, M.D.; Hagiwara, M.K.; Caragelasco, D.S.; Martorelli, C.R.; Mori, C.S. Evaluation of Oxidative Stress in the Anemia of Dogs with Chronic Kidney Disease. Vet. Clin. Pathol. 2015, 44, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Woolcock, A.D.; Serpa, P.B.S.; Santos, A.P.; Christian, J.A.; Moore, G.E. Reactive Oxygen Species, Glutathione, and Vitamin E Concentrations in Dogs with Hemolytic or Nonhemolytic Anemia. J. Vet. Intern. Med. 2020, 34, 2357–2364. [Google Scholar] [CrossRef]
- Anturaniemi, J.; Zaldívar-López, S.; Savelkoul, H.F.J.; Elo, K.; Hielm-Björkman, A. The Effect of Atopic Dermatitis and Diet on the Skin Transcriptome in Staffordshire Bull Terriers. Front. Vet. Sci. 2020, 7, 552251. [Google Scholar] [CrossRef] [PubMed]
- Kapun, A.P.; Salobir, J.; Levart, A.; Kotnik, T.; Svete, A.N. Oxidative Stress Markers in Canine Atopic Dermatitis. Res. Vet. Sci. 2012, 92, 469–470. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Vitamin E Supplementation in Canine Atopic Dermatitis: Improvement of Clinical Signs and Effects on Oxidative Stress Markers. Vet. Rec. 2014, 175, 560. [Google Scholar] [CrossRef]
- Macotpet, A.; Suksawat, F.; Sukon, P.; Pimpakdee, K.; Pattarapanwichien, E.; Tangrassameeprasert, R.; Boonsiri, P. Oxidative Stress in Cancer-Bearing Dogs Assessed by Measuring Serum Malondialdehyde. BMC Vet. Res. 2013, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Marquis, A.; Packer, R.A.; Borgens, R.B.; Duerstock, B.S. Increase in Oxidative Stress Biomarkers in Dogs with Ascending–Descending Myelomalacia Following Spinal Cord Injury. J. Neurol. Sci. 2015, 353, 63–69. [Google Scholar] [CrossRef]
- Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.; Martínez, M.S.; Míguez, M.P.; Pérez-Merino, E.M. Oxidative Stress in Dogs with Chronic Inflammatory Enteropathy Treated with Allogeneic Mesenchymal Stem Cells. Vet. Res. Commun. 2024, 48, 901–910. [Google Scholar] [CrossRef]
- Razavi, S.M.; Soltan, M.S.; Abbasian, K.; Karami, A.; Nazifi, S. Host Oxidative Stress in Piroplasmosis: A Review in Domestic Animals. Vet. Parasitol. 2023, 322, 110011. [Google Scholar] [CrossRef]
- Almeida, B.F.M.; Narciso, L.G.; Melo, L.M.; Preve, P.P.; Bosco, A.M.; Lima, V.M.F.; Ciarlini, P.C. Leishmaniasis Causes Oxidative Stress and Alteration of Oxidative Metabolism and Viability of Neutrophils in Dogs. Vet. J. 2013, 198, 599–605. [Google Scholar] [CrossRef]
- Pugliese, M.; Biondi, V.; Merola, G.; Landi, A.; Passantino, A. Oxidative Stress Evaluation in Dogs Affected with Canine Monocytic Ehrlichiosis. Antioxidants 2022, 11, 328. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Munhoz, T.D.; Faria, J.L.M.; Vargas-Hérnandez, G.; Machado, R.Z.; Almeida, T.C.; Moresco, R.N.; Stefani, L.M.; Tinucci-Costa, M. Increase Nitric Oxide and Oxidative Stress in Dogs Experimentally Infected by Ehrlichia Canis: Effect on the Pathogenesis of the Disease. Vet. Microbiol. 2013, 164, 366–369. [Google Scholar] [CrossRef]
- Crnogaj, M.; Cerón, J.J.; Šmit, I.; Kiš, I.; Gotić, J.; Brkljačić, M.; Matijatko, V.; Rubio, C.P.; Kučer, N.; Mrljak, V. Relation of Antioxidant Status at Admission and Disease Severity and Outcome in Dogs Naturally Infected with Babesia Canis Canis. BMC Vet. Res. 2017, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Tomsič, K.; Seliškar, A.; Lukanc, B.; Nemec Svete, A. Plasma Total Antioxidant Capacity and Activities of Blood Glutathione Peroxidase and Superoxide Dismutase Determined in Healthy Dogs by Using Commercially Available Kits. Acta Vet. 2016, 66, 534–548. [Google Scholar] [CrossRef]
- Erjavec, V.; Vovk, T.; Svete, A.N. Evaluation of Oxidative Stress Parameters in Dogs with Brachycephalic Obstructive Airway Syndrome Before and after Surgery. J. Vet. Res. 2021, 65, 201–208. [Google Scholar] [CrossRef]
- Vajdovich, P.; Gaál, T.; Szilágyi, A.; Harnos, A. Changes in Some Red Blood Cell and Clinical Laboratory Parameters in Young and Old Beagle Dogs. Vet. Res. Commun. 1997, 21, 463–470. [Google Scholar] [CrossRef]
- Todorova, I.; Simeonova, G.; Kyuchukova, D.; Dinev, D.; Gadjeva, V. Reference Values of Oxidative Stress Parameters (MDA, SOD, CAT) in Dogs and Cats. Comp. Clin. Path. 2005, 13, 190–194. [Google Scholar] [CrossRef]
- Porato, M.; Noël, S.; Pincemail, J.; Albert, A.; Cheramy-Bien, J.-P.; Le Goff, C.; Hamaide, A. Selected Biomarkers of Oxidative Stress in Healthy Beagle Dogs: A Preliminary Study. Front. Vet. Sci. 2023, 10, 1063216. [Google Scholar] [CrossRef]
- Stowe, H.D.; Lawler, D.F.; Kealy, R.D. Antioxidant Status of Pair-Fed Labrador Retrievers Is Affected by Diet Restriction and Aging. J. Nutr. 2006, 136, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, T.; Eppe, J.; Mugnier, A.; Delfour, F.; Meynadier, A. Enhancing Cognitive Functions in Aged Dogs and Cats: A Systematic Review of Enriched Diets and Nutraceuticals. GeroScience 2025, 47, 2925–2947. [Google Scholar] [CrossRef]
- Dowling, A.L.S.; Head, E. Antioxidants in the Canine Model of Human Aging. Biochim. Biophys. Acta 2012, 1822, 685–689. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.-F.; Lee, K.; Lopez, R.; Previs, S.F.; Willard, B.; McCullough, A.; Kasumov, T. A Western Diet Induced NAFLD in LDLR(-/)(-) Mice Is Associated with Reduced Hepatic Glutathione Synthesis. Free Radic. Biol. Med. 2016, 96, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Andrich, D.E.; Melbouci, L.; Ou, Y.; Auclair, N.; Mercier, J.; Grenier, J.-C.; Lira, F.S.; Barreiro, L.B.; Danialou, G.; Comtois, A.-S.; et al. A Short-Term High-Fat Diet Alters Glutathione Levels and IL-6 Gene Expression in Oxidative Skeletal Muscles of Young Rats. Front. Physiol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Vaanholt, L.M.; Milne, A.; Zheng, Y.; Hambly, C.; Mitchell, S.E.; Valencak, T.G.; Allison, D.B.; Speakman, J.R. Oxidative Costs of Reproduction: Oxidative Stress in Mice Fed Standard and Low Antioxidant Diets. Physiol. Behav. 2016, 154, 1–7. [Google Scholar] [CrossRef]
- Larsen, J.A.; Farcas, A. Nutrition of Aging Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Urfer, S.R.; Greer, K.; Wolf, N.S. Age-Related Cataract in Dogs: A Biomarker for Life Span and Its Relation to Body Size. AGE 2011, 33, 451–460. [Google Scholar] [CrossRef]
- Jimenez, A.G. Physiological Underpinnings in Life-History Trade-Offs in Man’s Most Popular Selection Experiment: The Dog. J. Comp. Physiol. B 2016, 186, 813–827. [Google Scholar] [CrossRef]
- Ruple, A.; MacLean, E.; Snyder-Mackler, N.; Creevy, K.E.; Promislow, D. Dog Models of Aging. Annu. Rev. Anim. Biosci. 2022, 10, 419–439. [Google Scholar] [CrossRef]
- Boyko, A.R.; Quignon, P.; Li, L.; Schoenebeck, J.J.; Degenhardt, J.D.; Lohmueller, K.E.; Zhao, K.; Brisbin, A.; Parker, H.G.; Vonholdt, B.M.; et al. A Simple Genetic Architecture Underlies Morphological Variation in Dogs. PLoS Biol. 2010, 8, e1000451. [Google Scholar] [CrossRef] [PubMed]
- Deeb, B.J.; Wolf, N.S. Studying Longevity and Morbidity in Giant and Small Breeds of Doga. Available online: https://www.researchgate.net/publication/293036806_Studying_longevity_and_morbidity_in_giant_and_small_breeds_of_dogs (accessed on 4 July 2025).
- Michell, A.R. Longevit of British Breeds of Dog and Its Relationships With-Sex, Size, Cardiovascular Variables and Disease. Vet. Rec. 1999, 145, 625–629. [Google Scholar] [CrossRef]
- Kraus, C.; Pavard, S.; Promislow, D.E.L. The Size–Life Span Trade-Off Decomposed: Why Large Dogs Die Young. Am. Nat. 2013, 181, 492–505. [Google Scholar] [CrossRef]
- Selman, C.; Blount, J.D.; Nussey, D.H.; Speakman, J.R. Oxidative Damage, Ageing, and Life-History Evolution: Where Now? Trends Ecol. Evol. 2012, 27, 570–577. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Winward, J.; Beattie, U.; Cipolli, W. Cellular Metabolism and Oxidative Stress as a Possible Determinant for Longevity in Small Breed and Large Breed Dogs. PLoS ONE 2018, 13, e0195832. [Google Scholar] [CrossRef]
- Greer, K.A.; Hughes, L.M.; Masternak, M.M. Connecting Serum IGF-1, Body Size, and Age in the Domestic Dog. AGE 2011, 33, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sutter, N.B.; Bustamante, C.D.; Chase, K.; Gray, M.M.; Zhao, K.; Zhu, L.; Padhukasahasram, B.; Karlins, E.; Davis, S.; Jones, P.G.; et al. A Single IGF1 Allele Is a Major Determinant of Small Size in Dogs. Science 2007, 316, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, J.E.; Amador, A.; Patterson, D.F. Insulin-like Growth Factor I Levels in Proportionate Dogs, Chondrodystrophic Dogs and in Giant Dogs. Acta Endocrinol. 1988, 118, 105–108. [Google Scholar] [CrossRef]
- Lewis, K.N.; Andziak, B.; Yang, T.; Buffenstein, R. The Naked Mole-Rat Response to Oxidative Stress: Just Deal with It. Antioxid. Redox Signal. 2013, 19, 1388–1399. [Google Scholar] [CrossRef]
- Kouda, K.; Iki, M. Beneficial Effects of Mild Stress (Hormetic Effects): Dietary Restriction and Health. J. Physiol. Anthropol. 2010, 29, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mehdi, M.M. Oxidative Stress, Inflammation and Hormesis: The Role of Dietary and Lifestyle Modifications on Aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Osakabe, N.; Di Paola, R.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Fritsch, T.; Abdelhameed, A.S.; et al. Hormesis Defines the Limits of Lifespan. Ageing Res. Rev. 2023, 91, 102074. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.G.; Downs, C.J. Untangling Life Span and Body Mass Discrepancies in Canids: Phylogenetic Comparison of Oxidative Stress in Blood from Domestic Dogs and Wild Canids. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, R203–R210. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Chapagain, D.; Range, F.; Huber, L.; Virányi, Z. Cognitive Aging in Dogs. Gerontology 2017, 64, 165–171. [Google Scholar] [CrossRef]
- Roudebush, P.; Zicker, S.C.; Cotman, C.W.; Milgram, N.W.; Muggenburg, B.A.; Head, E. Nutritional Management of Brain Aging in Dogs. J. Am. Vet. Med. Assoc. 2005, 227, 722–728. [Google Scholar] [CrossRef]
- Cotman, C.W.; Head, E.; Muggenburg, B.A.; Zicker, S.; Milgram, N.W. Brain Aging in the Canine: A Diet Enriched in Antioxidants Reduces Cognitive Dysfunction. Neurobiol. Aging 2002, 23, 809–818. [Google Scholar] [CrossRef]
- Head, E.; Rofina, J.; Zicker, S. Oxidative Stress, Aging, and Central Nervous System Disease in the Canine Model of Human Brain Aging. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 167–178. [Google Scholar] [CrossRef]
- Milgram, N.W.; Zicker, S.C.; Head, E.; Muggenburg, B.A.; Murphey, H.; Ikeda-Douglas, C.J.; Cotman, C.W. Dietary Enrichment Counteracts Age-Associated Cognitive Dysfunction in Canines. Neurobiol. Aging 2002, 23, 737–745. [Google Scholar] [CrossRef]
- Sechi, S.; Chiavolelli, F.; Spissu, N.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Cocco, R. An Antioxidant Dietary Supplement Improves Brain-Derived Neurotrophic Factor Levels in Serum of Aged Dogs: Preliminary Results. J. Vet. Med. 2015, 2015, 412501. [Google Scholar] [CrossRef]
- Selhub, J.; Troen, A.; Rosenberg, I.H. B Vitamins and the Aging Brain. Nutr. Rev. 2010, 68, S112–S118. [Google Scholar] [CrossRef]
- Cole, G.M.; Ma, Q.-L.; Frautschy, S.A. Omega-3 Fatty Acids and Dementia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Heath, S.E.; Barabas, S.; Craze, P.G. Nutritional Supplementation in Cases of Canine Cognitive Dysfunction—A Clinical Trial. Appl. Anim. Behav. Sci. 2007, 105, 284–296. [Google Scholar] [CrossRef]
- Pan, Y.; Araujo, J.A.; Burrows, J.; de Rivera, C.; Gore, A.; Bhatnagar, S.; Milgram, N.W. Cognitive Enhancement in Middle-Aged and Old Cats with Dietary Supplementation with a Nutrient Blend Containing Fish Oil, B Vitamins, Antioxidants and Arginine. Br. J. Nutr. 2013, 110, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. A Dual Activity of ROS and Oxidative Stress on Adult Neurogenesis and Alzheimer’s Disease. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 16–21. [Google Scholar] [CrossRef]
- Tangney, C.C.; Tang, Y.; Evans, D.A.; Morris, M.C. Biochemical Indicators of Vitamin B12 and Folate Insufficiency and Cognitive Decline. Neurology 2009, 72, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Head, E. Neurobiology of the Aging Dog. AGE 2011, 33, 485–496. [Google Scholar] [CrossRef]
- Head, E.; Nukala, V.N.; Fenoglio, K.A.; Muggenburg, B.A.; Cotman, C.W.; Sullivan, P.G. Effects of Age, Dietary, and Behavioral Enrichment on Brain Mitochondria in a Canine Model of Human Aging. Exp. Neurol. 2009, 220, 171–176. [Google Scholar] [CrossRef]
- Ames, B.N. Delaying the Mitochondrial Decay of Aging. Ann. N. Y. Acad. Sci. 2004, 1019, 406–411. [Google Scholar] [CrossRef]
- Barja, G. Mitochondrial Free Radical Production and Aging in Mammals and Birds. Ann. N. Y. Acad. Sci. 1998, 854, 224–238. [Google Scholar] [CrossRef]
- Poljšak, B.; Fink, R. The Protective Role of Antioxidants in the Defence against ROS/RNS-Mediated Environmental Pollution. Oxidative Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Casteilla, L.; Rigoulet, M.; Pénicaud, L. Mitochondrial ROS Metabolism: Modulation by Uncoupling Proteins. IUBMB Life 2001, 52, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G.; Hogue, B.A.; Mildaziene, V. Dependence of H2O2 Formation by Rat Heart Mitochondria on Substrate Availability and Donor Age. J. Bioenerg. Biomembr. 1997, 29, 89–95. [Google Scholar] [CrossRef]
- Staniek, K.; Nohl, H. H2O2 Detection from Intact Mitochondria as a Measure for One-Electron Reduction of Dioxygen Requires a Non-Invasive Assay System. Biochim. Biophys. Acta 1999, 1413, 70–80. [Google Scholar] [CrossRef]
- Snigdha, S.; de Rivera, C.; Milgram, N.W.; Cotman, C.W. Effect of Mitochondrial Cofactors and Antioxidants Supplementation on Cognition in the Aged Canine. Neurobiol. Aging 2016, 37, 171–178. [Google Scholar] [CrossRef]
- Lee, D.; Jo, M.G.; Kim, S.Y.; Chung, C.G.; Lee, S.B. Dietary Antioxidants and the Mitochondrial Quality Control: Their Potential Roles in Parkinson’s Disease Treatment. Antioxidants 2020, 9, 1056. [Google Scholar] [CrossRef]
- Sándor, S.; Jónás, D.; Tátrai, K.; Czeibert, K.; Kubinyi, E. Poly(A) RNA Sequencing Reveals Age-Related Differences in the Prefrontal Cortex of Dogs. GeroScience 2022, 44, 1269–1293. [Google Scholar] [CrossRef]
- Gonçalves, R.; De Decker, S.; Walmsley, G.; Butterfield, S.; Maddox, T.W. Inflammatory Disease Affecting the Central Nervous System in Dogs: A Retrospective Study in England (2010–2019). Front. Vet. Sci. 2022, 8, 819945. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive Dysfunction Syndrome: A Disease of Canine and Feline Brain Aging. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef]
- Gommeren, K.; Desmas, I.; Garcia, A.; Bauer, N.; Moritz, A.; Roth, J.; Peeters, D. Inflammatory Cytokine and C-Reactive Protein Concentrations in Dogs with Systemic Inflammatory Response Syndrome. J. Vet. Emerg. Crit. Care 2018, 28, 9–19. [Google Scholar] [CrossRef]
- Schmid, S.M.; Hoffman, J.M.; Prescott, J.; Ernst, H.; Promislow, D.E.L.; Dog Aging Project Consortium; Creevy, K.E. The Companion Dog as a Model for Inflammaging: A Cross-Sectional Pilot Study. Geroscience 2024, 46, 5395–5407. [Google Scholar] [CrossRef]
- O’Connor, E.; Mündel, T.; Barnes, M.J. Nutritional Compounds to Improve Post-Exercise Recovery. Nutrients 2022, 14, 5069. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef]
- Hall, J.A.; Tooley, K.A.; Gradin, J.L.; Jewell, D.E.; Wander, R.C. Effects of Dietary N-6 and n-3 Fatty Acids and Vitamin E on the Immune Response of Healthy Geriatric Dogs. Am. J. Vet. Res. 2003, 64, 762–772. [Google Scholar] [CrossRef]
- Fan, Z.; Bian, Z.; Huang, H.; Liu, T.; Ren, R.; Chen, X.; Zhang, X.; Wang, Y.; Deng, B.; Zhang, L. Dietary Strategies for Relieving Stress in Pet Dogs and Cats. Antioxidants 2023, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Ravić, B.; Debeljak-Martacić, J.; Pokimica, B.; Vidović, N.; Ranković, S.; Glibetić, M.; Stepanović, P.; Popović, T. The Effect of Fish Oil-Based Foods on Lipid and Oxidative Status Parameters in Police Dogs. Biomolecules 2022, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Jewell, D.E.; Motsinger, L.A.; Paetau-Robinson, I. Effect of Dietary Antioxidants on Free Radical Damage in Dogs and Cats. J. Anim. Sci. 2024, 102, skae153. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Martinez-Urbistondo, D.; Vargas-Nuñez, J.A.; Martinez, J.A. The Role of Nutrition on Meta-Inflammation: Insights and Potential Targets in Communicable and Chronic Disease Management. Curr. Obes. Rep. 2022, 11, 305–335. [Google Scholar] [CrossRef]
- Stockman, J. Nutrition and Aging in Dogs and Cats. In Nutrition and Metabolism of Dogs and Cats; Wu, G., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 203–215. ISBN 978-3-031-54192-6. [Google Scholar]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 Polyunsaturated Fatty Acids Exert Anti-Oxidant Effects through the Nuclear Factor (Erythroid-Derived 2)-Related Factor 2 Pathway in Immortalized Mouse Schwann Cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of N-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 Fatty Acids Supplementation and Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential Fatty Acids and the Brain: Possible Health Implications. Int. J. Dev. Neurosci. 2000, 18, 383–399. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 7, CD003177. [Google Scholar] [CrossRef]
- Ikizler, M.; Erkasap, N.; Dernek, S.; Kural, T.; Kaygisiz, Z. Dietary Polyphenol Quercetin Protects Rat Hearts during Reperfusion: Enhanced Antioxidant Capacity with Chronic Treatment. Anadolu Kardiyol. Derg. 2007, 7, 404–410. [Google Scholar]
- Abdukeyum, G.G.; Owen, A.J.; Larkin, T.A.; McLennan, P.L. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. J. Clin. Med. 2016, 5, 32. [Google Scholar] [CrossRef]
- Essential Fatty Acids|Linus Pauling Institute|Oregon State University. Available online: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids (accessed on 16 August 2025).
- Kearns, R.J.; Hayek, M.G.; Turek, J.J.; Meydani, M.; Burr, J.R.; Greene, R.J.; Marshall, C.A.; Adams, S.M.; Borgert, R.C.; Reinhart, G.A. Effect of Age, Breed and Dietary Omega-6 (n-6): Omega-3 (n-3) Fatty Acid Ratio on Immune Function, Eicosanoid Production, and Lipid Peroxidation in Young and Aged Dogs. Vet. Immunol. Immunopathol. 1999, 69, 165–183. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Coenzyme Q, Oxidative Stress and Aging. Mitochondrion 2007, 7, S103–S111. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and Antioxidant Properties of Coenzyme Q10: Recent Developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q--Biosynthesis and Functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The Antioxidant Role of Coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond Tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef]
- Marie, N.; Verdier, C.; Bot, B.L.; Burgot, G. Analysis of Sodium and Potassium in Total Parenteral Nutrition Bags by ICP-MS and ICP-AES:Critical Influence of the Ingredients. Am. J. Anal. Chem. 2011, 2, 573–581. [Google Scholar] [CrossRef]
- FEDIAF|Nutritional Guidelines. Available online: https://europeanpetfood.org/self-regulation/nutritional-guidelines/ (accessed on 23 August 2025).
- Nutritional Guidelines. Available online: https://europeanpetfood.org/wp-content/uploads/2024/09/FEDIAF-Nutritional-Guidelines_2024.pdf (accessed on 23 August 2025).
- Pan, Y.; Kennedy, A.D.; Jönsson, T.J.; Milgram, N.W. Cognitive Enhancement in Old Dogs from Dietary Supplementation with a Nutrient Blend Containing Arginine, Antioxidants, B Vitamins and Fish Oil. Br. J. Nutr. 2018, 119, 349–358. [Google Scholar] [CrossRef]
- Hesta, M.; Ottermans, C.; Krammer-Lukas, S.; Zentek, J.; Hellweg, P.; Buyse, J.; Janssens, G.P.J. The Effect of Vitamin C Supplementation in Healthy Dogs on Antioxidative Capacity and Immune Parameters. J. Anim. Physiol. Anim. Nutr. 2009, 93, 26–34. [Google Scholar] [CrossRef]
- Chapagain, D.; Wallis, L.J.; Range, F.; Affenzeller, N.; Serra, J.; Virányi, Z. Behavioural and Cognitive Changes in Aged Pet Dogs: No Effects of an Enriched Diet and Lifelong Training. PLoS ONE 2020, 15, e0238517. [Google Scholar] [CrossRef]
- Milgram, N.W.; Head, E.; Zicker, S.C.; Ikeda-Douglas, C.; Murphey, H.; Muggenberg, B.A.; Siwak, C.T.; Tapp, P.D.; Lowry, S.R.; Cotman, C.W. Long-Term Treatment with Antioxidants and a Program of Behavioral Enrichment Reduces Age-Dependent Impairment in Discrimination and Reversal Learning in Beagle Dogs. Exp. Gerontol. 2004, 39, 753–765. [Google Scholar] [CrossRef]
- McMichael, M.A. Oxidative Stress, Antioxidants, and Assessment of Oxidative Stress in Dogs and Cats. J. Am. Vet. Med. Assoc. 2007, 231, 714–720. [Google Scholar] [CrossRef]
- Araujo, J.A.; Landsberg, G.M.; Milgram, N.W.; Miolo, A. Improvement of Short-Term Memory Performance in Aged Beagles by a Nutraceutical Supplement Containing Phosphatidylserine, Ginkgo Biloba, Vitamin E, and Pyridoxine. Can. Vet. J. 2008, 49, 379–385. [Google Scholar]
- dLib.Si—24-Hour Follow-Up Study of Plasma Coenzyme Q10, Total Antioxidant Capacity and Selected Blood Parameters After a Single Oral Dose of Water-Soluble Coenzyme Q10 in Healthy Beagle Dogs. Available online: https://www.dlib.si/details/URN:NBN:SI:DOC-82XNDI4D (accessed on 23 August 2025).
- Prosek, M.; Butinar, J.; Lukanc, B.; Fir, M.M.; Milivojevic, L.; Krizman, M.; Smidovnik, A. Bioavailability of Water-Soluble CoQ10 in Beagle Dogs. J. Pharm. Biomed. Anal. 2008, 47, 918–922. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Cheng, C.-H.; Hsu, C.-L.; Lee, W.-J.; Huang, S.-C.; Huang, Y.-C. Role of Vitamin B6 Status on Antioxidant Defenses, Glutathione, and Related Enzyme Activities in Mice with Homocysteine-Induced Oxidative Stress. Food Nutr. Res. 2015, 59, 25702. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Rekka, E.A. The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients 2024, 16, 2740. [Google Scholar] [CrossRef]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding Mitochondria: Potential Role of Nutritional Components to Improve Critical Illness Convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Dieter, F.; Esselun, C.; Eckert, G.P. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. Int. J. Mol. Sci. 2022, 23, 9186. [Google Scholar] [CrossRef]
- Yamada, Y.; Ishimaru, T.; Ikeda, K.; Harashima, H. Validation of the Mitochondrial Delivery of Vitamin B1 to Enhance ATP Production Using SH-SY5Y Cells, a Model Neuroblast. J. Pharm. Sci. 2022, 111, 432–439. [Google Scholar] [CrossRef]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef]
- Gvozdjáková, A. Mitochondrial Medicine: Mitochondrial Metabolism, Diseases, Diagnosis and Therapy; Springer Science + Business Media, B.V.: Dordrecht, The Netherlands, 2008; Available online: https://www.scirp.org/reference/referencespapers?referenceid=1723686 (accessed on 16 August 2025).
- Li, P.; Wu, G. Characteristics of Nutrition and Metabolism in Dogs and Cats. In Nutrition and Metabolism of Dogs and Cats; Wu, G., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 55–98. ISBN 978-3-031-54192-6. [Google Scholar]
- Sechi, S.; Fiore, F.; Chiavolelli, F.; Dimauro, C.; Nudda, A.; Cocco, R. Oxidative Stress and Food Supplementation with Antioxidants in Therapy Dogs. Can. J. Vet. Res. 2017, 81, 206–216. [Google Scholar]
- Druzhaeva, N.; Nemec Svete, A.; Tavčar-Kalcher, G.; Babič, J.; Ihan, A.; Pohar, K.; Krapež, U.; Domanjko Petrič, A. Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants 2022, 11, 1427. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Drew, M.D.; Huang, Q.; Silver, T.I.; Weber, L.P. Postprandial Impairment of Flow-Mediated Dilation and Elevated Methylglyoxal after Simple but Not Complex Carbohydrate Consumption in Dogs. Nutr. Res. 2012, 32, 278–284. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of Glyoxal, Methylglyoxal and 3-Deoxyglucosone in the Glycation of Proteins by Glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Z.; Li, Y.; Liu, S.; Hu, P.; Luo, E. Advanced Glycation End Products and Reactive Oxygen Species: Uncovering the Potential Role of Ferroptosis in Diabetic Complications. Mol. Med. 2024, 30, 141. [Google Scholar] [CrossRef]
- Jimenez, A.G. Plasma Concentration of Advanced Glycation End-Products From Wild Canids and Domestic Dogs Does Not Change With Age or Across Body Masses. Front. Vet. Sci. 2021, 8, 637132. [Google Scholar] [CrossRef]
- Baynes, J.W. The Role of AGEs in Aging: Causation or Correlation. Exp. Gerontol. 2001, 36, 1527–1537. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span--from Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Bray, E.E.; Zheng, Z.; Tolbert, M.K.; McCoy, B.M.; Akey, J.M.; Benton, B.; Borenstein, E.; Castelhano, M.G.; Coleman, A.E.; Creevy, K.E.; et al. Once-Daily Feeding Is Associated with Better Health in Companion Dogs: Results from the Dog Aging Project. GeroScience 2022, 44, 1779–1790. [Google Scholar] [CrossRef]
- Kealy, R.D.; Lawler, D.F.; Ballam, J.M.; Mantz, S.L.; Biery, D.N.; Greeley, E.H.; Lust, G.; Segre, M.; Smith, G.K.; Stowe, H.D. Effects of Diet Restriction on Life Span and Age-Related Changes in Dogs. J. Am. Vet. Med. Assoc. 2002, 220, 1315–1320. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Toxicology of the Human Environment: The Critical Role of Free Radicals; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-7484-0916-7. [Google Scholar]
- Ristow, M.; Zarse, K. How Increased Oxidative Stress Promotes Longevity and Metabolic Health: The Concept of Mitochondrial Hormesis (Mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Hagen, D.M.; Ekena, J.L.; Geesaman, B.M.; Viviano, K.R. Antioxidant Supplementation during Illness in Dogs: Effect on Oxidative Stress and Outcome, an Exploratory Study. J. Small Anim. Pract. 2019, 60, 543–550. [Google Scholar] [CrossRef]
- Dündar, Y.; Aslan, R. Antioxidative Stress. East. J. Med. 2000, 5, 45–47. [Google Scholar]
- Poljsak, B.; Milisav, I. The Neglected Significance of “Antioxidative Stress”. Oxidative Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef]

| Antioxidant/Vitamin | Dog Category | Antioxidant/Vitamin Level | Reference |
|---|---|---|---|
| Selenium | Wet diets - Typical active adult dog - Inactive adult dog | - 23 µg/100 g DM - 27 µg/100 g DM | FEDIAF, 2024 [130] |
| Dry diets - Typical active adult dog - Inactive adult dog | - 18 µg/100 g DM - 22 µg/100 g DM | ||
| Old beagle dogs - control diet - test diet | - 0.59 mg/kg food - 0.53 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin A | - Typical active adult dog - Inactive adult dog | - 606 IU/100 g DM - 702 IU/100 g DM | FEDIAF, 2024 [130] |
| Adult beagle dogs, divided into two age groups (<2.5 years and >7 years) - control diet - test (enriched) diet | - 3.81 mg/kg DM - 3.81 mg/kg DM | Hesta et al., 2009 [132] | |
| Vitamin C | Old beagle dogs - control diet - test diet | - 0 mg/kg food - 84.7 mg/kg food | Pan et al., 2018 [131] |
| Adult beagle dogs, divided in two age groups (<2.5 years and >7 years) - control diet - test (enriched) diet | - 68.8 mg/kg DM - 68.8 mg/kg DM + 30 or 60 mg vitamin C per day (orally) | Hesta et al., 2009 [132] | |
| Old dogs (>6 years) - control diet - test (enriched) diet | - 0 ppm (0 mg/kg food) - 559 ppm (559 mg/kg food) | Chapagain et al., 2020 [133] | |
| Old and young beagle dogs, both receiving. - control diet - test (enriched) diet | - <30 ppm (<30 mg/kg food) - 80 ppm (80 mg/kg food) | Milgram et al., 2004 [134] | |
| Adult dogs | - 500–1000 mg PO/24 h | McMichael, 2007 [135] | |
| Vitamin D | - Typical active adult dog - Inactive adult dog | - 55.20 IU/100 g DM - 63.90 IU/100 g DM | FEDIAF, 2024 [130] |
| Adult beagle dogs, divided in two age groups (<2.5 years and >7 years) - control diet - test (enriched) diet | - 0.0254 mg/kg DM - 0.0254 mg/kg DM | Hesta et al., 2009 [132] | |
| Vitamin E | - Typical active adult dog - Inactive adult dog | - 3.60 IU/100 g DM - 4.17 IU/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 44 mg/kg food - 551 mg/kg food | Pan et al., 2018 [131] | |
| Adult beagle dogs, divided into two age groups (<2.5 years and >7 years) - control diet - test (enriched) diet | - 11.5 mg/kg DM - 11.5 mg/kg DM | Hesta et al., 2009 [132] | |
| Old dogs (>6 years) - control diet - test (enriched) diet | - 499 ppm (499 mg/kg food) - 839 ppm (839 mg/kg food) | Chapagain et al., 2020 [133] | |
| Old and young beagle dogs, both receiving. - control diet - test (enriched) diet | - 120 ppm (120 mg/kg food) - 1050 ppm (1050 mg/kg food) | Milgram et al., 2004 [134] | |
| Old beagle dogs | Based on weight (PO): - 5.01–10 kg: 67 mg daily - 10.01–15 kg: 100.5 mg daily - 15.01–20 kg: 134 mg daily | Araujo et al., 2008 [136] | |
| Dogs in general | - 400 IU PO/24 h | McMichael, 2007 [135] | |
| Vitamin B1 (Thiamine) | - Typical active adult dog - Inactive adult dog | - 0.21 mg/100 g DM - 0.25 mg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 10.39 mg/kg food - 18.67 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin B2 (Riboflavin) | - Typical active adult dog - Inactive adult dog | - 0.60 mg/100 g DM - 0.69 mg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 13.19 mg/kg food - 13.35 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin B5 (Pantothenic acid) | - Typical active adult dog - Inactive adult dog | - 1.42 mg/100 g DM - 1.64 mg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 18.57 mg/kg food - 34.07 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin B6 (Pyridoxine) | - Typical active adult dog - Inactive adult dog | - 0.15 mg/100 g DM - 0.17 mg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 6.11 mg/kg food - 11.05 mg/kg food | Pan et al., 2018 [131] | |
| Old beagle dogs | Based on weight (PO): - 5.01–10 kg: 41 mg daily - 10.01–15 kg: 61.5 mg daily - 15.01–20 kg: 82 mg daily | Araujo et al., 2008 [136] | |
| Vitamin B12 (Cyanocobalamin) | - Typical active adult dog - Inactive adult dog | - 3.35 µg/100 g DM - 3.87 µg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 0.053 mg/kg food - 0.100 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin B3 (Niacin) | - Typical active adult dog - Inactive adult dog | - 1.64 mg/100 g DM - 1.89 mg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 63.45 mg/kg food - 102.57 mg/kg food | Pan et al., 2018 [131] | |
| Vitamin B9 (Folic acid) | - Typical active adult dog - Inactive adult dog | - 25.80 µg/100 g DM - 29.90 µg/100 g DM | FEDIAF, 2024 [130] |
| Old beagle dogs - control diet - test diet | - 1.94 mg/kg food - 3.94 mg/kg food | Pan et al., 2018 [131] | |
| S-Adenosyl-L-Methionine (SAMe) | Dogs in general | 20 mg/kg BW/day (IV) | McMichael, 2007 [135] |
| N-acetylcysteine | Dogs in general | 50 mg/kg BW | McMichael, 2007 [135] |
| Ubiquinone (coenzyme Q10) | Dogs in general | 2 mg/kg BW | McMichael, 2007 [135] |
| Ubiquinone (coenzyme Q10) | Adult beagle dogs | 30 mg daily orally | Tomsič et al., 2009 [137] |
| Ubiquinone (coenzyme Q10) | Adult beagle dogs | 30 mg daily orally | Prošek et al., 2008 [138] |
| Antioxidant/Nutrient | Primary Mechanism of Action | Reported Effects in Dogs |
|---|---|---|
| Vitamin E | Lipid-soluble antioxidant; prevents lipid peroxidation in membranes; regenerates via glutathione peroxidase (selenium-dependent) | Improved skin health (atopic dermatitis), reduced oxidative stress markers, and better cognitive function in aged dogs |
| (α-tocopherol) | ||
| Vitamin C | Water-soluble antioxidant; scavenges ROS in cytosol; regenerates vitamin E | Limited benefit in young dogs; potential support in aged dogs; some studies show no effect |
| (ascorbic acid) | ||
| Coenzyme Q10 | Electron carrier in ETC, lipid-soluble antioxidant; stabilizes membranes; regenerates vitamin E; reduces lipid peroxidation | Improved energy metabolism, reduced oxidative damage; mixed results in cardiac disease trials |
| (ubiquinone/ubiquinol) | ||
| Polyphenols | Scavenge ROS; modulate redox-sensitive signaling pathways; anti-inflammatory | Improved cognition in aged dogs; possible protection in skin and metabolic disorders |
| (plant-derived) | ||
| Omega-3 fatty acids | Incorporated into membranes; modulate inflammation; improve mitochondrial function | Reduced oxidative stress markers; improved immune response; support in cognitive decline |
| (EPA, DHA) | ||
| B-vitamins | Cofactors in mitochondrial metabolism; reduce electron leakage from ETC; indirectly lower ROS | Support mitochondrial efficiency; improved antioxidant enzyme activity; indirect role in oxidative balance |
| (B1, B2, B3, B6, B9, B12) | ||
| S-Adenosyl-L-methionine (SAMe) | Methyl donor; supports glutathione synthesis | Improved hepatic function; enhanced antioxidant capacity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muršec, A.; Poljšak, B.; Nemec Svete, A.; Erjavec, V. Antioxidant Strategies for Age-Related Oxidative Damage in Dogs. Vet. Sci. 2025, 12, 962. https://doi.org/10.3390/vetsci12100962
Muršec A, Poljšak B, Nemec Svete A, Erjavec V. Antioxidant Strategies for Age-Related Oxidative Damage in Dogs. Veterinary Sciences. 2025; 12(10):962. https://doi.org/10.3390/vetsci12100962
Chicago/Turabian StyleMuršec, Aljaž, Borut Poljšak, Alenka Nemec Svete, and Vladimira Erjavec. 2025. "Antioxidant Strategies for Age-Related Oxidative Damage in Dogs" Veterinary Sciences 12, no. 10: 962. https://doi.org/10.3390/vetsci12100962
APA StyleMuršec, A., Poljšak, B., Nemec Svete, A., & Erjavec, V. (2025). Antioxidant Strategies for Age-Related Oxidative Damage in Dogs. Veterinary Sciences, 12(10), 962. https://doi.org/10.3390/vetsci12100962






