First Report on Cardiac Troponin T Detection in Canine Amniotic Fluid †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Procedures
2.2. cTnT Measurement in Amniotic Fluid
2.3. Statistical Analysis
3. Results
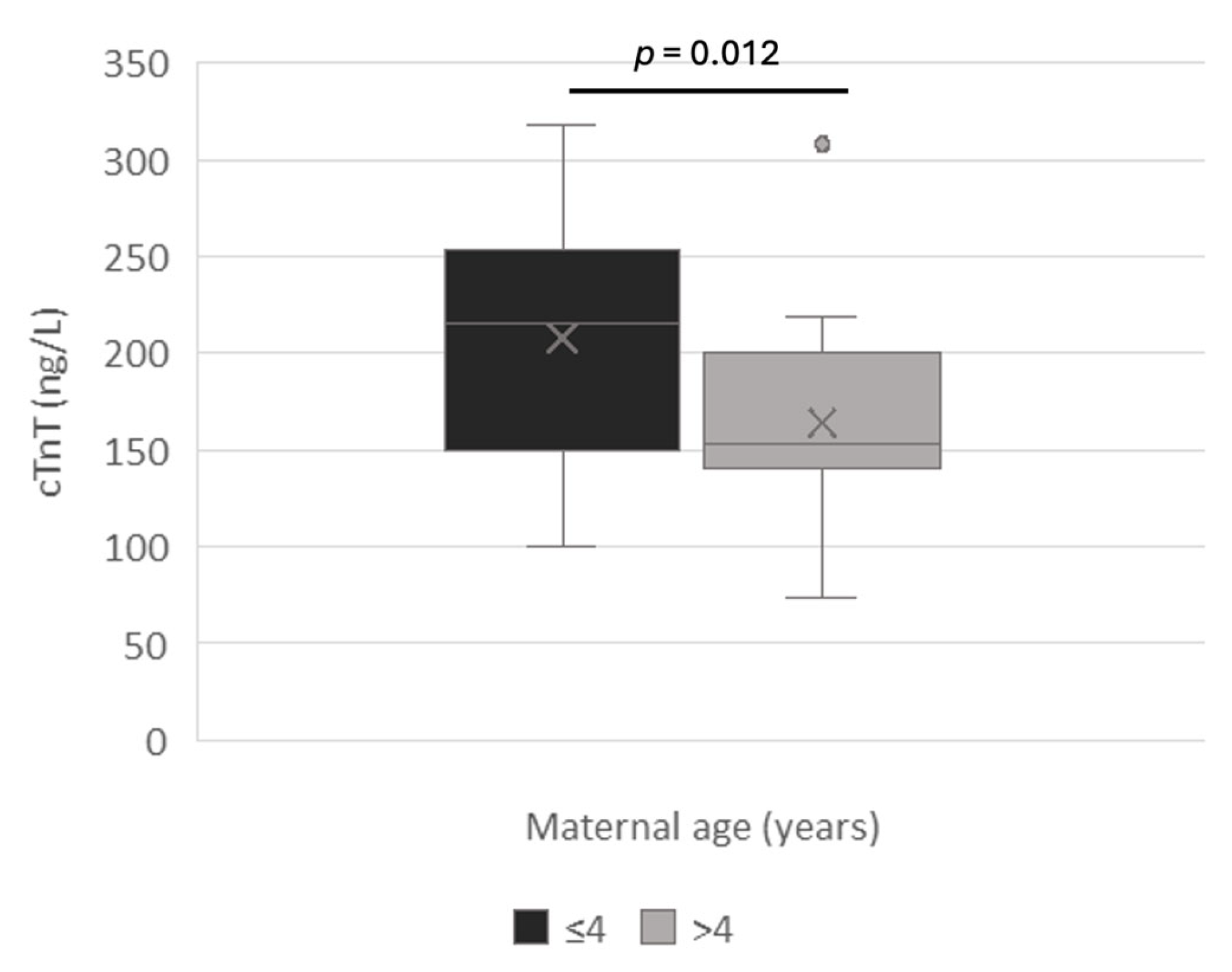
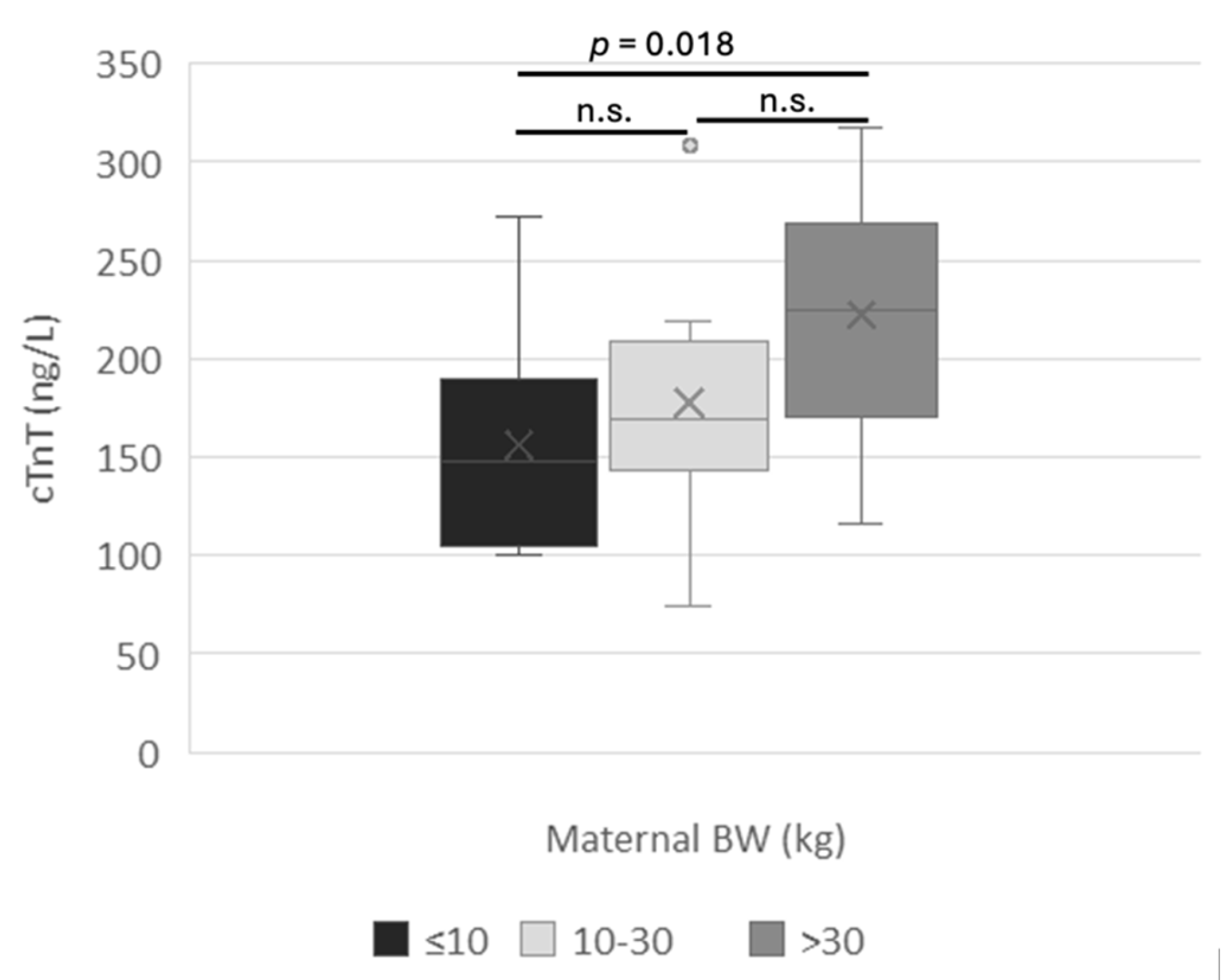
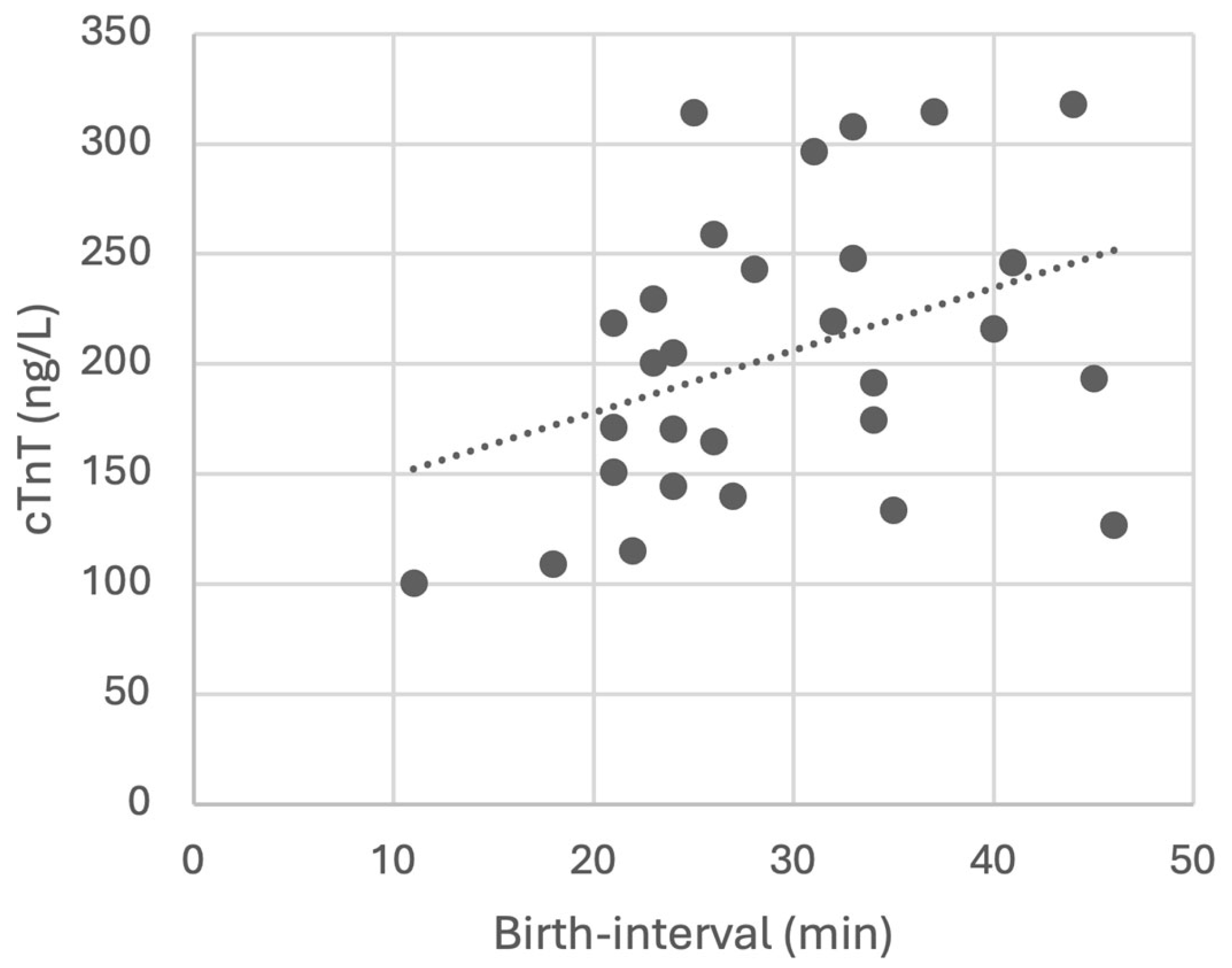
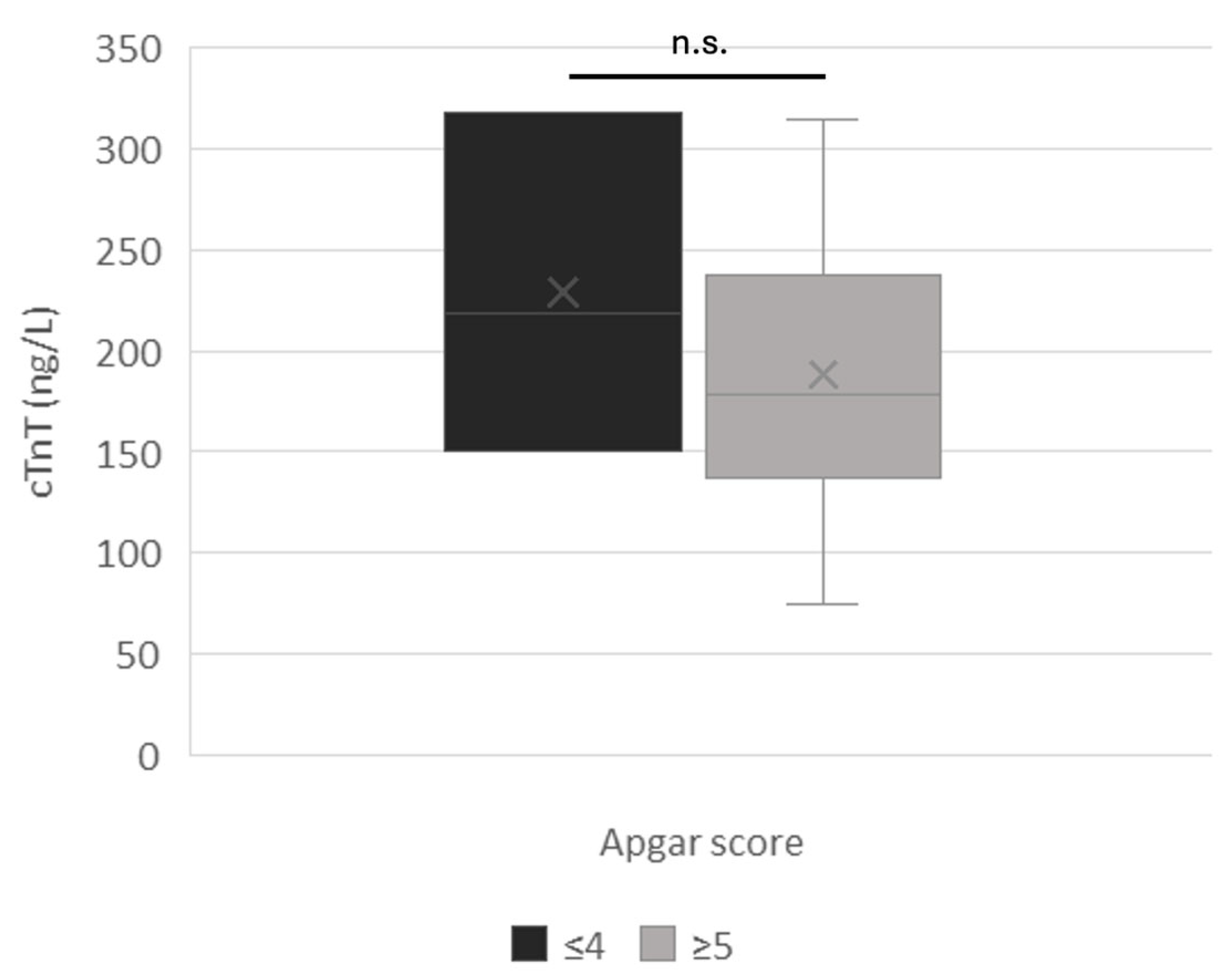
3.1. Clinical Outcomes
3.2. Amniotic cTnT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, K.H.N.P.; Hibaru, V.Y.; da Mata Fuchs, K.; dos Santos Correia, L.E.C.; Lopes, M.D.; Ferreira, J.C.P.; Lourenço, M.L.G. Use of cardiac troponin I (cTnI) levels to diagnose severe hypoxia and myocardial injury induced by perinatal asphyxia in neonatal dogs. Theriogenology 2022, 180, 146–153. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Dystocia in numbers–evidence-based parameters for intervention in the dog: Causes for dystocia and treatment recommendations. Reprod. Domest. Anim. 2009, 44, 141–147. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: Cornerstones of practical approach. Reprod. Domest. Anim. 2014, 49, 64–74. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Castagnetti, C.; Taverne, M.A.M. Preparazione alla nascita ed effetti del parto sul neonato. In Neonatologia Veterinaria; Veronesi, M.C., Castagnetti, C., Taverne, M.A.M., Eds.; EdiSES: Napoli, Italy, 2013; pp. 1–20. [Google Scholar]
- Pridhvidhar Reddy, Y.V.; Jayakumar, C.; Harshan, H.M.; Simon, S.; Raghavan Nair, S.A.; Sukumaran, S.I. Amniotic Fluid Analysis at Birth as a Predictor of Canine Neonatal Survival. Indian J. Anim. Reprod. 2024, 45, 71–74. [Google Scholar] [CrossRef]
- Greghi, J.R.; Favaron, P.O.; Trautwein, L.G.C.; da Silva, C.G.B.; de Lemos, G.A.A.; Martins, M.I.M. Emergency cesarean section in dogs: Usefulness of amniotic fluid biochemical parameters and placental morphology as indicators of neonatal viability. Theriogenology 2023, 211, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Plavec, T.; Knific, T.; Slapšak, A.; Raspor, S.; Lukanc, B.; Pipan, M.Z. Canine neonatal assessment by vitality score, AF, urine, and umbilical cord blood analysis of glucose, lactate, and cortisol: Possible influence of parturition type? Animals 2022, 12, 1247. [Google Scholar] [CrossRef]
- Groppetti, D.; Meazzi, S.; Filipe, J.F.; Colombani, C.; Panseri, S.; Zanzani, S.A.; Pecile, A. Maternal and neonatal canine cortisol measurement in multiple matrices during the perinatal period: A pilot study. PLoS ONE 2021, 16, e0254842. [Google Scholar] [CrossRef]
- Langhorn, R.; Willesen, J.L. Cardiac troponins in dogs and cats. J. Vet. Intern. Med. 2016, 30, 36–50. [Google Scholar] [CrossRef]
- Stefanovic, V.; Loukovaara, M. AF cardiac troponin T in pathological pregnancies with evidence of chronic fetal hypoxia. Croat. Med. J. 2005, 46, 801. [Google Scholar] [PubMed]
- Cain, J.; Davidson, A. Canine Cesarean Section: Emergency and Elective. Vet. Clin. Small Anim. Pract. 2023, 53, 1123–1146. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.P. The bitch around parturition. Theriogenology 2020, 150, 452–457. [Google Scholar] [CrossRef]
- Srithunyarat, T.; Jitpean, S.; Wipoosak, P.; Nonthakotr, C.; Boonbal, N.; Kunkitti, P.; Seesupa, S. Apgar scores in puppies following the induction of etomidate compared with alfaxalone or propofol for cesarean section. Vet. World 2024, 17, 527–534. [Google Scholar] [CrossRef]
- Groppetti, D.; Di Cesare, F.; Pecile, A.; Cagnardi, P.; Merlanti, R.; D’Urso, E.S.; Gioeni, D.; Boracchi, P.; Ravasio, G. Maternal and neonatal wellbeing during elective C-section induced with a combination of propofol and dexmedetomidine: How effective is the placental barrier in dogs? Theriogenology 2019, 129, 90–98. [Google Scholar] [CrossRef]
- Doebeli, A.; Michel, E.; Bettschart, R.; Hartnack, S.; Reichler, I.M. Apgar score after induction of anesthesia for canine cesarean section with alfaxalone versus propofol. Theriogenology 2013, 80, 850–854. [Google Scholar] [CrossRef]
- Rickard, V. Birth and the First 24 Hour. In Small Animal Pediatrics: The First 12 Months of Life, 1st ed.; Peterson, M.E., Kutzler, M., Eds.; Elsevier: St. Louis, MO, USA, 2010; pp. 11–19. [Google Scholar]
- Groppetti, D.; Pecile, A.; Del Carro, A.P.; Copley, K.; Minero, M.; Cremonesi, F. Evaluation of newborn canine viability by means of umbilical vein lactate measurement, apgar score and uterine tocodynamometry. Theriogenology 2010, 74, 1187–1196. [Google Scholar] [CrossRef]
- Kanaan, C.V.; Chiang, C.W. Cardiac troponins in pediatrics. Pediatr. Emerg. Care 2004, 20, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.V.; Potter, J.D.; Szczesna-Cordary, D. The role of troponins in muscle contraction. IUBMB Life 2002, 54, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Zecca, E.; De Rosa, G.; De Luca, D.; Barbato, G.; Pardeo, M.; Romagnoli, C. Is serum troponin T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr. 2007, 96, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Boo, N.Y.; Hafidz, H.; Nawawi, H.M.; Cheah, F.C.; Fadzil, Y.J.; Abdul-Aziz, B.B.; Ismail, Z. Comparison of serum cardiac troponin T and creatine kinase MB isoenzyme mass concentrations in asphyxiated term infants during the first 48 h of life. J. Paediatr. Child Health 2005, 41, 331–337. [Google Scholar] [CrossRef]
- Szymankiewicz, M.; Matuszczak-Wleklak, M.; Hodgman, J.E.; Gadzinowski, J. Usefulness of cardiac troponin T and echocardiography in the diagnosis of hypoxic myocardial injury of full-term neonates. Biol. Neonate 2005, 88, 19–23. [Google Scholar] [CrossRef]
- Gaze, D.C.; Collinson, O. Cardiac troponin determination in AF. Croat. Med. J. 2005, 46, 996–1004. [Google Scholar]
- Tharwat, M.; Al-Sobayil, F.; El-Sayed, M. Cardiac troponin I in healthy newborn goat kids and in goat kids with cardiac nutritional muscular dystrophy. Acta Vet. Hung. 2013, 61, 442–453. [Google Scholar] [CrossRef]
- Slack, J.A.; McGuirk, S.M.; Erb, H.N.; Lien, L.; Coombs, D.; Semrad, S.D.; Peek, S.F. Biochemical markers of cardiac injury in normal, surviving septic, or nonsurviving septic neonatal foals. J. Vet. Intern. Med. 2005, 19, 577–580. [Google Scholar] [CrossRef]
- van Mieghem, T.; Doné, E.; Gucciardo, L.; Klaritsch, P.; Allegaert, K.; Van Bree, R.; Lewi, L.; Deprest, J. Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2020, 202, 48.e1–48.e7. [Google Scholar] [CrossRef]
- Ostadal, B.; Ostadalova, I.; Dhalla, N.S. Development of cardiac sensitivity to oxygen deficiency: Comparative and ontogenetic aspects. Physiol. Rev. 1999, 79, 635–659. [Google Scholar] [CrossRef]
- Rohlicek, C.V.; Saiki, C.; Matsuoka, T.; Mortola, J. Oxygen transport in conscious newborn dogs during hypoxic hypometabolism. J. Appl. Physiol. 1998, 84, 763–768. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Bucci, R.; Probo, M.; Faustini, M.; Fusi, J. Apgar Score for Newborn Dog Viability Assessment: Differences between English and French Bulldogs Born via Cesarean Section. Animals 2023, 13, 3318. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Maronezi, M.C.; Padilha-Nakaghi, L.C.; Gasser, B.; Pavan, L.; Aires, L.P.N.; Feliciano, M.A.R. Contrast-enhanced ultrasound evaluation of placental perfusion in brachicephalic bitches. Theriogenology 2021, 173, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.H.N.P.; dos Santos Correia, L.E.C.; Oliveira, E.L.R.; Bernardo, R.B.; Jorge, M.L.N.; Gobato, M.L.M.; de Souza, F.F.; Sousa Rocha, N.; Chiacchio, S.B.; Lourenço, M.L.G. Incidence of congenital malformations and impact on the mortality of neonatal canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Futterman, I.D.; Jain, H.; McLaren, R.A., Jr.; Mays, J.K. Cord Blood Troponin I Levels: Biomarker Evidence of Fetal Cardiac Injury in Intrahepatic Cholestasis of Pregnancy. AJOG Glob. Rep. 2024, 4, 100356. [Google Scholar] [CrossRef]
- Shelton, S.D.; Fouse, B.L.; Holleman, C.M.; Sedor, F.A.; Herbert, W.N. Cardiac troponin T levels in umbilical cord blood. Am. J. Obstet. Gynecol. 1999, 181, 1259–1262. [Google Scholar] [CrossRef]
- Indrebø, A.; Trangerud, C.; Moe, L. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 2007, 49 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Tarducci, A.; Abate, O.; Borgarelli, M.; Borrelli, A.; Zanatta, R.; Cagnasso, A. Serum values of cardiac troponin-T in normal and cardiomyopathic dogs. Vet. Res. Commun. 2004, 28, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Rifai, N.; Sallan, S.E.; Lipsitz, S.R.; Dalton, V.; Sacks, D.B.; Ottlinger, M.E. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997, 96, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. Gender specificities of cardiac troponin serum levels: From formation mechanisms to the diagnostic role in case of acute coronary syndrome. Life 2023, 13, 267. [Google Scholar] [CrossRef]
- Giannitsis, E.; Mueller-Hennessen, M.; Zeller, T.; Schuebler, A.; Aurich, M.; Biener, M.; Vafaie, M.; Stoyanov, K.M.; Ochs, M.; Riffel, J.; et al. Gender-specific reference values for high-sensitivity cardiac troponin T and I in well-phenotyped healthy individuals and validity of high-sensitivity assay designation. Clin. Biochem. 2020, 78, 18–24. [Google Scholar] [CrossRef]
- Undhad, V.V.; Fefar, D.T.; Jivani, B.M.; Gupta, H.; Ghodasara, D.J.; Joshi, B.P.; Prajapati, K.S. Cardiac troponin: An emerging cardiac biomarker in animal health. Vet. World 2012, 5, 508–511. [Google Scholar] [CrossRef]

| ID. | Breed | Age (Year) | BW (kg) | Litter Size | Type of Parturition |
|---|---|---|---|---|---|
| 1 | Bernese Mountain dog | 5.6 | 45.1 | 2 | Elective CS |
| 2 | Bernese Mountain dog | 3.5 | 62.7 | 16 | Elective CS |
| 3 | Boxer | 5 | 29 | 2 | Elective CS |
| 4 | Chihuahua | 4.6 | 3.3 | 1 | Emergency CS |
| 5 | Chihuahua | 4.4 | 3 | 2 | Emergency CS |
| 6 | Chihuahua | 3.3 | 3.1 | 2 | Natural |
| 7 | Chihuahua | 7 | 3.8 | 1 | Emergency CS |
| 8 | Chihuahua | 5.6 | 2.4 | 2 | Elective CS |
| 9 | English Bull dog | 5.3 | 27.2 | 2 | Elective CS |
| 10 | Entlebucher Mountain dog | 6.5 | 26.4 | 2 | Elective CS |
| 11 | French Bull dog | 4.3 | 9 | 3 | Elective CS |
| 12 | Poodle | 4.6 | 4 | 1 | Elective CS |
| 13 | Staffordshire Bull terrier | 8.5 | 16.2 | 1 | Emergency CS |
| 14 | Welsh Corgi | 6 | 15.8 | 3 | Elective CS |
| Mean ± sd | 5.3 ± 1.4 | 17.9 ± 18.4 | 3 ± 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giussani, E.; Pecile, A.; Del Carro, A.P.; Bronzo, V.; Mazzola, S.M.; Groppetti, D. First Report on Cardiac Troponin T Detection in Canine Amniotic Fluid. Vet. Sci. 2025, 12, 952. https://doi.org/10.3390/vetsci12100952
Giussani E, Pecile A, Del Carro AP, Bronzo V, Mazzola SM, Groppetti D. First Report on Cardiac Troponin T Detection in Canine Amniotic Fluid. Veterinary Sciences. 2025; 12(10):952. https://doi.org/10.3390/vetsci12100952
Chicago/Turabian StyleGiussani, Elisa, Alessandro Pecile, Andrea Pasquale Del Carro, Valerio Bronzo, Silvia Michela Mazzola, and Debora Groppetti. 2025. "First Report on Cardiac Troponin T Detection in Canine Amniotic Fluid" Veterinary Sciences 12, no. 10: 952. https://doi.org/10.3390/vetsci12100952
APA StyleGiussani, E., Pecile, A., Del Carro, A. P., Bronzo, V., Mazzola, S. M., & Groppetti, D. (2025). First Report on Cardiac Troponin T Detection in Canine Amniotic Fluid. Veterinary Sciences, 12(10), 952. https://doi.org/10.3390/vetsci12100952






