Effect of Oxygen Tension Modification During Oocyte Maturation on Porcine Oocyte Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Cumulus Cell–Oocyte Complex (COC) Collection and In Vitro Maturation
2.3. Parthenogenetic Activation (PA) of Oocytes and In Vitro Culture (IVC) of Embryos
2.4. Evaluation of Metaphase II (MII) Oocyte Maturation Rate, Cleavage Rate, and Blastulation Rate
2.5. Evaluation of ATP Content in the Oocytes and Blastocyst
2.6. Evaluation of Glucose Consumption in Bi-Phasic IVM
2.7. Lipid Content in the MII Oocytes and Blastocysts
2.8. Measurement of the Mitochondrial DNA Copy Number (Mt-cn)
2.9. Evaluation of the Mitochondrial Membrane Potential (MMP)
2.10. Immunoblot Analysis
2.11. RNA-Sequence (RNA-Seq) of Cumulus Cells from IVM
2.12. Statistical Analysis
3. Results
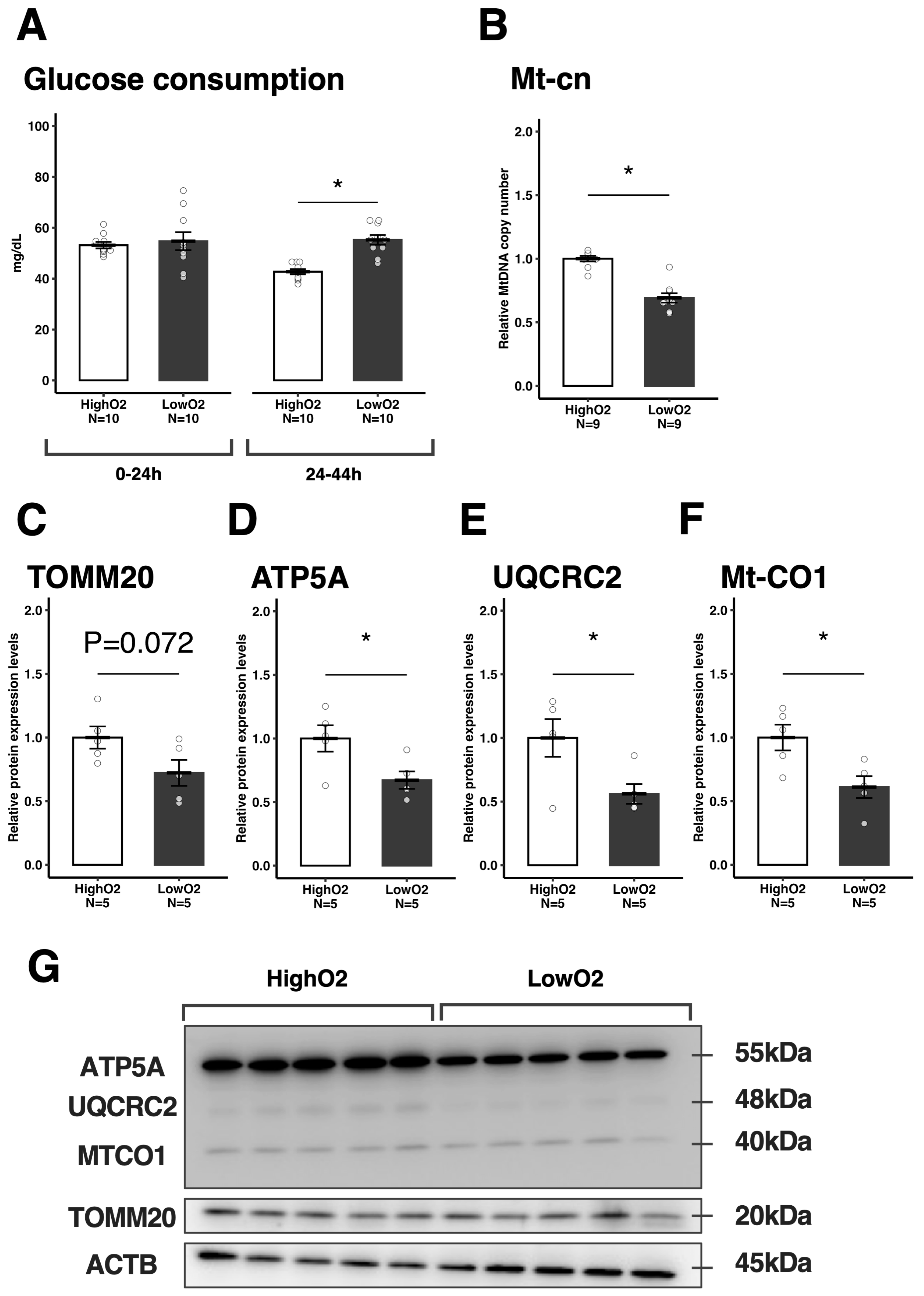
3.1. Developmental Ability and Mitochondrial Function in Oocytes
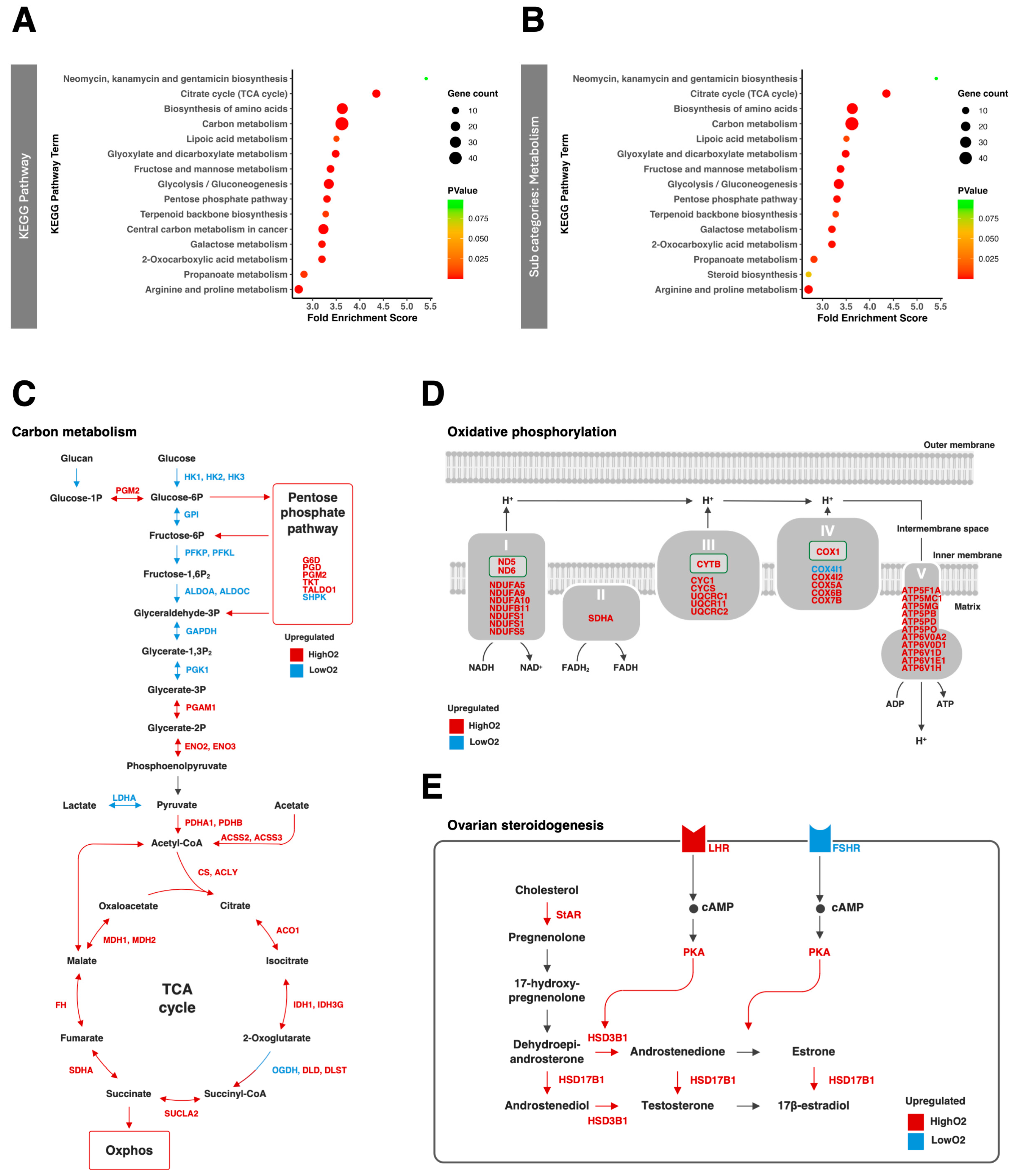
3.2. Metabolism of Cumulus Cells Under Two Oxygen Conditions
3.3. Novel Two-Step Culture Oxygen Condition Improves Oocyte Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Beta actin |
| ATP5A | ATP synthase lipid-binding protein V |
| Bi-O2 | 2-step O2 condition: Bi-O2 |
| CCs | Cumulus cells |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| COCs | Cumulus cell-oocyte complexes |
| dbcAMP | Dibutyryl-cAMP |
| DEGs | Differentially expressed genes |
| eCG | Equine chorionic gonadotropin |
| FDR | False Discovery Rate |
| FSH | Follicle stimulating hormone |
| FSHR | Follicle stimulating hormone receptor |
| GCs | Granulosa cells |
| hCG | Human chorionic gonadotropin |
| HighO2 | High oxygen tension |
| IVC | In vitro culture |
| IVM | In vitro maturation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LH | Luteinizing hormone |
| LHR | Luteinizing hormone receptor |
| LowO2 | Low oxygen tension (LowO2 |
| MII | Metaphase II |
| MMP | Mitochondrial membrane potential |
| Mt-cn | Mitochondrial DNA copy number |
| MT-CO1 | Cytochrome c oxidase subunit I |
| PA | Parthenogenetic activation |
| PBS | Phosphate-buffered saline |
| POM | Porcine oocyte medium |
| PVA | Polyvinyl alcohol |
| PZM-3 | Porcine zygote medium 3 |
| ROS | Reactive oxygen species |
| TCA | Tricarboxylic Acid |
| TOMM20 | Translocase of outer mitochondrial membrane 20 |
| TPM | Transcripts Per Million |
| UQCRC2 | Ubiquinol-cytochrome c reductase core protein II |
| VEGF | vascular endothelial growth factor |
| WB | Western blotting |
References
- Moor, R.; Dai, Y. Maturation of Pig Oocytes In Vivo and In Vitro. Reprod. Suppl. 2001, 58, 91–104. [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. In Vitro Maturation: A Committee Opinion. Fertil. Steril. 2021, 115, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Koertvelyessy, T.; Crawford, M.H.; Pap, M.; Szilagyi, K. The Influence of Religious Affiliation on Surname Repetition in Marriages in Tiszaszalka, Hungary. J. Biosoc. Sci. 1992, 24, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Alvarez, P.; Lonergan, P.; Rizos, D.; Gutiérrez-Adan, A. Low Oxygen Tension during IVM Improves Bovine Oocyte Competence and Enhances Anaerobic Glycolysis. Reprod. Biomed. Online 2010, 20, 341–349. [Google Scholar] [CrossRef]
- Iwamoto, M.; Onishi, A.; Fuchimoto, D.-I.; Somfai, T.; Takeda, K.; Tagami, T.; Hanada, H.; Noguchi, J.; Kaneko, H.; Nagai, T.; et al. Low Oxygen Tension during In Vitro Maturation of Porcine Follicular Oocytes Improves Parthenogenetic Activation and Subsequent Development to the Blastocyst Stage. Theriogenology 2005, 63, 1277–1289. [Google Scholar] [CrossRef]
- Kikuchi, K.; Onishi, A.; Kashiwazaki, N.; Iwamoto, M.; Noguchi, J.; Kaneko, H.; Akita, T.; Nagai, T. Successful Piglet Production after Transfer of Blastocysts Produced by a Modified In Vitro System. Biol. Reprod. 2002, 66, 1033–1041. [Google Scholar] [CrossRef]
- Preis, K.A.; Seidel, G.E., Jr.; Gardner, D.K. Reduced Oxygen Concentration Improves the Developmental Competence of Mouse Oocytes Following In Vitro Maturation. Mol. Reprod. Dev. 2007, 74, 893–903. [Google Scholar] [CrossRef]
- Rodrigues, B.A.; Rodrigues, C.A.; Salviano, M.B.; Willhelm, B.R.; Collares, F.J.F.; Rodrigues, J.L. Similar Patterns of Embryo Development in Canine Oocytes Cultured In Vitro at Oxygen Tensions of 5 and 20%. Theriogenology 2013, 79, 1224–1228. [Google Scholar] [CrossRef][Green Version]
- Kang, J.-T.; Atikuzzaman, M.; Kwon, D.-K.; Park, S.-J.; Kim, S.-J.; Moon, J.-H.; Koo, O.-J.; Jang, G.; Lee, B.-C. Developmental Competence of Porcine Oocytes after In Vitro Maturation and In Vitro Culture under Different Oxygen Concentrations. Zygote 2012, 20, 1–8. [Google Scholar] [CrossRef]
- Whitty, A.; Kind, K.L.; Dunning, K.R.; Thompson, J.G. Effect of Oxygen and Glucose Availability during In Vitro Maturation of Bovine Oocytes on Development and Gene Expression. J. Assist. Reprod. Genet. 2021, 38, 1349–1362. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic Co-Dependence of the Oocyte and Cumulus Cells: Essential Role in Determining Oocyte Developmental Competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Nagata, S.; Tatematsu, K.; Kansaku, K.; Inoue, Y.; Kobayashi, M.; Shirasuna, K.; Iwata, H. Effect of Aging on Mitochondria and Metabolism of Bovine Granulosa Cells. J. Reprod. Dev. 2020, 66, 547–554. [Google Scholar] [CrossRef]
- Hallberg, I.; Laskowski, D.; Sjunnesson, Y.C.B. In Vitro Maturation of Bovine and Porcine Oocytes as a Versatile Model for Toxicity and Metabolism Studies. Biol. Reprod. 2025, ioaf137. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hayashi, M.; Shirasuna, K.; Iwata, H. Acetic Acid Affects Porcine Oocyte Metabolism and Improves Oocyte Developmental Ability. Theriogenology 2024, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Suzuki, C.; Onishi, A. Defined System for In Vitro Production of Porcine Embryos Using a Single Basic Medium. J. Reprod. Dev. 2008, 54, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.-K.; Iwamura, S. Birth of Piglets Derived from Porcine Zygotes Cultured in a Chemically Defined Medium. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef]
- Iwata, H.; Goto, H.; Tanaka, H.; Sakaguchi, Y.; Kimura, K.; Kuwayama, T.; Monji, Y. Effect of Maternal Age on Mitochondrial DNA Copy Number, ATP Content and IVF Outcome of Bovine Oocytes. Reprod. Fertil. Dev. 2011, 23, 424–432. [Google Scholar] [CrossRef]
- Sirard, M.-A.; Desrosier, S.; Assidi, M. In Vivo and In Vitro Effects of FSH on Oocyte Maturation and Developmental Competence. Theriogenology 2007, 68 (Suppl. S1), S71–S76. [Google Scholar] [CrossRef]
- Morimoto, A.; Rose, R.D.; Smith, K.M.; Dinh, D.T.; Umehara, T.; Winstanley, Y.E.; Shibahara, H.; Russell, D.L.; Robker, R.L. Granulosa Cell Metabolism at Ovulation Correlates with Oocyte Competence and Is Disrupted by Obesity and Aging. Hum. Reprod. 2024, 39, 2053–2066. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Sugiura, K.; Eppig, J.J. Mouse Oocyte Control of Granulosa Cell Development and Function: Paracrine Regulation of Cumulus Cell Metabolism. Semin. Reprod. Med. 2009, 27, 32–42. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in Single Oocytes: Impact of Maturation and Cumulus Cells on Levels and Consumption: ATP in Mouse Oocytes. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef]
- Kansaku, K.; Itami, N.; Kawahara-Miki, R.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Differential Effects of Mitochondrial Inhibitors on Porcine Granulosa Cells and Oocytes. Theriogenology 2017, 103, 98–103. [Google Scholar] [CrossRef]
- Munakata, Y.; Ichinose, T.; Ogawa, K.; Itami, N.; Tasaki, H.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Relationship between the Number of Cells Surrounding Oocytes and Energy States of Oocytes. Theriogenology 2016, 86, 1789–1798.e1. [Google Scholar] [CrossRef]
- Silva, R.C.; Báo, S.N.; Jivago, J.L.P.R.; Lucci, C.M. Ultrastructural Characterization of Porcine Oocytes and Adjacent Follicular Cells during Follicle Development: Lipid Component Evolution. Theriogenology 2011, 76, 1647–1657. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, C.; Xiong, Q.; Yang, X.; Chi, D.; Li, P.; Liu, H.; Li, J.; Huang, R. Distribution and Content of Lipid Droplets and Mitochondria in Pig Parthenogenetically Activated Embryos after Delipation. Theriogenology 2015, 83, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.H.; Tran, V.Q.; Le, A.H.; Nguyen, D.L.; Pham, T.D.; Vu, A.L.; Le, T.K.; Le, H.L.; Huynh, B.G.; Ho, T.M.; et al. Impact of Low versus High Oxygen Tension on Human Oocyte Maturation during Biphasic Capacitation IVM (CAPA-IVM). J. Assist. Reprod. Genet. 2025, 42, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kawahara, M.; Abe, Y.; Yokoo, M.; Sasada, H.; Sato, E. Follicular Microvasculature and Angiogenic Factors in the Ovaries of Domestic Animals. J. Reprod. Dev. 2003, 49, 181–192. [Google Scholar] [CrossRef][Green Version]
- Basini, G.; Bianco, F.; Grasselli, F.; Tirelli, M.; Bussolati, S.; Tamanini, C. The Effects of Reduced Oxygen Tension on Swine Granulosa Cell. Regul. Pept. 2004, 120, 69–75. [Google Scholar] [CrossRef]
- Fischer, B.; Künzel, W.; Kleinstein, J.; Gips, H. Oxygen Tension in Follicular Fluid Falls with Follicle Maturation. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 43, 39–43. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Antczak, M.; Schrader, R. The Developmental Potential of the Human Oocyte Is Related to the Dissolved Oxygen Content of Follicular Fluid: Association with Vascular Endothelial Growth Factor Levels and Perifollicular Blood Flow Characteristics. Hum. Reprod. 1997, 12, 1047–1055. [Google Scholar] [CrossRef]
- Fischer, B.; Bavister, B.D. Oxygen Tension in the Oviduct and Uterus of Rhesus Monkeys, Hamsters and Rabbits. J. Reprod. Fertil. 1993, 99, 673–679. [Google Scholar] [CrossRef]
- Guzmán, A.; Hernández-Coronado, C.G.; Gutiérrez, C.G.; Rosales-Torres, A.M. The Vascular Endothelial Growth Factor (VEGF) System as a Key Regulator of Ovarian Follicle Angiogenesis and Growth. Mol. Reprod. Dev. 2023, 90, 201–217. [Google Scholar] [CrossRef]
- Shiratsuki, S.; Hara, T.; Munakata, Y.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Low Oxygen Level Increases Proliferation and Metabolic Changes in Bovine Granulosa Cells. Mol. Cell. Endocrinol. 2016, 437, 75–85. [Google Scholar] [CrossRef]
- Redding, G.P.; Bronlund, J.E.; Hart, A.L. Theoretical Investigation into the Dissolved Oxygen Levels in Follicular Fluid of the Developing Human Follicle Using Mathematical Modelling. Reprod. Fertil. Dev. 2008, 20, 408–417. [Google Scholar] [CrossRef]



| Groups | No. of Oocytes | No. of Trials | MII (%) | No of Oocytes | No. of Trials | 4 Cell (%) | Blastocysts (%) | Cell No. (No.) |
|---|---|---|---|---|---|---|---|---|
| HighO2 | 141 | 3 | 82.2 ± 2.8 | 176 | 17 | 58.0 ± 3.7 | 15.6 ± 1.4 | 38.6 ± 3.8 (23) |
| LowO2 | 146 | 3 | 80.0 ± 4.5 | 183 | 17 | 63.6 ± 3.2 | 19.2 ± 2.1 | 39.5 ± 2.9 (26) |
| Group | No. of Oocytes | No. of Trials | MII (%) | No. of Oocytes | No. of Trials | 4 Cell Rate (%) | Blastocyst Rate (%) | Cell No. (No.) | |
|---|---|---|---|---|---|---|---|---|---|
| HighO2 | 141 | 3 | 82.2 ± 2.8 | 320 | 32 | 43.8 ± 3.5 | 15.6 ± 2.5 | a | 55.3 ± 3.6 (39) |
| Bi-O2 | 153 | 3 | 91.6 ± 3.2 | 320 | 32 | 49.7 ± 3.3 | 24.1 ± 2.3 | b | 53.4 ± 2.4 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Akano, S.; Suzuki, Y.; Ushiroshoji, K.; Kamio, A.; Shirasuna, K.; Iwata, H. Effect of Oxygen Tension Modification During Oocyte Maturation on Porcine Oocyte Quality. Vet. Sci. 2025, 12, 954. https://doi.org/10.3390/vetsci12100954
Inoue Y, Akano S, Suzuki Y, Ushiroshoji K, Kamio A, Shirasuna K, Iwata H. Effect of Oxygen Tension Modification During Oocyte Maturation on Porcine Oocyte Quality. Veterinary Sciences. 2025; 12(10):954. https://doi.org/10.3390/vetsci12100954
Chicago/Turabian StyleInoue, Yuki, Saki Akano, Yuya Suzuki, Kota Ushiroshoji, Asuka Kamio, Koumei Shirasuna, and Hisataka Iwata. 2025. "Effect of Oxygen Tension Modification During Oocyte Maturation on Porcine Oocyte Quality" Veterinary Sciences 12, no. 10: 954. https://doi.org/10.3390/vetsci12100954
APA StyleInoue, Y., Akano, S., Suzuki, Y., Ushiroshoji, K., Kamio, A., Shirasuna, K., & Iwata, H. (2025). Effect of Oxygen Tension Modification During Oocyte Maturation on Porcine Oocyte Quality. Veterinary Sciences, 12(10), 954. https://doi.org/10.3390/vetsci12100954







