1. Introduction
As China’s economic development continues to improve, people’s demand for meat, eggs, milk and other livestock products has also risen. However, the rapid development of animal husbandry has made the imbalance between forage supply and demand increasingly prominent. Due to the limited area of arable land, it is a challenge to take into account the cultivation of forage crops while meeting the demand for human food, thus triggering the contradiction of “people and animals competing for food”, which not only restricts the sustainable development of animal husbandry, but also further exacerbates the shortage of forage; therefore, the development of new forage resources has become a major goal.
Stevia is an annual herbaceous plant of the Stevia genus in the Compositae family [
1], and its leaves are rich in a variety of active substances widely used in medicines and food [
2]. In China,
stevia is mainly grown in Xinjiang, Jiangsu, Anhui and other regions, and the cultivation area reached 300,000 mu (49,421 acres) in 2023, with an annual output of dry
stevia leaves of 75,200 tons and
stevia straw of 106,000 tons.
Stevia straw is the by-product remaining after leaf harvesting, and its utilization could alleviate the shortage of forage for cattle and sheep raising, and the nutrient levels were better than those of conventional straw roughage [
3]. The Bayanbulak sheep, also known as the Tianshan Yak Sheep or Bayanbulak Large-Tailed Sheep, is a unique breed primarily distributed in the Hejing County of the Bayingolin Mongol Autonomous Prefecture in Xinjiang Uygur Autonomous Region, China, specifically in the Bayanbulak Grassland. It belongs to the meat-and-fat dual-purpose coarse-wool sheep category and is renowned for its exceptional environmental adaptability, superior meat quality, and distinctive physical characteristics. Utilizing stevia straw as sheep feed is an innovative strategy that kills multiple birds with one stone. Economically, it creates new industrial chains and optimizes resource allocation. In terms of profitability, it provides farms with a powerful tool to reduce costs and enhance efficiency, provided that technical and managerial challenges are addressed. Environmentally, it represents a typical low-carbon, circular development path with significant ecological benefits.
In addition,
stevia straw contains bioactive compounds such as glycosides, chlorogenic acids and flavonoids, which have a positive effect on the growth and immune function of livestock and poultry and can be used as feed additives to promote their healthy growth [
4]. Shin et al. [
5] observed that dietary supplementation with stevioside at 1625 mg/kg BW significantly increased the average daily gain and reduced the drip loss and shear force of the longissimus dorsi muscle in beef cattle. Zhang et al. [
3] found that the addition of 1.0%
stevia straw to cattle feed increased feed conversion and promoted rumen fermentation. Adding 4% and 6%
stevia residue to laying hens’ diets increased the relative abundance of beneficial bacteria in the intestinal tract, decreased the number of harmful bacteria, and improved the amino acid and fatty acid compositions of the egg whites [
6]. Currently, a substantial amount of
stevia straw is wasted due to difficulties in harvesting and low utilization efficiency in feed processing. However,
stevia straw possesses considerable potential for animal feeding and medicinal applications. Therefore, this study aimed to evaluate how including varying levels of
stevia straw in sheep diets affects slaughter traits, meat quality, amino acid and fatty acid profiles, rumen fermentation parameters, and microbial diversity, thereby providing an empirical foundation for its use in ovine production.
2. Materials and Methods
2.1. Experimental Materials
Stevia straw was provided by the Xinjiang Shuomu Breeding Co. Ltd. in Heshuo County, Bayin’guoleng Mongol Autonomous Prefecture, Xinjiang, and was the residual product (mainly stems) of
stevia after leaf harvest. The nutrient composition of
stevia straw is shown in
Table 1. Samples of
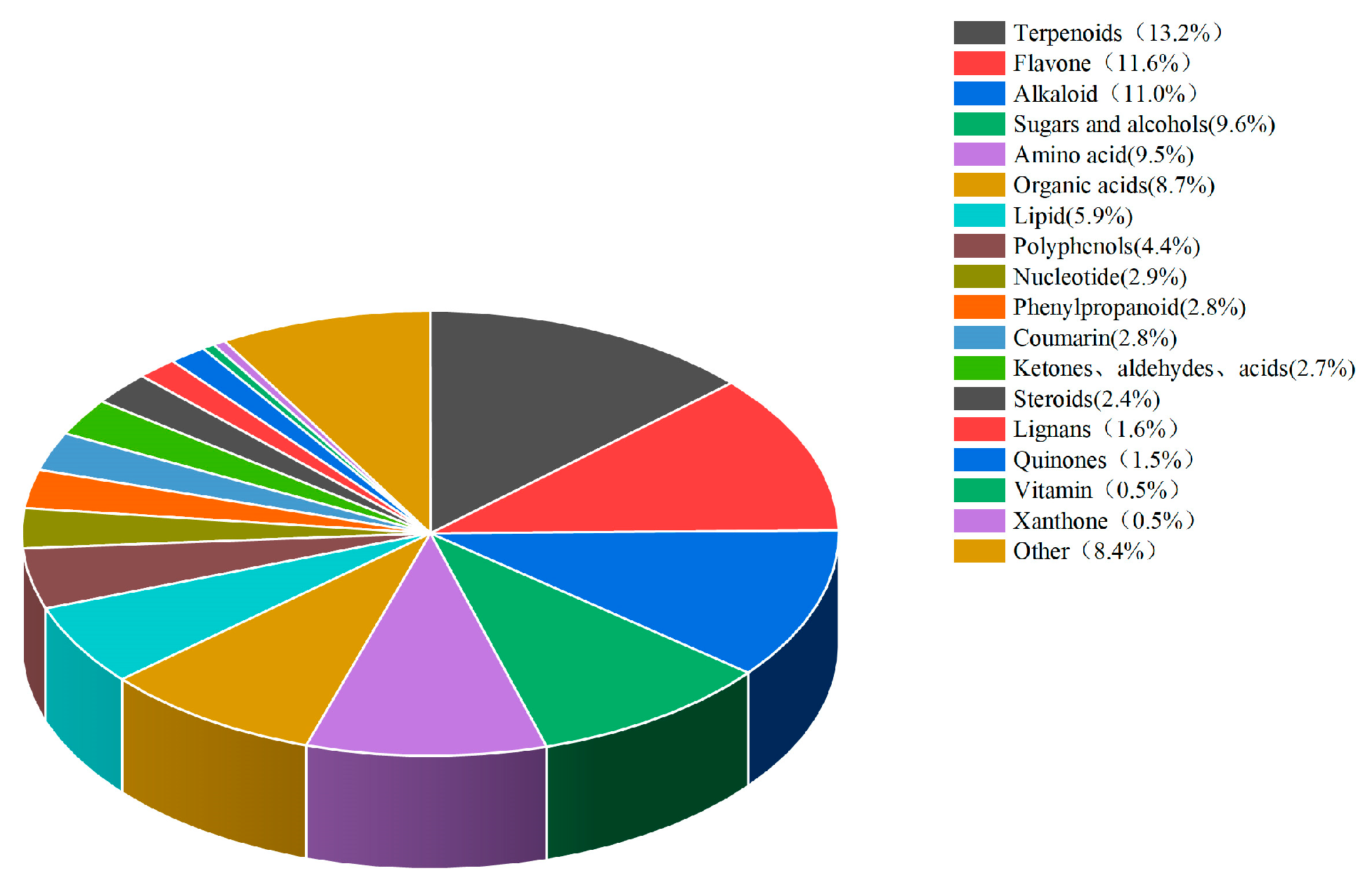
stevia straw were sent to Beijing Baimaike Biological Co. Ltd. for the quantification of widely targeted metabolites using liquid chromatography-mass spectrometry (LC-MS). A total of 1183 biologically active substances were detected, which were classified into 18 types, with terpenoids accounting for the highest proportion (13.2%), followed by flavonoids (11.6%), alkaloids (11.0%), sugars and alcohols (9.6%) in
Figure 1.
2.2. Animal Handling, Diets, and Experimental Design
All animal procedures in this study were approved by the review board of the Protocol Management and Review Committee of the Feed Research Institute of the Xinjiang Animal Science Academy (No. 11 20231227).
The study was designed as a completely randomized one-way trial. Fifty male Bayinbruk lambs, 3–4 months of age and weighing 27.01 ± 3.8 kg were randomly grouped based on their initial weight, and were divided into five groups, with ten sheep in each group. An isoenergetic and isonitrogenous diet during the fattening period, and with a standard nutritional requirement for a 250 g daily gain, sheep weighing 30 kg were selected to receive a mixed pelleted ration containing 0% (CK), 5%, 15%, 25% and 35%
stevia straw; the specific compositions and nutrient levels of the various groups are shown in
Table 2.
Prior to the formal trial period, the enclosures were disinfected, and the sheep were dewormed. Five of these groups were raised in five different enclosures. The sheep were then reared in groups in the same pen. They were fed ad libitum (twice a day at 9:00 and 19:00) and had free access to water. The total experimental period lasted 82 days, including a 10-day pre-feeding period and 72-day trial period.
2.3. Sample Collection
Upon completion of the trial, following 12 h of fasting and 2 h of water restriction, five lambs from each group were randomly selected and transported to the slaughterhouse. After humane slaughter, the backfat thickness was measured vertically to the skin surface at the junction of the 12th and 13th ribs, 5 cm from the dorsal midline, using a vernier caliper with an accuracy of 0.1 mm. Upon completion, the longissimus dorsi muscle was collected from between the 12th and 13th ribs and preserved in liquid nitrogen. Rumen fluid samples were collected: the rumen was removed after slaughter, and the rumen fluid was collected from the upper opening of the left side. In order to avoid contamination of the rumen fluid, the first 50 mL of rumen fluid was discarded, and 30 mL of rumen fluid was collected after 4 layers of gauze filtration, and the rumen pH was measured immediately using a portable pH meter ((Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China), Shanghai, China). A total of 10 mL of rumen fluid was transferred to −20 °C and stored in the freezer. for subsequent detection and analysis. Another 5 mL of rumen fluid was collected into a freezing tube and stored at −80 °C after liquid nitrogen flash-freezing for the determination of rumen microbiota.
2.4. Measurement of Indicators
2.4.1. Carcass Traits
The live weight (kg) of each experimental sheep was measured and recorded just before slaughter. The test sheep were bled through the carotid artery and jugular vein, and the head, hoof, skin, and viscera of the test sheep were removed, while the kidneys were retained. The carcass weight was measured and recorded immediately after slaughter, and slaughter percentage was calculated as carcass yield = (carcass weight/live weight) × 100%. A longitudinal incision was made on the longissimus dorsi (LD) muscle between the 12th and 13th ribs to obtain the area of the eye muscle. A Vernier caliper was used to measure the length of the longest and widest parts of the eye muscle. The eye muscle area is the product of the length and width of the eye muscle multiplied by 0.7.
2.4.2. Meat Quality
Meat quality was assessed through both physical and chemical indicators. Physical measurements comprised pH, water loss (%), and cooking loss (%), while chemical analyses included moisture, protein, fat, and ash content.
Muscle pH was measured using a portable muscle tissue pH meter (PHS-3E; Shanghai YiDian Scientific Instrument Co., Ltd., Shanghai, China), calibrated using standard buffer solutions at pH = 4, pH = 6.86, and pH = 9.18. To calculate water loss (%), longissimus dorsi muscle samples were cut perpendicular to the muscle fiber direction into 1 cm thick slices. A circular sampler with a diameter of 3 cm (DL-100; Sunspring Sanquan Zhongshi, Shandong, China) was used to obtain samples. The samples were placed between six layers of filter paper and pressed under 35 kg of pressure for 5 min. The weights before and after pressing were measured, and the water loss rate was calculated using the following formula: water loss rate (%) = (Weight before pressing − Weight after pressing)/Weight before pressing × 100. Cooking loss was evaluated by drying a 3 × 3 × 5 cm meat sample with a paper towel, recording its initial weight (W1, g), sealing it in a bag, and heating it in an 85 °C water bath for 40 min. After cooling and drying, the sample was reweighed (W2, g); cooking loss (%) = (W1 − W2)/W1 × 100. Moisture, crude protein, crude fat, and ash contents were quantified following standard AOAC methods [
7]: 950.46, 928.08, 960.39, and 920.153, respectively.
2.4.3. Cholesterol, Inosinic Acid, Thiamine
Weigh about 2 g of sample in a 50 mL centrifuge tube, homogenize with 20 mL of 5% perchloric acid solution three times, and centrifuge the homogenate at 4000 r/min for l0 min at 4 °C. Then, transfer the supernatant into a 50 mL beaker, and continue to wash the precipitate with 5% perchloric acid and centrifuge it two times, merge the supernatant, adjust the pH with KOH solution to 6.5, and shake the supernatant well with distilled water until it is 50 mL. Then, filter it through a 0.45 μm filter membrane and put it into a bottle for on-line testing. Filter with a 0.45 μm membrane and put into a bottle for testing. Chromatographic column: C18 (length 250 mm, inner diameter 4.6 mm, particle size 5 μm); column temperature: 25 °C; injection volume: 20 μL; flow rate: l mL/min; detection wavelength: 254 nm; mobile phase: take 3.5 mL of phosphoric acid solution and add 200 mL of water and 7.2 mL of triethylamine mixing, and then adjust the pH of the solution to 6.5 with triethylamine, and then take 950 mL and 50 mL of methanol mixing. A total of 50 mL of methanol was mixed, filtered through 0.45 μm and degassed by ultrasonic.
2.4.4. Amino Acids
To determine amino acid profiles, 100 mg of lyophilized tissue was homogenized in 1.2 mL of 10% sulfosalicylic acid and centrifuged at 13,500× g for 15 min at 4 °C. The supernatant was collected, filtered through a 0.22 μm membrane, transferred to a 2.0 mL glass vial, and analyzed using a high-speed amino acid analyzer (L-8900; Hitachi High-Tech Corporation, Tokyo, Japan).
2.4.5. Fatty Acids
Total fatty acids (FAs) were extracted from frozen meat samples following the procedure of Liang et al. [
8]. FA separation was performed using gas chromatography (GC-450; Varian Co., Walnut Creek, CA, USA), with peaks identified based on retention time. Individual FA concentrations were quantified against standard curves prepared from a known methyl ester mixture (C4–C24; Sigma-Aldrich, St. Louis, MO, USA)
2.4.6. Rumen Fluid Sample Collection and Analysis
DNA was purified from rumen fluid by the cetyltrimethylammonium bromide (CTAB) method and used as template for PCR amplification with specific primers [
8]. The PCR products were quantified by fluorometry (Qubit 3.0), and sequenced on an Illumina PE300 platform at the Beijing Baimaike Biological Co. The raw data from 16S rRNA gene sequencing was QC processed with QIIME2, to remove low-quality reads and potential contaminants. The SILVA database was selected for alignment, and a 97% sequence similarity cut-off was used to identify operational taxonomic units (OTUs). Alpha diversity of the microbial community was assessed by Chao1, ACE and Shannon indexes in QIIME2. Differences in relative bacterial abundance were evaluated by the nonparametric Kruskal–Wallis rank-sum test, and the difference between groups was analyzed by Student’s
t-test. Bacterial markers differentiating the various microbial communities were determined by linear discriminant analysis (LDA) effect size (LEfSe) (LDA > 3.5,
p < 0.05).
2.5. Statistical Analysis of Data
Data are expressed as means ± standard errors of the mean. Data were preliminarily sorted using Excel2010, and SPSS24.0 was used for performing one-way ANOVA. If the difference between treatments was significant, a Duncan multiple-range test was used for comparisons, and orthogonal polynomials were used to evaluate the linear and secondary effects of changes in relevant data. We used 0.05 ≤ p < 0.10, p < 0.05, and p < 0.01 to indicate a significant trend, significant difference, and highly significant differences, respectively.
4. Discussion
Slaughter performance is an important index for assessing the production of meat animals and poultry. It clearly reflects the proportion of edible meat in an adult sheep, and it is also a core criterion for measuring the production of lamb meat. It not only reflects the meat production capacity and organ development of livestock and poultry, but also indicates the digestion and utilization capacity of the ration by the animal [
9]. Our investigation showed that with increasing
stevia straw addition, the pre-slaughter live weight and carcass weight showed a quadratic curve change with an initial increase and then a decrease. The average carcass weight of each
stevia straw group was higher than that of the CK group, but the difference was not significant, and the other indices were not significantly different. This indicates that the addition of
stevia straw to the ration can improve the meat production performance of lambs, which may be related to the fact that
stevia straw is relatively rich in chlorogenic acid. Chlorogenic acid is a natural bioactive substance that can enhance the bioavailability of dietary nutrients and promote the expression of transporter carriers, thus enhancing an animal’s ability to transport and absorb minerals, which could ultimately improve their slaughter performance [
10,
11].
After slaughter, the pH of lamb meat gradually decreases with increasing time and glycolytic reactions. This is due to the depletion of muscle glycogen for anaerobic respiration by muscle cells after slaughter, which produces excess lactic acid leading to a decrease in pH [
12,
13]. The cooking loss and water loss rates are important indicators of muscle water-holding capacity; the higher the cooking loss and the lower the water loss rate, the higher the water-holding capacity of the muscle and the better the meat quality [
14]. The water-holding capacity of muscle is closely related to pH, and a lower pH may lead to changes in muscle structure that affect its water retention [
15]. Therefore, a decrease in pH can indirectly reduce meat quality by increasing its water loss rate.
The results of our experiments showed that the water loss rate and cooking loss of the 15% and 25%
stevia straw groups were significantly lower than the control group. The pH of the 15% group was significantly higher than that of the CK group and the 25% group, and it showed a quadratic response of increasing and then decreasing with the increased addition of
stevia straw. This supports our hypothesis that the feeding of
stevia straw improves the water retention capacity of lamb meat. This may be related to the presence of active substances such as flavonoids and alkaloids in
stevia straw, which are able to slow down the rate of oxidation of muscle lipids and maintain the structural integrity of muscle cell membranes [
16].
Stevia straw is rich in flavonoids and polyphenols, which not only have antioxidant effects, but can also effectively improve the nutrient composition of livestock muscle and enhance the meat’s nutritional value [
17]. Duan et al. [
18] added residual black wolfberry fruit to the diets of sheep and reported a significant increase in the content of crude fat in the muscle, which improved its nutritional value. Our study showed that the crude fat content of each experimental group was significantly higher than that of the CK group, and linearly increased with
stevia straw addition. Based on the above, we hypothesize that the dietary inclusion of stevia straw reduced both the cooking loss percentage and water loss percentage in the mutton longissimus dorsi, which may be attributed to the significant increase in intramuscular fat content. The elevated fat content enhances the muscle’s water-holding capacity, as moisture in the muscle is partially physically replaced by fat, thereby reducing water loss during cooking and resulting in a lower cooking loss percentage [
19,
20].
In our experiment, the longissimus dorsi muscle of lambs was selected for fatty acid composition testing, and it was found that the addition of
stevia straw to the ration increased the C12:0, C18:2n6c, C18:1n9c, and C20:1 content of the muscle, and decreased the C16:0 and C18:0 levels. This may be due to the fact that
stevia straw is rich in chlorogenic acid and phenolics, which can regulate the activity of enzymes involved in fat metabolism by activating the AMPK signaling pathway, thus controlling the synthesis and metabolism of fatty acids in the organism [
21]. This phenomenon confirms stevia as an anti-aging product [
22]; indeed, increase in C:16 has been reported in the meat of older animals [
23].
Stevia supplementation can also promote the activity of a desaturase located in the endoplasmic reticulum. By the generation of acetyl-coenzyme A from acetic acid, it can catalyze the conversion of stearic acid to oleic acid, thus reducing the saturated fatty acid content and increasing the unsaturated fatty acid content which improves lamb meat quality. When
stevia straw was added at 25%, it significantly increased the content of unsaturated fatty acids and decreased the content of saturated fatty acids in the longissimus dorsi muscle. This is consistent with the results of Kwiecien et al. [
24], who found that the bioactive components in alfalfa could increase the saturated fatty acid enzyme activity and promote the conversion of saturated fatty acids to polyunsaturated fatty acids. This may be the result of flavonoid activity that inhibits the synthesis of phospholipids and free fatty acids, which promotes an increase in unsaturated fatty acids and improved meat quality. Moreover, the results of this study showed that the addition of 15%
stevia straw could significantly increase the glutamic acid content in the longissimus dorsi muscle of lambs.
Stevia straw is rich in glycosides, which can significantly increase the content of flavor-promoting amino acids in the muscle and improve the taste of the lamb [
25,
26].
The rumen microbiota of ruminants comprise a highly complex ecosystem with multiple functions such as fiber catabolism, fat degradation, plant protein hydrolysis and microbial protein synthesis [
27,
28]. These microorganisms play a key role in nutrient absorption and distribution, metabolism, and the immune responses of the organism [
28,
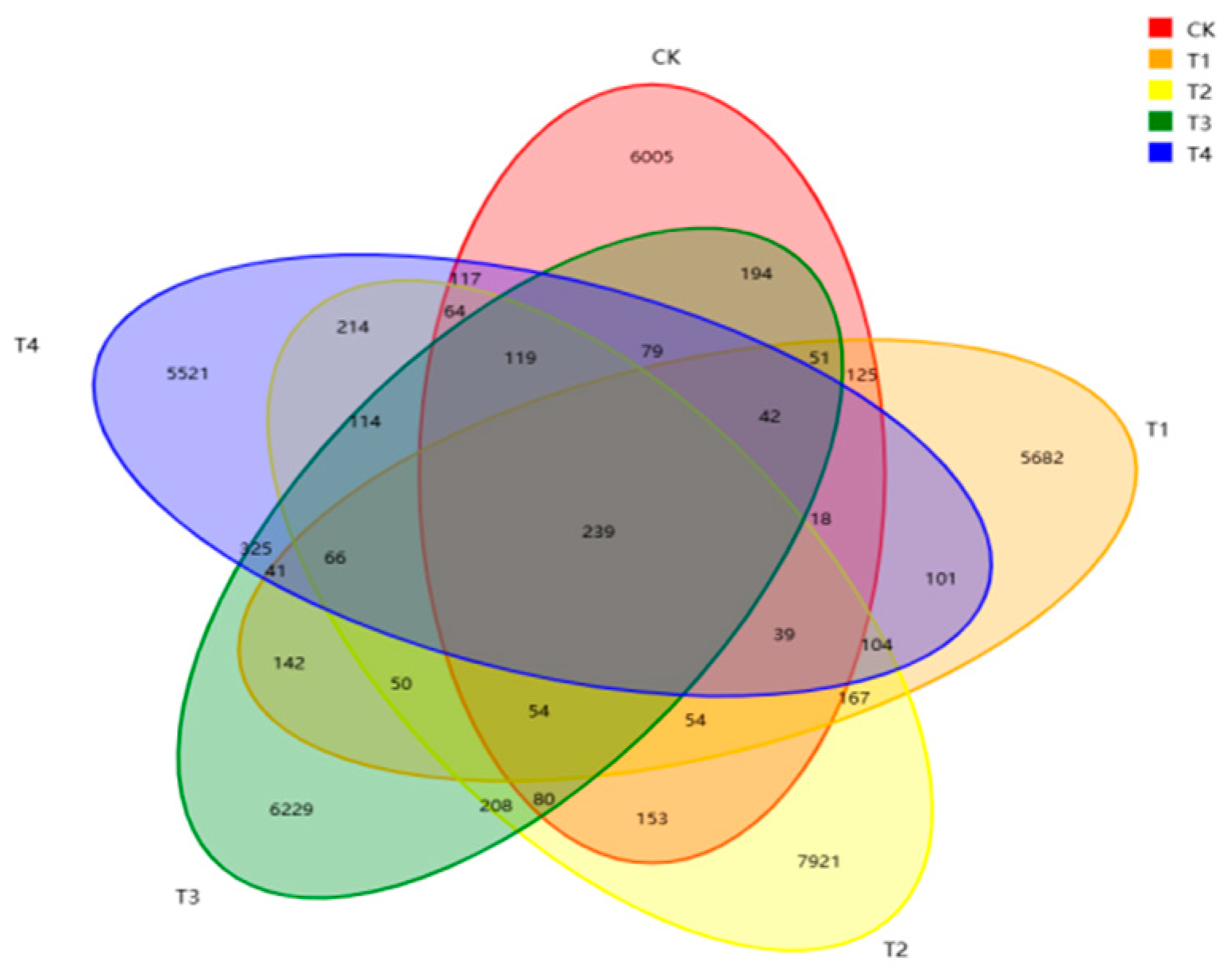
29]. Our Venn diagram analysis based on the level of OTUs in this study found that the number of rumen-specific OTUs was higher in the 15% group than in the other groups. The level of rumen microbial diversity was also higher than that of the other groups, suggesting that feeding
stevia straw can increase rumen microbial diversity. The rumen microbiota composition is an important part of the digestive system of ruminants, and the structure and type of diet is one of the key factors affecting rumen microecological balance. In this study, the relative abundances of Firmicutes and Bacteroidetes were the highest, which is consistent with the rumen fermentation results. Bacteroidetes primarily convert non-fibrous substances into acetic acid [
30], propionic acid, and other compounds. Firmicutes can produce various digestive enzymes to break down nutrients and are key bacterial groups for improving fiber utilization [
31]. We hypothesize that the high fiber content in stevia straw leads to changes in the rumen environment, which can increase the relative abundance of cellulose-degrading bacteria. These bacteria secrete large amounts of cellulase to break down the cellulose in the straw, thereby increasing the concentration of propionic acid and promoting energy supply.