Inside the Tumor: Decoding the Feline Mammary Tumor Microenvironment and Its Prognostic Value—A Review
Abstract
Simple Summary
Abstract
1. Introduction
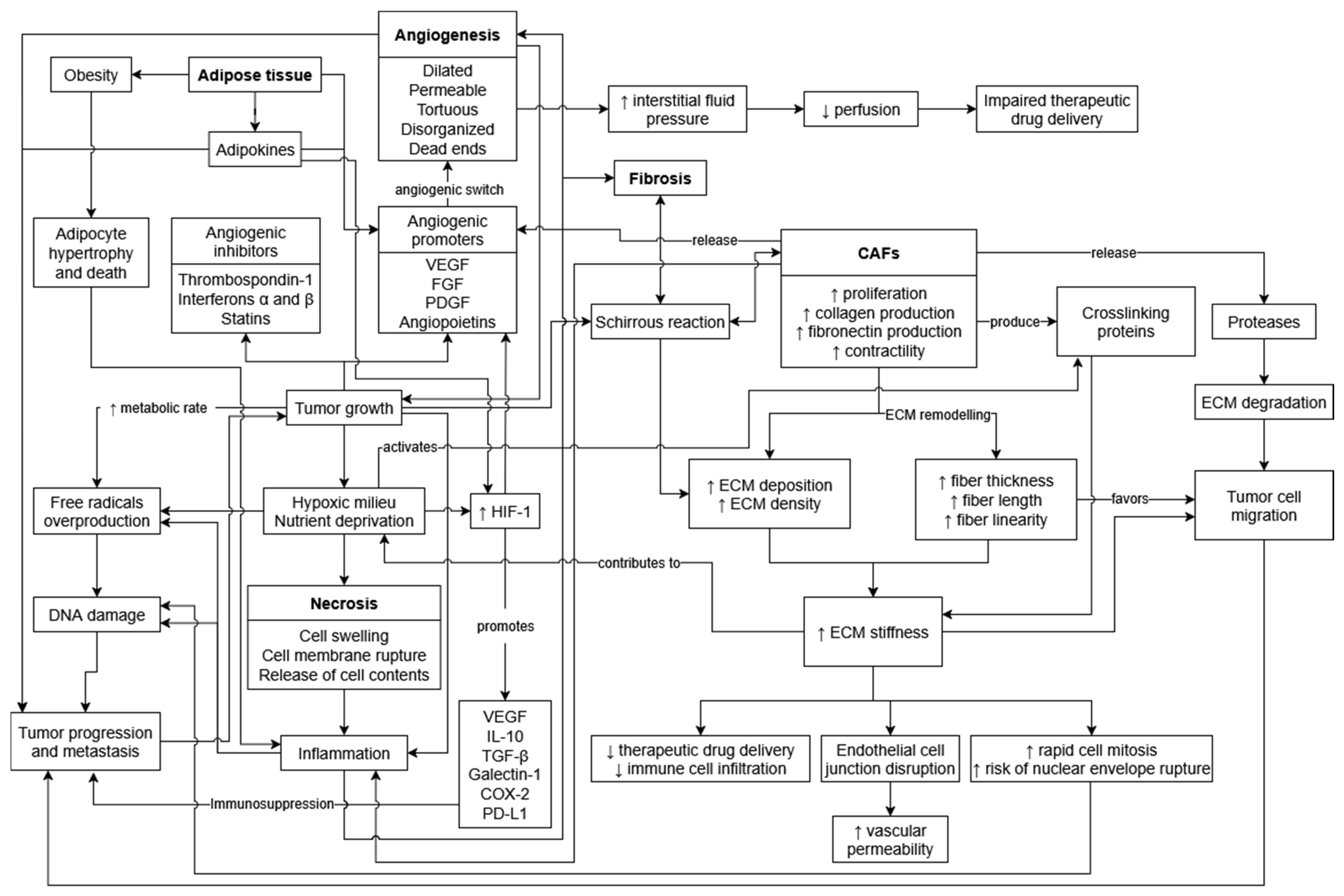
2. Tumor Necrosis
3. Tumor Angiogenesis
4. Tumor Fibrosis
5. Adipose Tissue
6. Tumor-Associated Inflammation
6.1. Tumor-Infiltrating Lymphocytes
6.2. Tumor-Infiltrating Macrophages
6.3. Other Immune Cells
6.4. Immune Phenotypes
6.5. Immune Checkpoints
7. Extracellular Vesicles
8. Epithelial–Mesenchymal Transition
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paget, S. Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Witz, I.P. The Tumor Microenvironment: The Making of a Paradigm. Cancer Microenviron. 2009, 2, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Pas de Deux: Control of Anti-Tumor Immunity by Cancer-Associated Inflammation. Immunity 2019, 51, 15–26. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Chang, W.-A.; Huang, M.-S.; Kuo, P.-L. Tumor Microenvironment: A New Treatment Target for Cancer. ISRN Biochem. 2014, 2014, 351959. [Google Scholar] [CrossRef]
- Caliari, D.; Zappulli, V.; Rasotto, R.; Cardazzo, B.; Frassineti, F.; Goldschmidt, M.H.; Castagnaro, M. Triple-Negative Vimentin-Positive Heterogeneous Feline Mammary Carcinomas as a Potential Comparative Model for Breast Cancer. BMC Vet. Res. 2014, 10, 185. [Google Scholar] [CrossRef]
- Cannon, C.M. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef]
- Frénel, J.-S.; Nguyen, F. Mammary Carcinoma: Comparative Oncology between Small Animals and Humans—New Therapeutic Tools. Reprod. Domest. Anim. 2023, 58 (Suppl. S2), 102–108. [Google Scholar] [CrossRef]
- Gameiro, A.; Urbano, A.C.; Ferreira, F. Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Vet. Sci. 2021, 8, 164. [Google Scholar] [CrossRef]
- Nascimento, C.; Ferreira, F. Tumor Microenvironment of Human Breast Cancer, and Feline Mammary Carcinoma as a Potential Study Model. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188587. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Madeira, S.; Correia, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F. Molecular Based Subtyping of Feline Mammary Carcinomas and Clinicopathological Characterization. Breast 2016, 27, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, L.; Howard, J.; Evans, S.; Kelly, P.; McCann, A. Comparative Gene Expression Study Highlights Molecular Similarities between Triple Negative Breast Cancer Tumours and Feline Mammary Carcinomas. Vet. Comp. Oncol. 2022, 20, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Wiese, D.A.; Thaiwong, T.; Yuzbasiyan-Gurkan, V.; Kiupel, M. Feline Mammary Basal-like Adenocarcinomas: A Potential Model for Human Triple-Negative Breast Cancer (TNBC) with Basal-like Subtype. BMC Cancer 2013, 13, 403. [Google Scholar] [CrossRef]
- Zappulli, V.; Zan, G.D.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline Mammary Tumours in Comparative Oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. General Pathology—Chapter 1: Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In Pathologic Basis of Veterinary Disease; Elsevier: St. Louis, MI, USA, 2017; pp. 2–43. ISBN 978-0-323-35775-3. [Google Scholar]
- Richards, C.H.; Mohammed, Z.; Qayyum, T.; Horgan, P.G.; McMillan, D.C. The Prognostic Value of Histological Tumor Necrosis in Solid Organ Malignant Disease: A Systematic Review. Future Oncol. 2011, 7, 1223–1235. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Marioli-Sapsakou, G.-K.; Kourti, M. Targeting Production of Reactive Oxygen Species as an Anticancer Strategy. Anticancer Res. 2021, 41, 5881–5902. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Yee, P.P.; Li, W. Tumor Necrosis: A Synergistic Consequence of Metabolic Stress and Inflammation. BioEssays 2021, 43, e2100029. [Google Scholar] [CrossRef]
- Thakur, B.; Kumar, Y.; Bhatia, A. Programmed Necrosis and Its Role in Management of Breast Cancer. Pathol. Res. Pract. 2019, 215, 152652. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Moore, F.M.; Williams, B.; Bertram, C.A.; Donovan, T.A.; Klopfleisch, R.; Meuten, D.J.; Santos, R.L. Tumor Necrosis Guideline, Version 1.1. Veterinary Cancer Guidelines and Protocols. 2021. Available online: http://vetcancerprotocols.org (accessed on 9 August 2023).
- Chen, B.; Lin, S.J.-H.; Li, W.-T.; Chang, H.-W.; Pang, V.F.; Chu, P.-Y.; Lee, C.-C.; Nakayama, H.; Wu, C.-H.; Jeng, C.-R. Expression of HIF-1α and VEGF in Feline Mammary Gland Carcinomas: Association with Pathological Characteristics and Clinical Outcomes. BMC Vet. Res. 2020, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Dagher, E.; Abadie, J.; Loussouarn, D.; Campone, M.; Nguyen, F. Feline Invasive Mammary Carcinomas: Prognostic Value of Histological Grading. Vet. Pathol. 2019, 56, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Gregorio, R.M.; Fisher, B.; Redmond, C.; Vellios, F.; Sommers, S.C. The Pathology of Invasive Breast Cancer. A Syllabus Derived from Findings of the National Surgical Adjuvant Breast Project (Protocol No. 4). Cancer 1975, 36, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Palekar, A.S.; Gregorio, R.M.; Redmond, C.; Fisher, B. Pathological Findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). IV. Significance of Tumor Necrosis. Hum. Pathol. 1978, 9, 523–530. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Correia, J.; Ferreira, F. HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models. Cancers 2021, 13, 2007. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Urbano, A.C.; Correia, J.; Ferreira, F. Serum and Tissue Expression Levels of Leptin and Leptin Receptor Are Putative Markers of Specific Feline Mammary Carcinoma Subtypes. Front. Vet. Sci. 2021, 8, 83. [Google Scholar] [CrossRef]
- Leek, R.D.; Landers2, R.J.; Harris, A.L.; Lewis, C.E. Necrosis Correlates with High Vascular Density and Focal Macrophage Infiltration in Invasive Carcinoma of the Breast. Br. J. Cancer 1999, 79, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; Gameiro, A.; Correia, J.; Ferreira, J.; Ferreira, F. The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications. Cells 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Jesus, J.; Canadas-Sousa, A.; Santos, M.; Oliveira, P.; Figueira, A.C.; Marrinhas, C.; Petrucci, G.N.; Gregório, H.; Tinoco, F.; Goulart, A.; et al. Level of Necrosis in Feline Mammary Tumors: How to Quantify, Why and for What Purpose? Animals 2024, 14, 3280. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals—Volume 2: Mammary Tumors, 3rd ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019; Volume 2, ISBN 978-1-7337491-1-4. [Google Scholar]
- Mills, S.W.; Musil, K.M.; Davies, J.L.; Hendrick, S.; Duncan, C.; Jackson, M.L.; Kidney, B.; Philibert, H.; Wobeser, B.K.; Simko, E. Prognostic Value of Histologic Grading for Feline Mammary Carcinoma: A Retrospective Survival Analysis. Vet. Pathol. 2015, 52, 238–249. [Google Scholar] [CrossRef]
- Seixas, F.; Palmeira, C.; Pires, M.A.; Bento, M.J.; Lopes, C. Grade Is an Independent Prognostic Factor for Feline Mammary Carcinomas: A Clinicopathological and Survival Analysis. Vet. J. 2011, 187, 65–71. [Google Scholar] [CrossRef]
- Weijer, K.; Hart, A.A. Prognostic Factors in Feline Mammary Carcinoma. J. Natl. Cancer Inst. 1983, 70, 709–716. [Google Scholar]
- Aaltomaa, S.; Lipponen, P.; Eskelinen, M.; Kosma, V.M.; Mari, S.; Alhava, E.; Syrjänen, K. Histological Assessment of the Prognostic Factors in Female Breast Cancer. Oncology 1992, 49, 1–8. [Google Scholar] [CrossRef]
- Carlomagno, C.; Perrone, F.; Lauria, R.; de Laurentiis, M.; Gallo, C.; Morabito, A.; Pettinato, G.; Panico, L.; Bellelli, T.; Apicella, A. Prognostic Significance of Necrosis, Elastosis, Fibrosis and Inflammatory Cell Reaction in Operable Breast Cancer. Oncology 1995, 52, 272–277. [Google Scholar] [CrossRef]
- Carter, D.; Pipkin, R.D.; Shepard, R.H.; Elkins, R.C.; Abbey, H. Relationship of Necrosis and Tumor Border to Lymph Node Metastases and 10-Year Survival in Carcinoma of the Breast. Am. J. Surg. Pathol. 1978, 2, 39–46. [Google Scholar] [CrossRef]
- Gilchrist, K.W.; Gray, R.; Fowble, B.; Tormey, D.C.; Taylor, S.G. Tumor Necrosis Is a Prognostic Predictor for Early Recurrence and Death in Lymph Node-Positive Breast Cancer: A 10-Year Follow-up Study of 728 Eastern Cooperative Oncology Group Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 1929–1935. [Google Scholar] [CrossRef]
- Millanta, F.; Lazzeri, G.; Vannozzi, I.; Viacava, P.; Poli, A. Correlation of Vascular Endothelial Growth Factor Expression to Overall Survival in Feline Invasive Mammary Carcinomas. Vet. Pathol. 2002, 39, 690–696. [Google Scholar] [CrossRef]
- De Campos, C.B.; Damasceno, K.A.; Gamba, C.O.; Ribeiro, A.M.; Machado, C.J.; Lavalle, G.E.; Cassali, G.D. Evaluation of Prognostic Factors and Survival Rates in Malignant Feline Mammary Gland Neoplasms. J. Feline Med. Surg. 2015, 18, 1003–1012. [Google Scholar] [CrossRef]
- Rosen, S.; Brisson, B.K.; Durham, A.C.; Munroe, C.M.; McNeill, C.J.; Stefanovski, D.; Sørenmo, K.U.; Volk, S.W. Intratumoral Collagen Signatures Predict Clinical Outcomes in Feline Mammary Carcinoma. PLoS ONE 2020, 15, e0236516. [Google Scholar] [CrossRef]
- Guimarães, J.C.M.; Petrucci, G.; Prada, J.; Pires, I.; Queiroga, F.L. Immunohistochemical Expression and Prognostic Value of COX-2 and Alpha-Smooth Muscle Actin-Positive Cancer-Associated Fibroblasts in Feline Mammary Cancer. In Vivo 2024, 38, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Koti, M.; Siemens, D.R.; Graham, C.H. Mechanisms of Hypoxia-Mediated Immune Escape in Cancer. Cancer Res. 2014, 74, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The Role of Hypoxia-Inducible Factor 1 in Tumor Immune Evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Chapter 7: Neoplasia. In Robbins & Cotran Pathologic Basis of Disease; Elsevier: Philadelphia, PA, USA, 2021; pp. 267–338. ISBN 978-0-323-53113-9. [Google Scholar]
- Ribatti, D.; Nico, B.; Crivellato, E.; Roccaro, A.M.; Vacca, A. The History of the Angiogenic Switch Concept. Leukemia 2007, 21, 44–52. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Newkirk, K.M.; Brannick, E.M.; Kusewitt, D.F. General Pathology—Chapter 6: Neoplasia and Tumor Biology. In Pathologic Basis of Veterinary Disease; Elsevier: St. Louis, MO, USA, 2017; pp. 286–321. ISBN 978-0-323-35775-3. [Google Scholar]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2019, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Silvestri, G.; Vaselli, C.; Citi, S.; Pisani, G.; Lorenzi, D.; Poli, A. The Role of Vascular Endothelial Growth Factor and Its Receptor Flk-1/KDR in Promoting Tumour Angiogenesis in Feline and Canine Mammary Carcinomas: A Preliminary Study of Autocrine and Paracrine Loops. Res. Vet. Sci. 2006, 81, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Diagnostic Value of VEGF-A, VEGFR-1 and VEGFR-2 in Feline Mammary Carcinoma. Cancers 2021, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Matsumoto, M.; Hidaka, R.; Miyoshi, N.; Yasuda, N. Expression of NOS and VEGF in Feline Mammary Tumours and Their Correlation with Angiogenesis. Vet. J. 2012, 192, 338–344. [Google Scholar] [CrossRef]
- Sarli, G.; Sassi, F.; Brunetti, B.; Rizzo, A.; Diracca, L.; Benazzi, C. Lymphatic Vessels Assessment in Feline Mammary Tumours. BMC Cancer 2007, 7, 7. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Chapter 3: Inflammation and Repair. In Robbins & Cotran Pathologic Basis of Disease; Elsevier: Philadelphia, PA, USA, 2021; pp. 71–113. ISBN 978-0-323-53113-9. [Google Scholar]
- Ackermann, M.R. General Pathology—Chapter 3: Inflammation and Healing. In Pathologic Basis of Veterinary Disease; Elsevier: St. Louis, MO, USA, 2017; pp. 2–321. ISBN 978-0-323-35775-3. [Google Scholar]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The Double Edge Sword of Fibrosis in Cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.-K.; Weaver, V.M. Fibrosis and Cancer: A Strained Relationship. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Rybinski, B.; Franco-Barraza, J.; Cukierman, E. The Wound Healing, Chronic Fibrosis, and Cancer Progression Triad. Physiol. Genomics 2014, 46, 223–244. [Google Scholar] [CrossRef]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- McCormack, V.A.; Dos Santos Silva, I. Breast Density and Parenchymal Patterns as Markers of Breast Cancer Risk: A Meta-Analysis. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Barbazán, J.; Matic Vignjevic, D. Cancer Associated Fibroblasts: Is the Force the Path to the Dark Side? Curr. Opin. Cell Biol. 2019, 56, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; DeNardo, D.G. Tumor-Associated Fibrosis as a Regulator of Tumor Immunity and Response to Immunotherapy. Cancer Immunol. Immunother. 2017, 66, 1037–1048. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human Breast Cancer Invasion and Aggression Correlates with ECM Stiffening and Immune Cell Infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Ariga, N.; Sato, E.; Ohuchi, N.; Nagura, H.; Ohtani, H. Stromal Expression of Fibroblast Activation Protein/Seprase, a Cell Membrane Serine Proteinase and Gelatinase, Is Associated with Longer Survival in Patients with Invasive Ductal Carcinoma of Breast. Int. J. Cancer 2001, 95, 67–72. [Google Scholar] [CrossRef]
- Cai, D.; Wu, X.; Hong, T.; Mao, Y.; Ge, X.; Hua, D. CD61+ and CAF+ Were Found to Be Good Prognosis Factors for Invasive Breast Cancer Patients. Pathol. Res. Pract. 2017, 213, 1296–1301. [Google Scholar] [CrossRef]
- Martinez, L.M.; Labovsky, V.; de Luján Calcagno, M.; Davies, K.M.; Garcia Rivello, H.; Bianchi, M.S.; Wernicke, A.; Fernández Vallone, V.B.; Chasseing, N.A. CD105 Expression on CD34-Negative Spindle-Shaped Stromal Cells of Primary Tumor Is an Unfavorable Prognostic Marker in Early Breast Cancer Patients. PLoS ONE 2015, 10, e0121421. [Google Scholar] [CrossRef]
- Park, C.K.; Jung, W.H.; Koo, J.S. Expression of Cancer-Associated Fibroblast-Related Proteins Differs between Invasive Lobular Carcinoma and Invasive Ductal Carcinoma. Breast Cancer Res. Treat. 2016, 159, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.; Sjöblom, T.; Micke, P.; Pontén, F.; Landberg, G.; Heldin, C.-H.; Bergh, J.; Brennan, D.J.; Jirström, K.; Östman, A. Prognostic Significance of Stromal Platelet-Derived Growth Factor β-Receptor Expression in Human Breast Cancer. Am. J. Pathol. 2009, 175, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Pula, B.; Jethon, A.; Piotrowska, A.; Gomulkiewicz, A.; Owczarek, T.; Calik, J.; Wojnar, A.; Witkiewicz, W.; Rys, J.; Ugorski, M.; et al. Podoplanin Expression by Cancer-Associated Fibroblasts Predicts Poor Outcome in Invasive Ductal Breast Carcinoma. Histopathology 2011, 59, 1249–1260. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Berghoff, A.; Dinhof, C.; Jakesz, R.; Gnant, M.; Dubsky, P.; Jesch, B.; Heinzl, H.; Birner, P. Podoplanin-Expressing Cancer-Associated Fibroblasts Are Associated with Poor Prognosis in Invasive Breast Cancer. Breast Cancer Res. Treat. 2012, 134, 237–244. [Google Scholar] [CrossRef]
- Surowiak, P.; Murawa, D.; Materna, V.; Maciejczyk, A.; Pudelko, M.; Ciesla, S.; Breborowicz, J.; Murawa, P.; Zabel, M.; Dietel, M.; et al. Occurence of Stromal Myofibroblasts in the Invasive Ductal Breast Cancer Tissue Is an Unfavourable Prognostic Factor. Anticancer Res. 2007, 27, 2917–2924. [Google Scholar]
- Yamashita, M.; Ogawa, T.; Zhang, X.; Hanamura, N.; Kashikura, Y.; Takamura, M.; Yoneda, M.; Shiraishi, T. Role of Stromal Myofibroblasts in Invasive Breast Cancer: Stromal Expression of Alpha-Smooth Muscle Actin Correlates with Worse Clinical Outcome. Breast Cancer 2012, 19, 170–176. [Google Scholar] [CrossRef]
- Yang, Z.; Ni, W.; Cui, C.; Fang, L.; Xuan, Y. Tenascin C Is a Prognostic Determinant and Potential Cancer-Associated Fibroblasts Marker for Breast Ductal Carcinoma. Exp. Mol. Pathol. 2017, 102, 262–267. [Google Scholar] [CrossRef]
- De Kruijf, E.M.; Van Nes, J.G.H.; Van De Velde, C.J.H.; Putter, H.; Smit, V.T.H.B.M.; Liefers, G.J.; Kuppen, P.J.K.; Tollenaar, R.A.E.M.; Mesker, W.E. Tumor–Stroma Ratio in the Primary Tumor Is a Prognostic Factor in Early Breast Cancer Patients, Especially in Triple-Negative Carcinoma Patients. Breast Cancer Res. Treat. 2011, 125, 687–696. [Google Scholar] [CrossRef]
- Roeke, T.; Sobral-Leite, M.; Dekker, T.J.A.; Wesseling, J.; Smit, V.T.H.B.M.; Tollenaar, R.A.E.M.; Schmidt, M.K.; Mesker, W.E. The Prognostic Value of the Tumour-Stroma Ratio in Primary Operable Invasive Cancer of the Breast: A Validation Study. Breast Cancer Res. Treat. 2017, 166, 435–445. [Google Scholar] [CrossRef]
- Vangangelt, K.M.H.; van Pelt, G.W.; Engels, C.C.; Putter, H.; Liefers, G.J.; Smit, V.T.H.B.M.; Tollenaar, R.a.E.M.; Kuppen, P.J.K.; Mesker, W.E. Prognostic Value of Tumor-Stroma Ratio Combined with the Immune Status of Tumors in Invasive Breast Carcinoma. Breast Cancer Res. Treat. 2018, 168, 601–612. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, J.-P.; Chen, Y.-Y.; Zhang, H.-Y.; Wang, L.-W.; Xiong, B. Prognostic Significance of the Tumor-Stromal Ratio in Invasive Breast Cancer and a Proposal of a New Ts-TNM Staging System. J. Oncol. 2020, 2020, 9050631. [Google Scholar] [CrossRef]
- Hagenaars, S.C.; Vangangelt, K.M.H.; Van Pelt, G.W.; Karancsi, Z.; Tollenaar, R.A.E.M.; Green, A.R.; Rakha, E.A.; Kulka, J.; Mesker, W.E. Standardization of the Tumor-Stroma Ratio Scoring Method for Breast Cancer Research. Breast Cancer Res. Treat. 2022, 193, 545–553. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.A.; De Campos, C.B.; De Biasi Bassani Gonçalves, A.; Nunes, F.C.; Monteiro, L.N.; De Oliveira Vasconcelos, R.; Cassali, G.D. Relationship between the Inflammatory Tumor Microenvironment and Different Histologic Types of Canine Mammary Tumors. Res. Vet. Sci. 2018, 119, 209–214. [Google Scholar] [CrossRef]
- Case, A.; Brisson, B.K.; Durham, A.C.; Rosen, S.; Monslow, J.; Buza, E.; Salah, P.; Gillem, J.; Ruthel, G.; Veluvolu, S.; et al. Identification of Prognostic Collagen Signatures and Potential Therapeutic Stromal Targets in Canine Mammary Gland Carcinoma. PLoS ONE 2017, 12, e0180448. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Cunningham, S.; Lund, E.M.; Khanna, C.; Naramore, R.; Patel, A.; Day, M.J. Obesity and Associated Comorbidities in People and Companion Animals: A One Health Perspective. J. Comp. Pathol. 2017, 156, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, C.; Pérez, C.; Oyarzún, C.; Torres-Arévalo, Á. Overweight and Obesity in Domestic Cats: Epidemiological Risk Factors and Associated Pathologies. J. Feline Med. Surg. 2024, 26, 1098612X241285519. [Google Scholar] [CrossRef]
- Marshall, T.; Chen, J.; Viloria-Petit, A.M. Adipocyte-Derived Adipokines and Other Obesity-Associated Molecules in Feline Mammary Cancer. Biomedicines 2023, 11, 2309. [Google Scholar] [CrossRef]
- García-Estevez, L.; González-Martínez, S.; Moreno-Bueno, G. The Leptin Axis and Its Association With the Adaptive Immune System in Breast Cancer. Front. Immunol. 2021, 12, 784823. [Google Scholar] [CrossRef]
- Lagarde, C.B.; Thapa, K.; Cullen, N.M.; Hawes, M.L.; Salim, K.; Benz, M.C.; Dietrich, S.R.; Burow, B.E.; Bunnell, B.A.; Martin, E.C.; et al. Obesity and Leptin in Breast Cancer Angiogenesis. Front. Endocrinol. 2024, 15, 1465727. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, Q.; Fares, H.M.; Wang, Y.; Han, Y.; Sun, L. Contribution of Adipocytes in the Tumor Microenvironment to Breast Cancer Metabolism. Cancer Lett. 2022, 534, 215616. [Google Scholar] [CrossRef] [PubMed]
- Burnet, M. Cancer—A Biological Approach. Br. Med. J. 1957, 1, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Burnet, M. Immunological Factors In The Process Of Carcinogenesis. Br. Med. Bull. 1964, 20, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Degnim, A.C.; Brahmbhatt, R.D.; Radisky, D.C.; Hoskin, T.L.; Stallings-Mann, M.; Laudenschlager, M.; Mansfield, A.; Frost, M.H.; Murphy, L.; Knutson, K.; et al. Immune Cell Quantitation in Normal Breast Tissue Lobules with and without Lobulitis. Breast Cancer Res. Treat. 2014, 144, 539–549. [Google Scholar] [CrossRef]
- Degnim, A.C.; Hoskin, T.L.; Arshad, M.; Frost, M.H.; Winham, S.J.; Brahmbhatt, R.A.; Pena, A.; Carter, J.M.; Stallings-Mann, M.L.; Murphy, L.M.; et al. Alterations in the Immune Cell Composition in Premalignant Breast Tissue That Precede Breast Cancer Development. Clin. Cancer Res. 2017, 23, 3945–3952. [Google Scholar] [CrossRef]
- Weijer, K.; Head, K.W.; Misdorp, W.; Hampe, J.F. Feline Malignant Mammary Tumors. I. Morphology and Biology: Some Comparisons with Human and Canine Mammary Carcinomas. J. Natl. Cancer Inst. 1972, 49, 1697–1704. [Google Scholar] [CrossRef]
- Chocteau, F.; Boulay, M.-M.; Besnard, F.; Valeau, G.; Loussouarn, D.; Nguyen, F. Proposal for a Histological Staging System of Mammary Carcinomas in Dogs and Cats. Part 2: Feline Mammary Carcinomas. Front. Vet. Sci. 2019, 6, 387. [Google Scholar] [CrossRef]
- Dagher, E.; Abadie, J.; Loussouarn, D.; Fanuel, D.; Campone, M.; Nguyen, F. Bcl-2 Expression and Prognostic Significance in Feline Invasive Mammary Carcinomas: A Retrospective Observational Study. BMC Vet. Res. 2019, 15, 25. [Google Scholar] [CrossRef]
- Rodrigues-Jesus, J.; Canadas-Sousa, A.; Oliveira, P.; Figueira, A.C.; Marrinhas, C.; Petrucci, G.N.; Gregório, H.; Tinoco, F.; Goulart, A.; Felga, H.; et al. Distribution of Inflammatory Infiltrate in Feline Mammary Lesions: Relationship With Clinicopathological Features. Vet. Comp. Oncol. 2024, 22, 398–409. [Google Scholar] [CrossRef]
- Dagher, E.; Simbault, L.; Abadie, J.; Loussouarn, D.; Campone, M.; Nguyen, F. Identification of an Immune-Suppressed Subtype of Feline Triple-Negative Basal-like Invasive Mammary Carcinomas, Spontaneous Models of Breast Cancer. Tumour Biol. 2020, 42, 1010428319901052. [Google Scholar] [CrossRef]
- Sammarco, A.; Finesso, G.; Zanetti, R.; Ferro, S.; Rasotto, R.; Caliari, D.; Goldschmidt, M.H.; Orvieto, E.; Castagnaro, M.; Cavicchioli, L.; et al. Biphasic Feline Mammary Carcinomas Including Carcinoma and Malignant Myoepithelioma. Vet. Pathol. 2020, 57, 377–387. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the Serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef] [PubMed]
- Rogozynski, N.P.; Dixon, B. The Th1/Th2 Paradigm: A Misrepresentation of Helper T Cell Plasticity. Immunol. Lett. 2024, 268, 106870. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Coussens, L.M. Inflammation and Breast Cancer. Balancing Immune Response: Crosstalk between Adaptive and Innate Immune Cells during Breast Cancer Progression. Breast Cancer Res. BCR 2007, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hsu, T.-W.; Li, M.O. Immunity beyond Cancer Cells: Perspective from Tumor Tissue. Trends Cancer 2021, 7, 1010–1019. [Google Scholar] [CrossRef]
- Setrerrahmane, S.; Xu, H. Tumor-Related Interleukins: Old Validated Targets for New Anti-Cancer Drug Development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef]
- Al-Ghurabi, B.H. IL-2 and IL-4 Serum Levels in Breast Cancer. J. Fac. Med. Baghdad 2009, 51, 300–303. [Google Scholar] [CrossRef]
- König, A.; Vilsmaier, T.; Rack, B.; Friese, K.; Janni, W.; Jeschke, U.; Andergassen, U.; Trapp, E.; Jückstock, J.; Jäger, B.; et al. Determination of Interleukin-4, -5, -6, -8 and -13 in Serum of Patients with Breast Cancer Before Treatment and Its Correlation to Circulating Tumor Cells. Anticancer Res. 2016, 36, 3123–3130. [Google Scholar]
- Paccagnella, M.; Abbona, A.; Michelotti, A.; Geuna, E.; Ruatta, F.; Landucci, E.; Denaro, N.; Vanella, P.; Lo Nigro, C.; Galizia, D.; et al. Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression. Vaccines 2022, 10, 78. [Google Scholar] [CrossRef]
- Estrela-Lima, A.; Araújo, M.S.S.; Soares, R.P.; Ribeiro, L.G.R.; Damasceno, K.A.; Costa, A.T.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Cassali, G.D. Plasma Biomarkers Profile of Female Dogs with Mammary Carcinoma and Its Association with Clinical and Pathological Features. Vet. Comp. Oncol. 2013, 14, 88–100. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of Specific Cytokine Expression Patterns in Patients with Breast Cancer. Sci. Rep. 2019, 9, 2924. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Espinoza, J.A.; Torland, L.A.; Zucknick, M.; Kumar, S.; Haakensen, V.D.; Lüders, T.; Engebraaten, O.; Børresen-Dale, A.-L.; Kyte, J.A.; et al. Noninvasive Profiling of Serum Cytokines in Breast Cancer Patients and Clinicopathological Characteristics. OncoImmunology 2019, 8, e1537691. [Google Scholar] [CrossRef] [PubMed]
- Althobiti, M.; Aleskandarany, M.A.; Joseph, C.; Toss, M.; Mongan, N.; Diez-Rodriguez, M.; Nolan, C.C.; Ashankyty, I.; Ellis, I.O.; Green, A.R.; et al. Heterogeneity of Tumour-Infiltrating Lymphocytes in Breast Cancer and Its Prognostic Significance. Histopathology 2018, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Millar, E.; Browne, L.; Slapetova, I.; Shang, F.; Ren, Y.; Bradshaw, R.; Ann Brauer, H.; O’Toole, S.; Beretov, J.; Whan, R.; et al. TILs Immunophenotype in Breast Cancer Predicts Local Failure and Overall Survival: Analysis in a Large Radiotherapy Trial with Long-Term Follow-Up. Cancers 2020, 12, 2365. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Srivastava, A.N.; Makker, A.; Goel, M.M. Presence of CD3+ Tumor Infiltrating Lymphocytes Is Significantly Associated with Good Prognosis in Infiltrating Ductal Carcinoma of Breast. Indian J. Cancer 2013, 50, 239–244. [Google Scholar]
- Mahmoud, S.M.A.; Lee, A.H.S.; Paish, E.C.; Macmillan, R.D.; Ellis, I.O.; Green, A.R. The Prognostic Significance of B Lymphocytes in Invasive Carcinoma of the Breast. Breast Cancer Res. Treat. 2012, 132, 545–553. [Google Scholar] [CrossRef]
- Mohammed, Z.M.A.; Going, J.J.; Edwards, J.; Elsberger, B.; McMillan, D.C. The Relationship between Lymphocyte Subsets and Clinico-Pathological Determinants of Survival in Patients with Primary Operable Invasive Ductal Breast Cancer. Br. J. Cancer 2013, 109, 1676–1684. [Google Scholar] [CrossRef]
- Kim, S.; Lee, A.; Lim, W.; Park, S.; Cho, M.S.; Koo, H.; Moon, B.-I.; Sung, S.H. Zonal Difference and Prognostic Significance of Foxp3 Regulatory T Cell Infiltration in Breast Cancer. J. Breast Cancer 2014, 17, 8–17. [Google Scholar] [CrossRef]
- Baker, K.; Lachapelle, J.; Zlobec, I.; Bismar, T.A.; Terracciano, L.; Foulkes, W.D. Prognostic Significance of CD8+ T Lymphocytes in Breast Cancer Depends upon Both Oestrogen Receptor Status and Histological Grade. Histopathology 2011, 58, 1107–1116. [Google Scholar] [CrossRef]
- Kim, S.T.; Jeong, H.; Woo, O.H.; Seo, J.H.; Kim, A.; Lee, E.S.; Shin, S.W.; Kim, Y.H.; Kim, J.S.; Park, K.H. Tumor-Infiltrating Lymphocytes, Tumor Characteristics, and Recurrence in Patients with Early Breast Cancer. Am. J. Clin. Oncol. 2013, 36, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-Cell Infiltration and Breast Cancer Survival in 12 439 Patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, X.; Zhou, E.; Chen, G.; Qian, K.; Wu, X.; Miao, X.; Tang, Z. Intratumoral CD8+ Cytotoxic Lymphocyte Is a Favorable Prognostic Marker in Node-Negative Breast Cancer. PLoS ONE 2014, 9, e95475. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Brady, N.J.; Chuntova, P.; Schwertfeger, K.L. Macrophages: Regulators of the Inflammatory Microenvironment during Mammary Gland Development and Breast Cancer. Mediators Inflamm. 2016, 2016, 4549676. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Van Dalen, F.J.; Van Stevendaal, M.H.M.E.; Fennemann, F.L.; Verdoes, M.; Ilina, O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules 2019, 24, 9. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Liu, X.; Liu, Y. The Role of Tumor-Associated Macrophages in Breast Carcinoma Invasion and Metastasis. Int. J. Clin. Exp. Pathol. 2015, 8, 6656–6664. [Google Scholar]
- Gwak, J.M.; Jang, M.H.; Kim, D.I.; Seo, A.N.; Park, S.Y. Prognostic Value of Tumor-Associated Macrophages According to Histologic Locations and Hormone Receptor Status in Breast Cancer. PLoS ONE 2015, 10, e0125728. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human Breast Cancer Cells Educate Macrophages toward the M2 Activation Status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The Presence of Tumor Associated Macrophages in Tumor Stroma as a Prognostic Marker for Breast Cancer Patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Liu, Z.; Wang, L.; Liu, S.; Zhang, Q. Jagged1 Modulated Tumor-Associated Macrophage Differentiation Predicts Poor Prognosis in Patients with Invasive Micropapillary Carcinoma of the Breast. Medicine 2017, 96, e6663. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, W.; Yang, P.; Lin, R.; Pu, L.; Zhang, H. Dual Roles of Innate Immune Cells and Cytokines in Shaping the Breast Cancer Microenvironment. Front. Immunol. 2025, 16, 1654947. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.U. Exploring Neutrophil Functionality in Breast Cancer Progression: A Review. Medicine 2024, 103, e37654. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Aghebati Maleki, L.; Alexander, M.; Mikhailova, M.V.; Masjedi, A.; Ahmadpour, M.; Hashemi, V.; Jadidi-Niaragh, F. Tumor-Associated Neutrophils as New Players in Immunosuppressive Process of the Tumor Microenvironment in Breast Cancer. Life Sci. 2021, 264, 118699. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, J.; Sun, Q.; Lu, N.; Pan, Y.; Han, X. Role of Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy: A Meta-Analysis. BMJ Open 2021, 11, e047957. [Google Scholar] [CrossRef]
- Petrucci, G.; Lobo, L.; Queiroga, F.; Martins, J.; Prada, J.; Pires, I.; Henriques, J. Neutrophil-to-Lymphocyte Ratio Is an Independent Prognostic Marker for Feline Mammary Carcinomas. Vet. Comp. Oncol. 2021, 19, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Yuki, M.; Hirano, T.; Kainuma, D.; Aoyama, R. Prognostic Utility of Preoperative Neutrophil-Lymphocyte Ratio in Cats with Malignant Mammary Tumors. Res. Vet. Sci. 2021, 135, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Tamma, R. Controversial Role of Mast Cells in Breast Cancer Tumor Progression and Angiogenesis. Clin. Breast Cancer 2021, 21, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Yavas, Ö.; Yavas, S.; Cangül, İ.; Sönmez, G. Antitumorogenic Effect of Mast Cells: Insights from an Experimentally-Induced Mammary Carcinoma Model in Rats and Feline and Canine Mammary Tumors. Pak. Vet. J. 2024, 44, 1131–1141. [Google Scholar] [CrossRef]
- Gupta, Y.H.; Khanom, A.; Acton, S.E. Control of Dendritic Cell Function Within the Tumour Microenvironment. Front. Immunol. 2022, 13, 733800. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer–Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Tiwari, A.; Oravecz, T.; Dillon, L.A.; Italiano, A.; Audoly, L.; Fridman, W.H.; Clifton, G.T. Towards a Consensus Definition of Immune Exclusion in Cancer. Front. Immunol. 2023, 14, 1084887. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 1–65. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. T-Lymphocytic Infiltrate in Canine Mammary Tumours: Clinic and Prognostic Implications. In Vivo 2011, 25, 963–969. [Google Scholar]
- Vilela, T.; Valente, S.; Correia, J.; Ferreira, F. Advances in Immunotherapy for Breast Cancer and Feline Mammary Carcinoma: From Molecular Basis to Novel Therapeutic Targets. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189144. [Google Scholar] [CrossRef]
- Nascimento, C.; Urbano, A.C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Correia, J.; Ferreira, F. VISTA Is a Diagnostic Biomarker and Immunotherapy Target of Aggressive Feline Mammary Carcinoma Subtypes. Cancers 2021, 13, 5559. [Google Scholar] [CrossRef]
- Valente, S.; Nascimento, C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. TIM-3 Is a Potential Immune Checkpoint Target in Cats with Mammary Carcinoma. Cancers 2023, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Asano, Y.; Otsuka, T.; Aoki, E.; Takeuchi, H.; Kato, Y.; Kaneko, M.K.; Yamada, S.; Kagawa, Y.; et al. Molecular Characterization of Feline Immune Checkpoint Molecules and Establishment of PD-L1 Immunohistochemistry for Feline Tumors. PLoS ONE 2023, 18, e0281143. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Seixas, F.; dos Anjos Pires, M.; Alves, A.; Santos, A.; Marrinhas, C.; Vilhena, H.; Santos, J.; Faísca, P.; Dias-Pereira, P.; et al. PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors. Vet. Sci. 2025, 12, 731. [Google Scholar] [CrossRef]
- Diomaiuto, E.; Principe, V.; De Luca, A.; Laperuta, F.; Alterisio, C.; Di Loria, A. Exosomes in Dogs and Cats: An Innovative Approach to Neoplastic and Non-Neoplastic Diseases. Pharmaceuticals 2021, 14, 766. [Google Scholar] [CrossRef]
- Sullivan, R.; Maresh, G.; Zhang, X.; Salomon, C.; Hooper, J.; Margolin, D.; Li, L. The Emerging Roles of Extracellular Vesicles As Communication Vehicles within the Tumor Microenvironment and Beyond. Front. Endocrinol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Howard, J.; Wyse, C.; Argyle, D.; Quinn, C.; Kelly, P.; McCann, A. Exosomes as Biomarkers of Human and Feline Mammary Tumours; A Comparative Medicine Approach to Unravelling the Aggressiveness of TNBC. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188431. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, F.; Fang, Y.; Yang, Y.; Zhou, Q.; Yuan, W.; Gu, X.; Hu, J.; Yang, S. Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication. Signal Transduct. Target. Ther. 2024, 9, 236. [Google Scholar] [CrossRef]
- Sammarco, A.; Finesso, G.; Cavicchioli, L.; Ferro, S.; Caicci, F.; Zanetti, R.; Sacchetto, R.; Zappulli, V. Preliminary Investigation of Extracellular Vesicles in Mammary Cancer of Dogs and Cats: Identification and Characterization. Vet. Comp. Oncol. 2018, 16, 489–496. [Google Scholar] [CrossRef]
- Howard, J.; Browne, J.; Bollard, S.; Peters, S.; Sweeney, C.; Wynne, K.; Potter, S.; McCann, A.; Kelly, P. The Protein and miRNA Profile of Plasma Extracellular Vesicles (EVs) Can Distinguish Feline Mammary Adenocarcinoma Patients from Healthy Feline Controls. Sci. Rep. 2023, 13, 9178. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Liu, H.-P.; Chuang, H.-L.; Liao, J.-W.; Kao, P.-L.; Chan, H.-L.; Chen, T.-H.; Wang, Y.-C. Feline Mammary Carcinoma-Derived Extracellular Vesicle Promotes Liver Metastasis via Sphingosine Kinase-1-Mediated Premetastatic Niche Formation. Lab. Anim. Res. 2023, 39, 27. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chuang, H.-L.; Yin, J.-H.; Liao, J.-W.; Chen, T.-H.; Wang, Y.-C. Significance of Sphingosine Kinase 1 Expression in Feline Mammary Tumors. BMC Vet. Res. 2019, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Sammarco, A.; Gomiero, C.; Beffagna, G.; Cavicchioli, L.; Ferro, S.; Michieletto, S.; Orvieto, E.; Patruno, M.; Zappulli, V. Epithelial-to-Mesenchymal Transition and Phenotypic Marker Evaluation in Human, Canine, and Feline Mammary Gland Tumors. Animals 2023, 13, 878. [Google Scholar] [CrossRef]
- Baptista, C.S.; Santos, S.; Laso, A.; Bastos, E.; Ávila, S.; Guedes-Pinto, H.; Gärtner, F.; Gut, I.G.; Castrillo, J.L.; Chaves, R. Sequence Variation and mRNA Expression of the TWIST1 Gene in Cats with Mammary Hyperplasia and Neoplasia. Vet. J. 2012, 191, 203–207. [Google Scholar] [CrossRef]
- Peñafiel-Verdu, C.; Buendia, A.J.; Navarro, J.A.; Ramirez, G.A.; Vilafranca, M.; Altimira, J.; Sanchez, J. Reduced Expression of E-Cadherin and β-Catenin and High Expression of Basal Cytokeratins in Feline Mammary Carcinomas with Regional Metastasis. Vet. Pathol. 2012, 49, 979–987. [Google Scholar] [CrossRef]
- Martín de las Mulas, J.; Espinosa de los Monteros, A.; Bautista, M.J.; Gómez-Villamandos, J.C.; Morales, C. Immunohistochemical Distribution Pattern of Intermediate Filament Proteins and Muscle Actin in Feline and Human Mammary Carcinomas. J. Comp. Pathol. 1994, 111, 365–381. [Google Scholar] [CrossRef]
- Granados-Soler, J.L.; Junginger, J.; Hewicker-Trautwein, M.; Bornemann-Kolatzki, K.; Beck, J.; Brenig, B.; Betz, D.; Schille, J.T.; Murua Escobar, H.; Nolte, I. TiHo-0906: A New Feline Mammary Cancer Cell Line with Molecular, Morphological, and Immunocytological Characteristics of Epithelial to Mesenchymal Transition. Sci. Rep. 2018, 8, 13231. [Google Scholar] [CrossRef]
- Figueira, A.C.; Teodósio, A.S.; Carvalheira, J.; Lacerda, M.; de Matos, A.; Gärtner, F. P-Cadherin Expression in Feline Mammary Tissues. Vet. Med. Int. 2012, 2012, 687424. [Google Scholar] [CrossRef]
- Figueira, A.C.; Gomes, C.; de Oliveira, J.T.; Vilhena, H.; Carvalheira, J.; de Matos, A.J.F.; Pereira, P.D.; Gärtner, F. Aberrant P-Cadherin Expression Is Associated to Aggressive Feline Mammary Carcinomas. BMC Vet. Res. 2014, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Dias Pereira, P.; Gärtner, F. Expression of E-Cadherin in Normal, Hyperplastic and Neoplastic Feline Mammary Tissue. Vet. Rec. 2003, 153, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Figueira, A.C.; Gomes, C.; Vilhena, H.; Miranda, S.; Carvalheira, J.; Matos, A.J.F.D.; Dias-Pereira, P.; Gärtner, F. Characterization of α-, β- and P120-Catenin Expression in Feline Mammary Tissues and Their Relation with E- and P-Cadherin. Anticancer Res. 2015, 35, 3361–3369. [Google Scholar] [PubMed]
- Zappulli, V.; De Cecco, S.; Trez, D.; Caliari, D.; Aresu, L.; Castagnaro, M. Immunohistochemical Expression of E-Cadherin and β-Catenin in Feline Mammary Tumours. J. Comp. Pathol. 2012, 147, 161–170. [Google Scholar] [CrossRef]
- Furusawa, Y.; Takahashi, M.; Shima-Sawa, M.; Hatai, H.; Miyoshi, N.; Yamato, O.; Yabuki, A. Immunocytochemical Evaluation of Epithelial-Mesenchymal Transition in Epithelial Tumors of Dogs and Cats. J. Vet. Med. Sci. 2021, 83, 1363–1368. [Google Scholar] [CrossRef]
- Harms, P.W.; Frankel, T.L.; Moutafi, M.; Rao, A.; Rimm, D.L.; Taube, J.M.; Thomas, D.; Chan, M.P.; Pantanowitz, L. Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists. Mod. Pathol. 2023, 36, 100197. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An Introduction to Spatial Transcriptomics for Biomedical Research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Jin, Y.; Zuo, Y.; Li, G.; Liu, W.; Pan, Y.; Fan, T.; Fu, X.; Yao, X.; Peng, Y. Advances in Spatial Transcriptomics and Its Applications in Cancer Research. Mol. Cancer 2024, 23, 129. [Google Scholar] [CrossRef]
| Marker (Technique) | Categories/Thresholds | Significance | Outcome | Statistical Model | Reference |
|---|---|---|---|---|---|
| Tumor necrosis | |||||
| Necrosis (HE) | Absence, some necrosis, ≈30%, 60%, ≥90% | Yes | OS | MV | [39] |
| 25% cutoff | Yes | 1-year OS | UV | [27] | |
| Absent, isolated cells, extensive | No | TSS/DFI | UV | [38] | |
| 25% cutoff | No | TSS | UV | [37] | |
| Central necrosis of any type: present, absent | No | OS/TSS | UV | [28] | |
| Ischemic central necrosis: present, absent | No | OS/TSS/DFI | UV | [35] | |
| Comedo necrosis: present, absent Random necrosis: present, absent 25% cutoff | No | OS/TSS/DFI | UV | ||
| Necrosis (HE stereology) | 0.130 cutoff | No | OS/TSS/DFI | UV | |
| HIF-1α (IHC) | Overexpression: nuclear immunoreactivity in any tumor cells | Yes | 1-year OS | UV | [27] |
| Tumor angiogenesis | |||||
| VEGF (IHC) | 72.1% cutoff | Yes | OS | UV | [44] |
| Score 0 (absence of staining), score 1 (weak immunoreactivity in <50% of tumor cells), score 2 (weak immunoreactivity in ≥50% or strong immunoreactivity in <50% of tumor cells), score 3 (strong immunoreactivity in ≥50% of tumor cells) Overexpression: scores 2 and 3 | No | TSS | UV | [45] | |
| No | 1-year OS | UV | [27] | ||
| MVD (IHC) | 29.5 cutoff | No | OS | UV | [44] |
| Tumor fibrosis | |||||
| Collagen fibers (SHG) * | Collagen density (mean cutoff) | Yes | OS/DFI | UV | [46] |
| Tumor-stroma boundary: N (not enough collagen to score), score 0 (distinct division), score 1 (discrete) Tumor-stroma boundary (mean cutoff) | Yes | OS/DFI | UV | ||
| Yes | OS/DFI | MV | |||
| Collagen fiber number (mean cutoff) | No | OS/DFI | UV | ||
| Yes | OS/DFI | MV | |||
| Collagen fiber length (mean cutoff) | Yes | OS/DFI | UV | ||
| Collagen fiber width (mean cutoff) | Yes | OS/DFI | UV | ||
| Yes | DFI | MV | |||
| Collagen fiber straightness (mean cutoff) | Yes | OS/DFI | UV | ||
| Yes | DFI | MV | |||
| Stromal response (HE) | None to mild, peritumoral, intratumoral | Yes | 1-year OS | UV | [27] |
| α-SMA (IHC) | Immunoreactivity percentage: score 0 (absence of staining), score 1 (1–10% positive CAFs), score 2 (11–50% positive CAFs), score 3 (51–80% positive CAFs), score 4 (81–100% positive CAFs) Immunoreactivity intensity: score 0 (no staining), score 1 (weak), score 2 (moderate), score 3 (intense/strong) Final score = percentage score + intensity score Low immunoreactivity: final score < 6; high immunoreactivity: final score ≥ 6 | Yes | TSS/DFI | UV | [47] |
| Adipose tissue | |||||
| Leptin (ELISA) | 4.17 pg/mL cutoff | Yes | DFI | UV | [32] |
| n | Categories | % of Cases | Survival Times (Median) | Significance | Survival Variable | Statistical Model | Reference | |
|---|---|---|---|---|---|---|---|---|
| 170 | Lymphocytes and plasma cells | - | - | No | - | - | [106] | |
| 202 | Quantity of acute and chronic cellular inflammation | - | - | Yes | OS | UV | [39] | |
| No | OS | MV | ||||||
| 24 | Absent to negligible | 37.5% | - | - | - | - | [15] | |
| Few moderate peritumoral lymphoid aggregates | 41.7% | - | - | - | - | |||
| Moderate peritumoral lymphoid aggregates | 20.8% | - | - | - | - | |||
| 108 | Absent or mild | 31.5% | 18 months | No | TSS | UV | [37] | |
| Lymphoplasmacytic | 54.6% | 15 months | ||||||
| Neutrophilic or pleocellular | 13.9% | 9 months | ||||||
| 342 | Mononuclear | Absent to mild | 48.5% | 13.2 months | Yes | OS/TSS | UV | [28] |
| Moderate to severe | 51.4% | 8.5 months | ||||||
| 180 | Mononuclear | Absent to mild | 42.8% | - | - | - | - | [108] |
| Moderate to severe | 57.2% | |||||||
| 395 | Absent to mild (mononuclear) | 52.7% | - | Yes | DFI/OS/TSS | UV | [107] | |
| Moderate to severe (mononuclear) | 47.3% | TSS | MV | |||||
| 180 | Intratumoral Tregs (≥2 vs. <2/mm2) | 82.2% | - | Yes | DFI/OS/TSS | MV | [110] | |
| Stromal Tregs (≥6 vs. <6/mm2) | 91.7% | DFI/OS/TSS | MV | |||||
| Peritumoral Tregs (≥575 vs. <575/mm2) | 97.2% | DFI/OS/TSS | UV/MV | |||||
| 10 | Peripheral lymphocytes | 80.0% | - | - | - | - | [111] | |
| 49 | Positive score interstitial FoxP3+ lymphocytes | 71.4% | - | - | - | - | [112] | |
| None seen | 14.3% | - | - | - | - | |||
| <1% of FoxP3+ interstitial lymphocytes | 8.2% | - | - | - | - | |||
| 1–5% of FoxP3+ interstitial lymphocytes | 42.9% | - | - | - | - | |||
| 6–30% of FoxP3+ interstitial lymphocytes | 26.5% | - | - | - | - | |||
| >30% of FoxP3+ interstitial lymphocytes | 8.2% | - | - | - | - | |||
| 40 | Lymphocytic infiltration (yes vs. no) | 67.5% | - | - | - | - | [31] | |
| 56 | Lymphocytic infiltration (yes vs. no) | 66.1% | - | - | - | - | [32] | |
| 73 | Lymphocytic infiltration (yes vs. no) | 78.1% | - | - | - | - | [34] | |
| Intratumoral, stromal and total cells: CD3+, CD4+ and CD8+ * T cells, Tregs, B cells, NK cells, CD68+ and CD163+ macrophages | - | - | No | DFI/OS | UV | |||
| Low stromal CD8+ T cells | - | 15.5 months | Yes | DFI/OS | UV | |||
| High stromal CD8+ T cells | - | 31.0 months | ||||||
| 150 | Peritumoral inflammation distribution | Absent from focal | 5.3% | - | - | - | - | [109] |
| Multifocal to diffuse | 94.7% | - | - | - | - | |||
| Intratumoral inflammation distribution | Absent from focal | 28.7% | - | - | - | - | ||
| Multifocal to diffuse | 71.3% | - | - | - | - | |||
| Perilesional inflammation intensity | Discrete | 34.2% | - | - | - | - | ||
| Moderate to marked | 65.8% | - | - | - | - | |||
| Intratumoral inflammation intensity | Discrete | 58.2% | - | - | - | - | ||
| Moderate to marked | 41.8% | - | - | - | - | |||
| Tertiary lymphoid structures (present vs. absent) | 11.7% | - | - | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues-Jesus, J.; Canadas-Sousa, A.; Vilhena, H.; Dias-Pereira, P. Inside the Tumor: Decoding the Feline Mammary Tumor Microenvironment and Its Prognostic Value—A Review. Vet. Sci. 2025, 12, 959. https://doi.org/10.3390/vetsci12100959
Rodrigues-Jesus J, Canadas-Sousa A, Vilhena H, Dias-Pereira P. Inside the Tumor: Decoding the Feline Mammary Tumor Microenvironment and Its Prognostic Value—A Review. Veterinary Sciences. 2025; 12(10):959. https://doi.org/10.3390/vetsci12100959
Chicago/Turabian StyleRodrigues-Jesus, Joana, Ana Canadas-Sousa, Hugo Vilhena, and Patrícia Dias-Pereira. 2025. "Inside the Tumor: Decoding the Feline Mammary Tumor Microenvironment and Its Prognostic Value—A Review" Veterinary Sciences 12, no. 10: 959. https://doi.org/10.3390/vetsci12100959
APA StyleRodrigues-Jesus, J., Canadas-Sousa, A., Vilhena, H., & Dias-Pereira, P. (2025). Inside the Tumor: Decoding the Feline Mammary Tumor Microenvironment and Its Prognostic Value—A Review. Veterinary Sciences, 12(10), 959. https://doi.org/10.3390/vetsci12100959






