Effects of Production System With or Without Growth-Promoting Technologies on Growth and Blood Expression of (Cyto)Chemokines and Heat Shock and Tight Junction Proteins in Bos taurus and indicus Breeds During Summer Season
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cattle Breeds and Experimental Design
2.3. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
2.4. Statistical Analysis
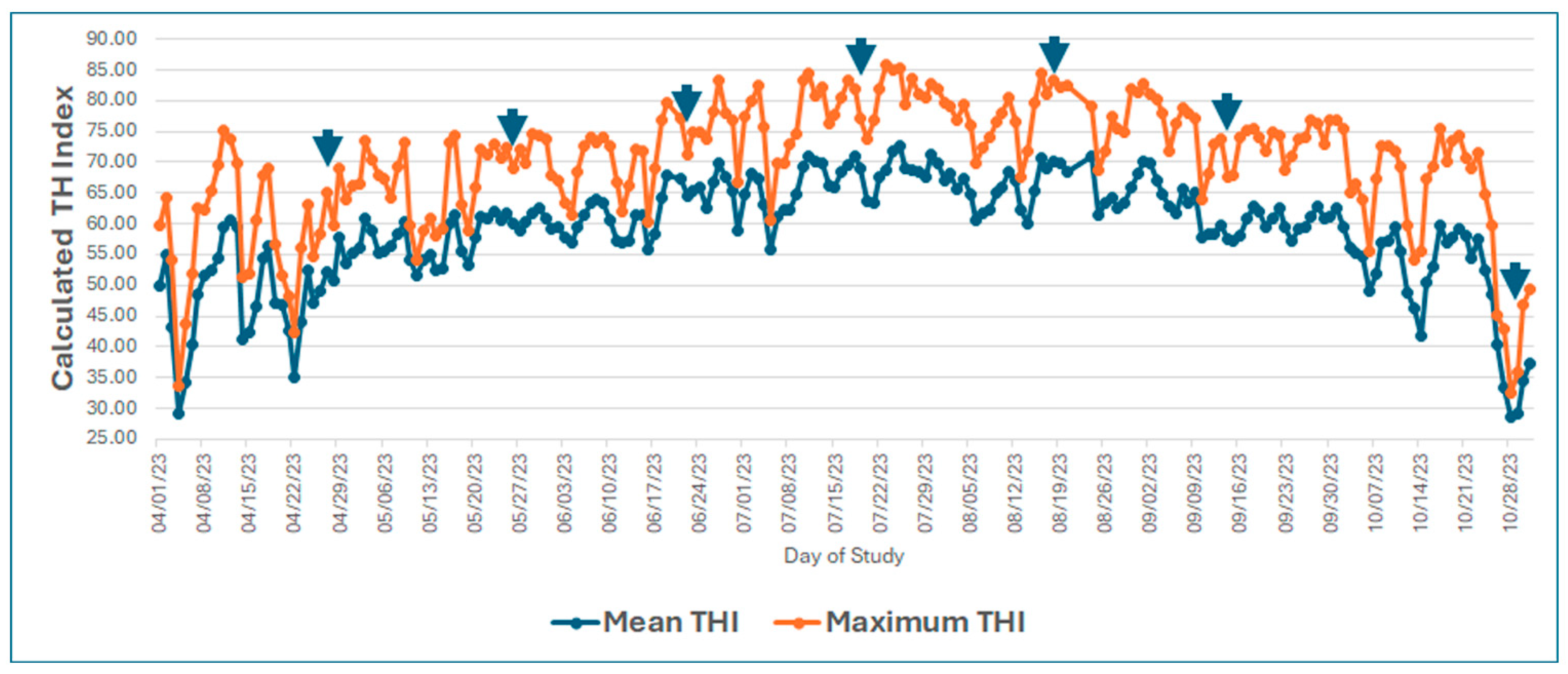
3. Results
3.1. Growth Performance (Body Weight, Body Weight Gain, and Hot Carcass Weight)
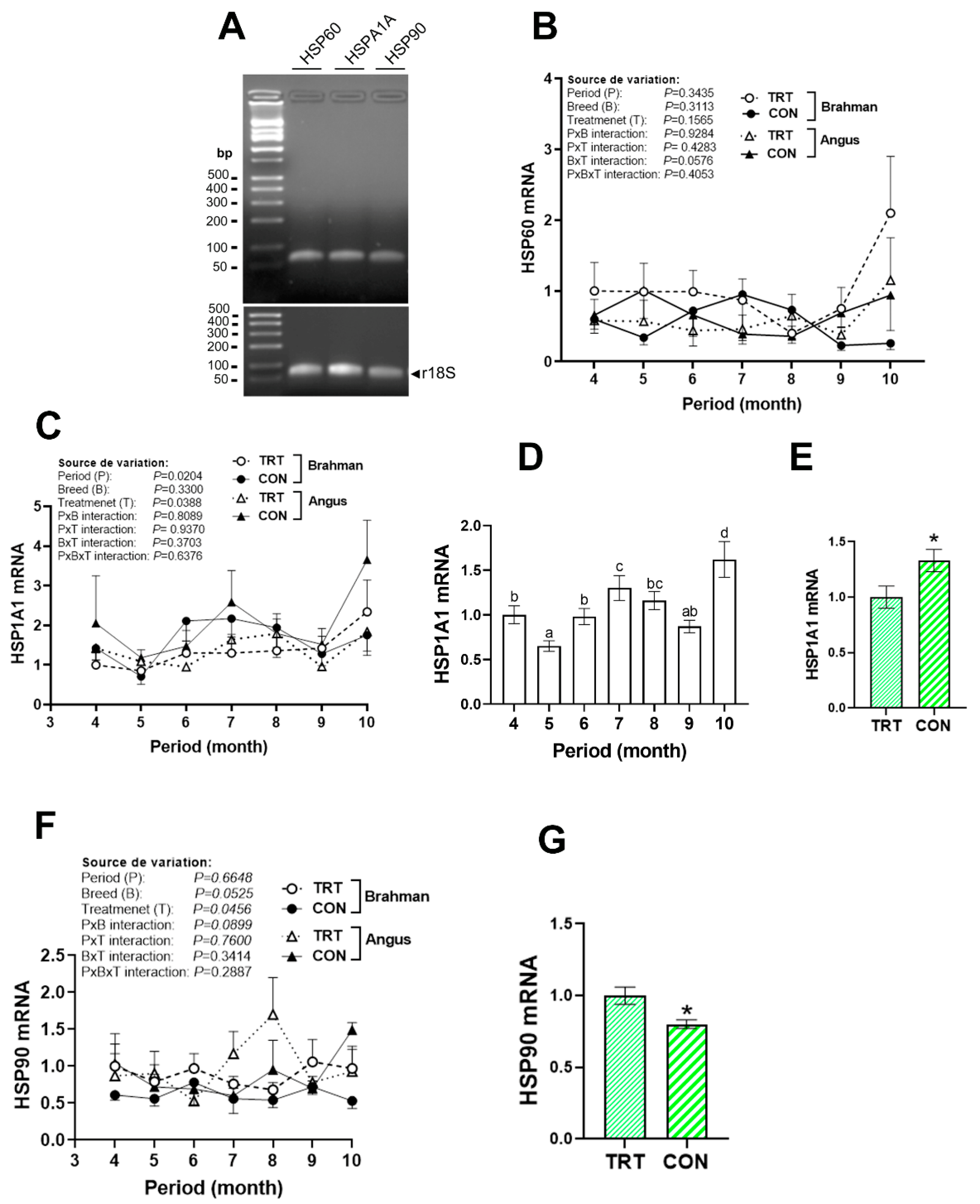
3.2. HSP Gene Expression Profile
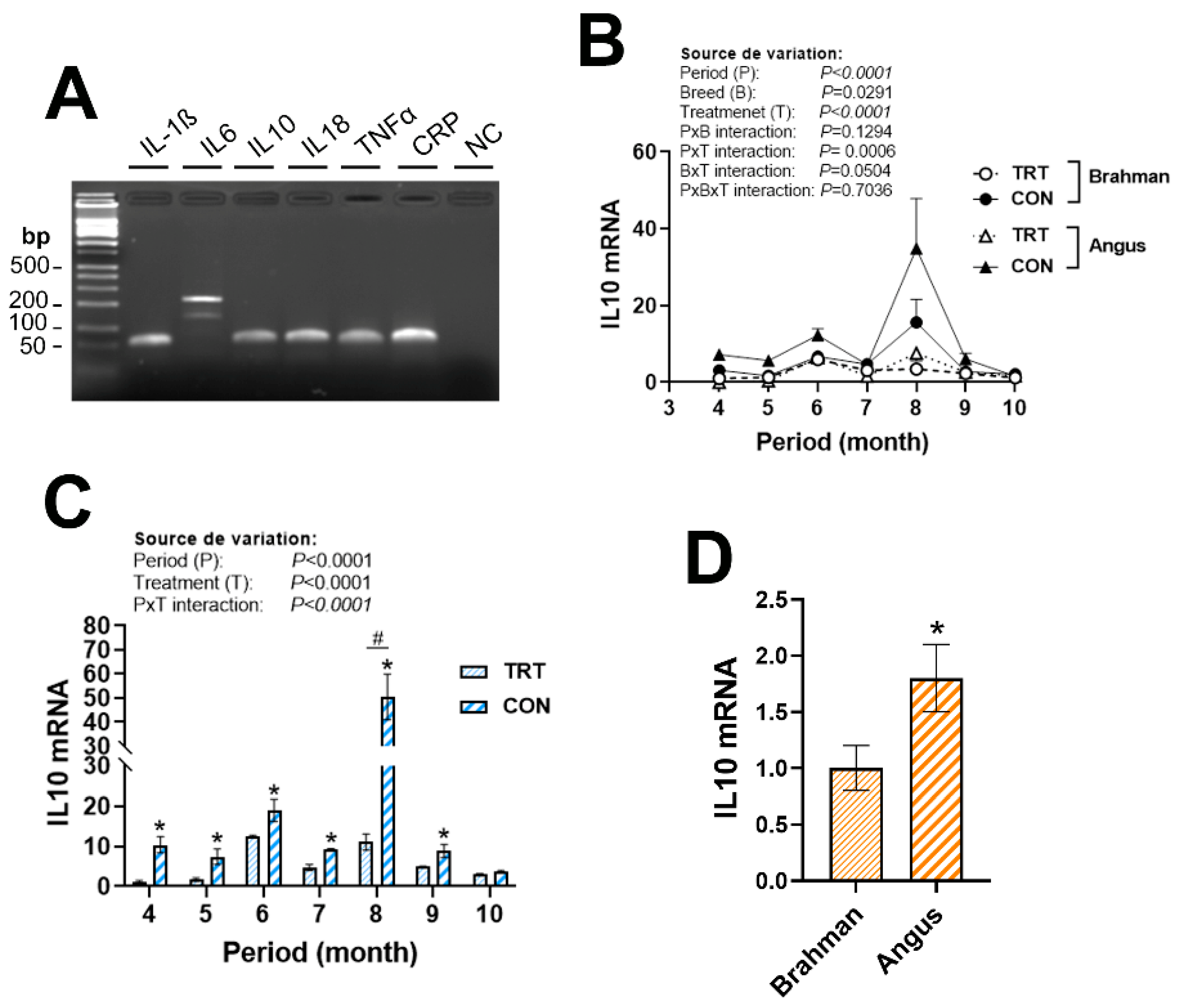
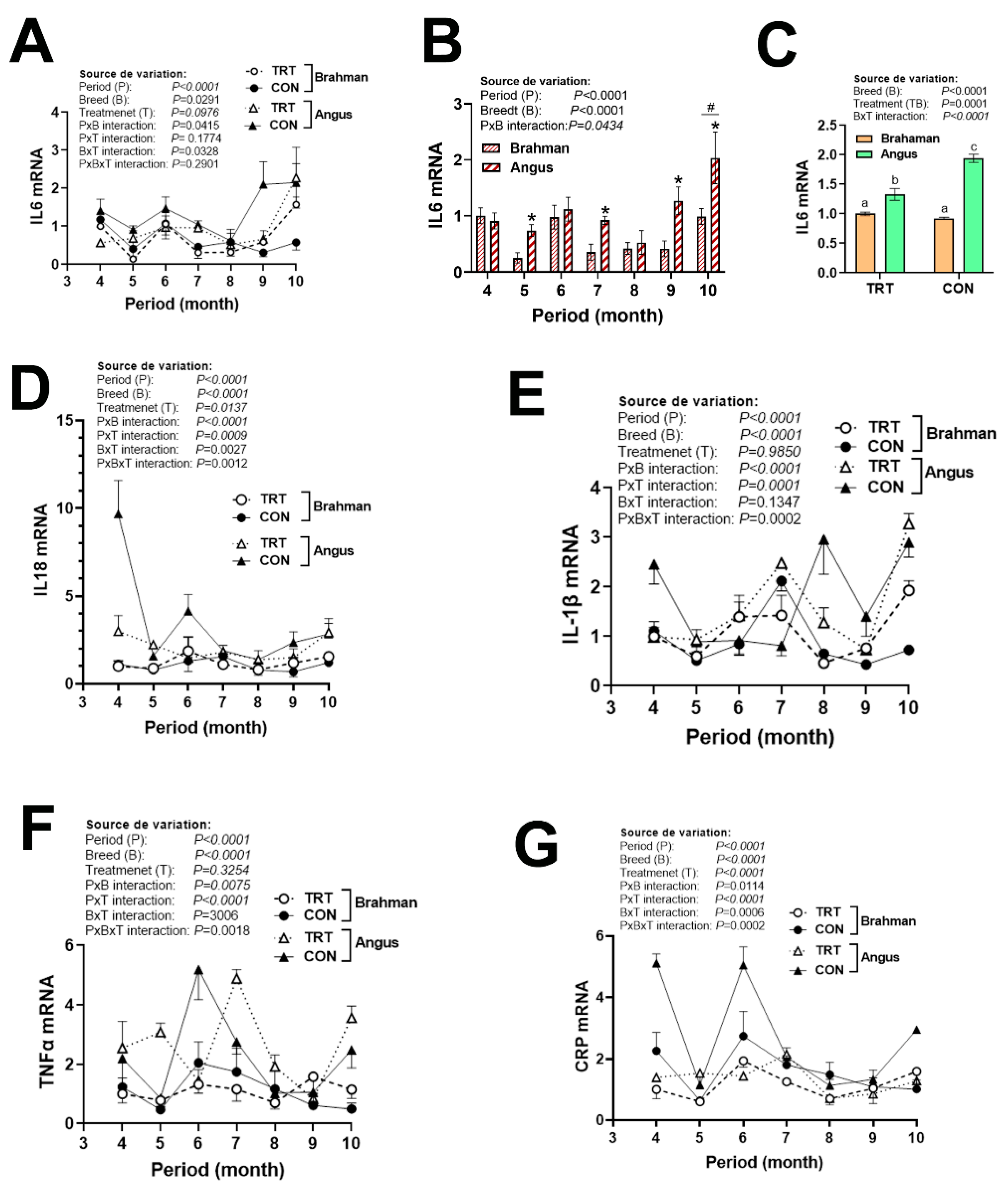
3.3. Gene Expression Profile of (Anti)Pro-Inflammatory Cytokines
3.4. Gene Expression Profile of Chemokine Ligands and Receptors
3.5. Gene Expression Profile of Tight Junction Proteins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alley, R.B.; Clark, P.U.; Huybrechts, P.; Joughin, I. Ice-sheet and sea-level changes. Science 2005, 310, 456–460. [Google Scholar] [CrossRef]
- Esper, J.; Torbenson, M.; Buntgen, U. 2023 summer warmth unparalleled over the past 2000 yars. Nature 2024, 631, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Judd, E.J.; Tierney, J.E.; Lunt, D.J.; Montanez, I.P.; Huber, B.T.; Wing, S.L.; Valdes, P.J. A 485-million-year history of Earth’s surface temperature. Science 2024, 385, eadk3705. [Google Scholar] [CrossRef]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef]
- Tollefson, J. Top climate scientists are sceptical that nations will rein in global warming. Nature 2021, 599, 22–24. [Google Scholar] [CrossRef]
- Armstrong McKay, D.I.; Staal, A.; Abrams, J.F.; Winkelmann, R.; Sakschewski, B.; Loriani, S.; Fetzer, I.; Cornell, S.E.; Rockstrom, J.; Lenton, T.M. Exceeding 1.5 degrees C global warming could trigger multiple climate tipping points. Science 2022, 377, eabn7950. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, A.M.; Blard, P.H.; Sherwood, S.C.; Kageyama, M. Terrestrial amplification of past, present, and future climate change. Sci. Adv. 2023, 9, eadf8119. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Jagermeyr, J.; Muller, C.; Ruane, A.C.; Elliott, J.; Balkovic, J.; Castillo, O.; Faye, B.; Foster, I.; Folberth, C.; Franke, J.A.; et al. Climate impacts on global agriculture emerge earlier in new generation of climate and crop models. Nat. Food 2021, 2, 873–885. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Knight, R.; Taylor, H. Livestock, Dairy, and Poultry Outlook: February 2024, LDP-M-356, February 14, 2024 USDA, Economic Research Service. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=37550 (accessed on 13 January 2025).
- Drouillard, J.S. Current situation and future trends for beef production in the United States of America—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. How to Feed the World 2050; Food and Agriculture Organization: Rome, Italy, 2009. [Google Scholar]
- Bousfield, C.G.; Morton, O.; Edwards, D.P. Climate change will exacerbate land conflict between agriculture and timber production. Nat. Clim. Change 2024, 14, 1071–1077. [Google Scholar] [CrossRef]
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 2021, 12, 2501. [Google Scholar] [CrossRef]
- Jones, E.R.; Bierkens, M.F.P.; Van Vliet, M.T.H. Current and future global water scarcity intensifies when accounting for surface water quality. Nat. Clim. Change 2024, 14, 629–635. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Achakulwisut, P.; Erickson, P.; Guivarch, C.; Schaeffer, R.; Brutschin, E.; Pye, S. Global fossil fuel reduction pathways under different climate mitigation strategies and ambitions. Nat. Commun. 2023, 14, 5425. [Google Scholar] [CrossRef]
- Goldthau, A.; Tagliapietra, S. Energy crisis: Five questions that must be answered in 2023. Nature 2022, 612, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of heat stress on global cattle production during the 21st century: A modelling study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Zhang, M.; Osei-Amponsah, R.; Clarke, I.; Sejian, V.; Warner, R.; Dunshea, F.R. Impact of heat stress on ruminant livestock production and meat quality, and strategies for amelioration. Anim. Front. 2023, 13, 60–68. [Google Scholar] [CrossRef]
- Meneses, J.A.M.; de Sá, O.A.A.L.; Coelho, C.F.; Pereira, R.N.; Batista, E.D.; Ladeira, M.M.; Casagrande, D.R.; Gionbelli, M.P. Effect of heat stress on ingestive, digestive, ruminal and physiological parameters of Nellore cattle feeding low- or high-energy diets. Livest. Sci. 2021, 252, 104676. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S. Effect of management strategies on reducing heat stress of feedlot cattle: Feed and water intake. J. Anim. Sci. 2004, 82, 3077–3087. [Google Scholar] [CrossRef]
- Curtis, A.K.; Scharf, B.; Eichen, P.A.; Spiers, D.E. Relationships between ambient conditions, thermal status, and feed intake of cattle during summer heat stress with access to shade. J. Therm. Biol. 2017, 63, 104–111. [Google Scholar] [CrossRef]
- Mitlohner, F.M.; Morrow, J.L.; Dailey, J.W.; Wilson, S.C.; Galyean, M.L.; Miller, M.F.; McGlone, J.J. Shade and water misting effects on behavior, physiology, performance, and carcass traits of heat-stressed feedlot cattle. J. Anim. Sci. 2001, 79, 2327–2335. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Sarlo-Davila, K.M.; Dikmen, S.; Rodriguez, E.; Oltenacu, P.A. The effect of Brahman genes on body temperature plasticity of heifers on pasture under heat stress. J. Anim. Sci. 2020, 98, skaa126. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ceron, J.; Chase, C.C., Jr.; Hansen, P.J. Differences in heat tolerance between preimplantation embryos from Brahman, Romosinuano, and Angus breeds. J. Dairy Sci. 2004, 87, 53–58. [Google Scholar] [CrossRef]
- Kregel, K.C. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Heled, Y.; Fleischmann, C.; Epstein, Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hatabu, T.; Yamamoto, Y.; Kimura, K. Alteration of chemokine production in bovine endometrial epithelial and stromal cells under heat stress conditions. Physiol. Rep. 2020, 8, e14640. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef]
- Dridi, S.; Hirano, Y.; Tarallo, V.; Kim, Y.; Fowler, B.J.; Ambati, B.K.; Bogdanovich, S.; Chiodo, V.A.; Hauswirth, W.W.; Kugel, J.F.; et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 13781–13786. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Xu, Y.; Ramanathan, V.; Victor, D.G. Global warming will happen faster than we think. Nature 2018, 564, 30–32. [Google Scholar] [CrossRef]
- Song, J.; Tong, G.; Chao, J.; Chung, J.; Zhang, M.; Lin, W.; Zhang, T.; Bentler, P.M.; Zhu, W. Data driven pathway analysis and forecast of global warming and sea level rise. Sci. Rep. 2023, 13, 5536. [Google Scholar] [CrossRef]
- Al-Husseini, W.; Gondro, C.; Quinn, K.; Cafe, L.M.; Herd, R.M.; Gibson, J.P.; Greenwood, P.L.; Chen, Y. Hormonal growth implants affect feed efficiency and expression of residual feed intake-associated genes in beef cattle. Anim. Prod. Sci. 2014, 54, 550–556. [Google Scholar] [CrossRef]
- Boyles, S.L.; Riley, J.G. Feedlot performance of Brahman x Angus versus Angus steers during cold weather. J. Anim. Sci. 1991, 69, 2677–2684. [Google Scholar] [CrossRef]
- Pfau, A.P.; Shepherd, E.A.; Martin, M.G.; Ascolese, S.; Mason, K.M.; Egert-McLean, A.M.; Voy, B.H.; Myer, P.R. Beta-Adrenergic Agonists, Dietary Protein, and Rumen Bacterial Community Interactions in Beef Cattle: A Review. Vet. Sci. 2023, 10, 579. [Google Scholar] [CrossRef]
- Mersmann, H.J. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 1998, 76, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, R.J.; Mehaffey, J.M.; Baxa, T.J.; Nichols, W.T.; Yates, D.A.; Hutcheson, J.P.; Brooks, J.C.; Johnson, B.J.; Miller, M.F. Effects of duration of zilpaterol hydrochloride and days on the finishing diet on carcass cutability, composition, tenderness, and skeletal muscle gene expression in feedlot steers. J. Anim. Sci. 2009, 87, 3686–3701. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, J.M.; Carter, J.N.; Johnson, D.D.; Ouellette, S.E.; Johnson, S.E. Effect of ractopamine-hydrochloride and trenbolone acetate on longissimus muscle fiber area, diameter, and satellite cell numbers in cull beef cows. J. Anim. Sci. 2007, 85, 1893–1901. [Google Scholar] [CrossRef]
- Kim, W.S.; Ghassemi Nejad, J.; Roh, S.G.; Lee, H.G. Heat-Shock Proteins Gene Expression in Peripheral Blood Mononuclear Cells as an Indicator of Heat Stress in Beef Calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef]
- Lindholm-Perry, A.K.; Kuehn, L.A.; McDaneld, T.G.; Miles, J.R.; Workman, A.M.; Chitko-McKown, C.G.; Keele, J.W. Complete blood count data and leukocyte expression of cytokine genes and cytokine receptor genes associated with bovine respiratory disease in calves. BMC Res. Notes 2018, 11, 786. [Google Scholar] [CrossRef]
- Zhu, J.C.; Si, M.Y.; Li, Y.Z.; Chen, H.Z.; Fan, Z.C.; Xie, Q.D.; Jiao, X.Y. Circulating tight junction proteins mirror blood-brain barrier integrity in leukaemia central nervous system metastasis. Hematol. Oncol. 2017, 35, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Taborda-Charris, J.C.; Rodriguez-Hernandez, R.; Herrera-Sanchez, M.P.; Uribe-Garcia, H.F.; Otero-Arroyo, R.J.; Naranjo-Gomez, J.S.; Lozano-Villegas, K.J.; Rondon-Barragan, I.S. Expression profiling of heat shock protein genes in whole blood of Romosinuano cattle breed. Vet. World 2023, 16, 601–606. [Google Scholar] [CrossRef]
- Kim, H.M.; Suman, S.P.; Li, S.; Dilger, A.C.; Nair, M.N.; Zhai, C.; Edenburn, B.M.; Beach, C.M.; Felix, T.L.; Boler, D.D. Ractopamine-induced changes in the proteome of post-mortem beef longissimus lumborum muscle. S. Afr. J. Anim. Sci. 2019, 49, 424–431. [Google Scholar] [CrossRef]
- Currie, S.; Reddin, K.; McGinn, P.; McConnell, T.; Perry, S.F. Beta-adrenergic stimulation enhances the heat-shock response in fish. Physiol. Biochem. Zool. 2008, 81, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Rendell, J.L.; Fowler, S.; Cockshutt, A.; Currie, S. Development-dependent differences in intracellular localization of stress proteins (hsps) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 238–252. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Chen, C.H.; Guo, B.C.; Hu, P.A.; Lee, H.T.; Hu, H.Y.; Hsu, M.C.; Chen, W.H.; Lee, T.S. Ractopamine at legal residue dosage accelerates atherosclerosis by inducing endothelial dysfunction and promoting macrophage foam cell formation. Environ. Pollut. 2022, 313, 120080. [Google Scholar] [CrossRef]
- Cao, X.Y.; Dong, M.; Shen, J.Z.; Wu, B.B.; Wu, C.M.; Du, X.D.; Wang, Z.; Qi, Y.T.; Li, B.Y. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 2006, 27, 431–438. [Google Scholar] [CrossRef]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Cereijo, R.; Gavalda-Navarro, A.; Cairo, M.; Quesada-Lopez, T.; Villarroya, J.; Moron-Ros, S.; Sanchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a Brown Adipokine that Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab. 2018, 28, 750–763.e756. [Google Scholar] [CrossRef]
- Nara, N.; Nakayama, Y.; Okamoto, S.; Tamura, H.; Kiyono, M.; Muraoka, M.; Tanaka, K.; Taya, C.; Shitara, H.; Ishii, R.; et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 2007, 282, 30794–30803. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Thomas, M.G.; Hallford, D.M.; Keisler, D.H.; Petersen, M.K.; Bryant, W.D.; Garcia, M.D.; Narro, L.; Lopez, R. Metabolic characteristics of multiparous Angus and Brahman cows grazing in the Chihuahuan Desert. J. Anim. Sci. 2002, 80, 2223–2233. [Google Scholar] [CrossRef]
- Chan, O.; Burke, J.D.; Gao, D.F.; Fish, E.N. The chemokine CCL5 regulates glucose uptake and AMP kinase signaling in activated T cells to facilitate chemotaxis. J. Biol. Chem. 2012, 287, 29406–29416. [Google Scholar] [CrossRef]
- Ajoy, R.; Lo, Y.C.; Ho, M.H.; Chen, Y.Y.; Wang, Y.; Chen, Y.H.; Jing-Yuan, C.; Changou, C.A.; Hsiung, Y.C.; Chen, H.M.; et al. CCL5 promotion of bioenergy metabolism is crucial for hippocampal synapse complex and memory formation. Mol. Psychiatry 2021, 26, 6451–6468. [Google Scholar] [CrossRef]
- Lorchner, H.; Canes Esteve, L.; Goes, M.E.; Harzenetter, R.; Brachmann, N.; Gajawada, P.; Gunther, S.; Doll, N.; Poling, J.; Braun, T. Neutrophils for Revascularization Require Activation of CCR6 and CCL20 by TNFalpha. Circ. Res. 2023, 133, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Roman-Ponce, H.; Thatcher, W.W.; Caton, D.; Barron, D.H.; Wilcox, C.J. Thermal stress effects on uterine blood flow in dairy cows. J. Anim. Sci. 1978, 46, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat Stress Impacts Immune Status in Cows Across the Life Cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Shashikanth, N.; France, M.M.; Xiao, R.; Haest, X.; Rizzo, H.E.; Yeste, J.; Reiner, J.; Turner, J.R. Tight junction channel regulation by interclaudin interference. Nat. Commun. 2022, 13, 3780. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Schafer, J.; Lyck, R.; Makrides, V.; Brunner, S.; Schaeren-Wiemers, N.; Deutsch, U.; Engelhardt, B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011, 122, 601–614. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, K.J.; Qi, Z. Occludin regulation of blood-brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020, 6, 152–162. [Google Scholar] [CrossRef]
- Mandel, I.; Paperna, T.; Glass-Marmor, L.; Volkowich, A.; Badarny, S.; Schwartz, I.; Vardi, P.; Koren, I.; Miller, A. Tight junction proteins expression and modulation in immune cells and multiple sclerosis. J. Cell Mol. Med. 2012, 16, 765–775. [Google Scholar] [CrossRef]
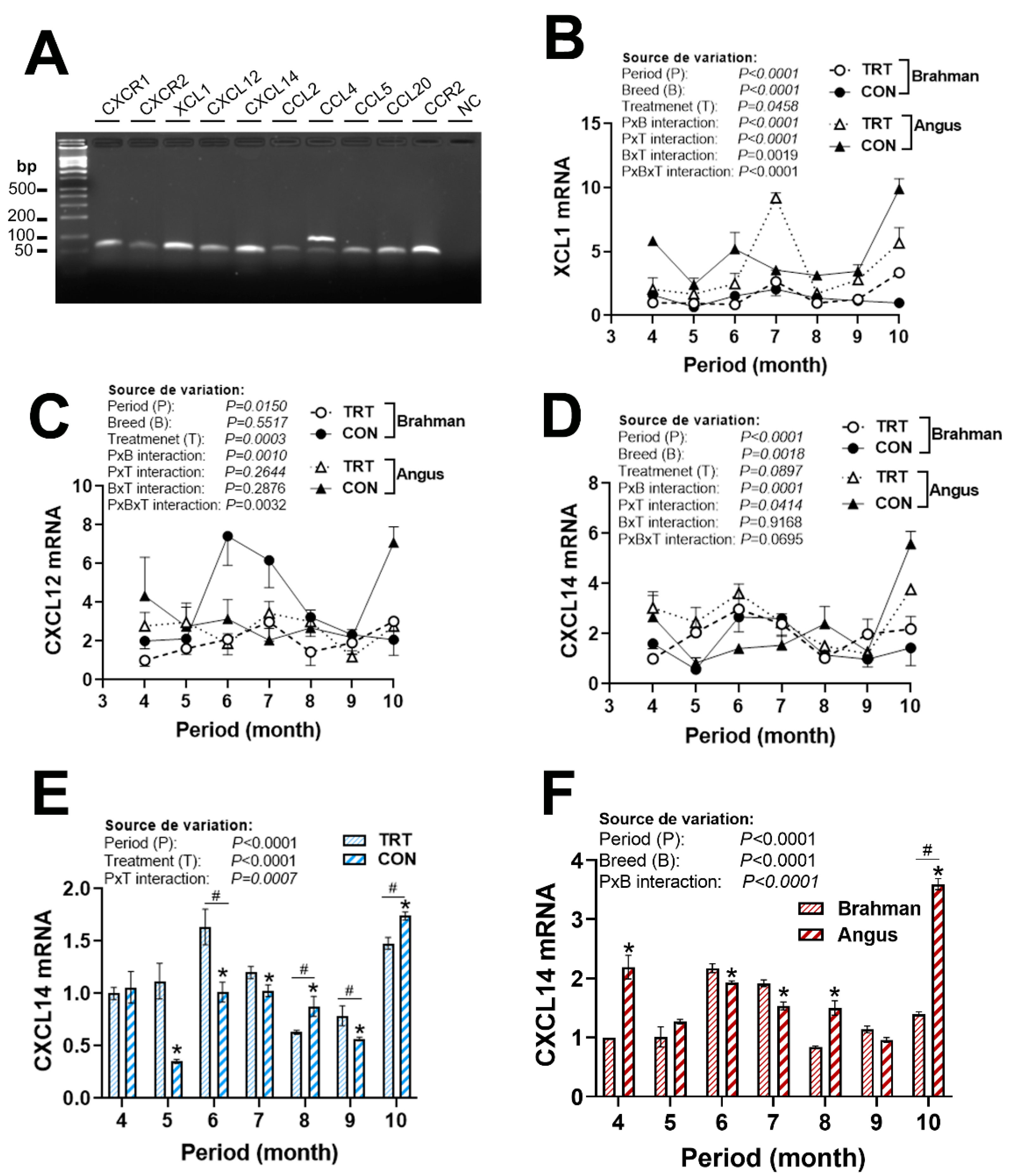
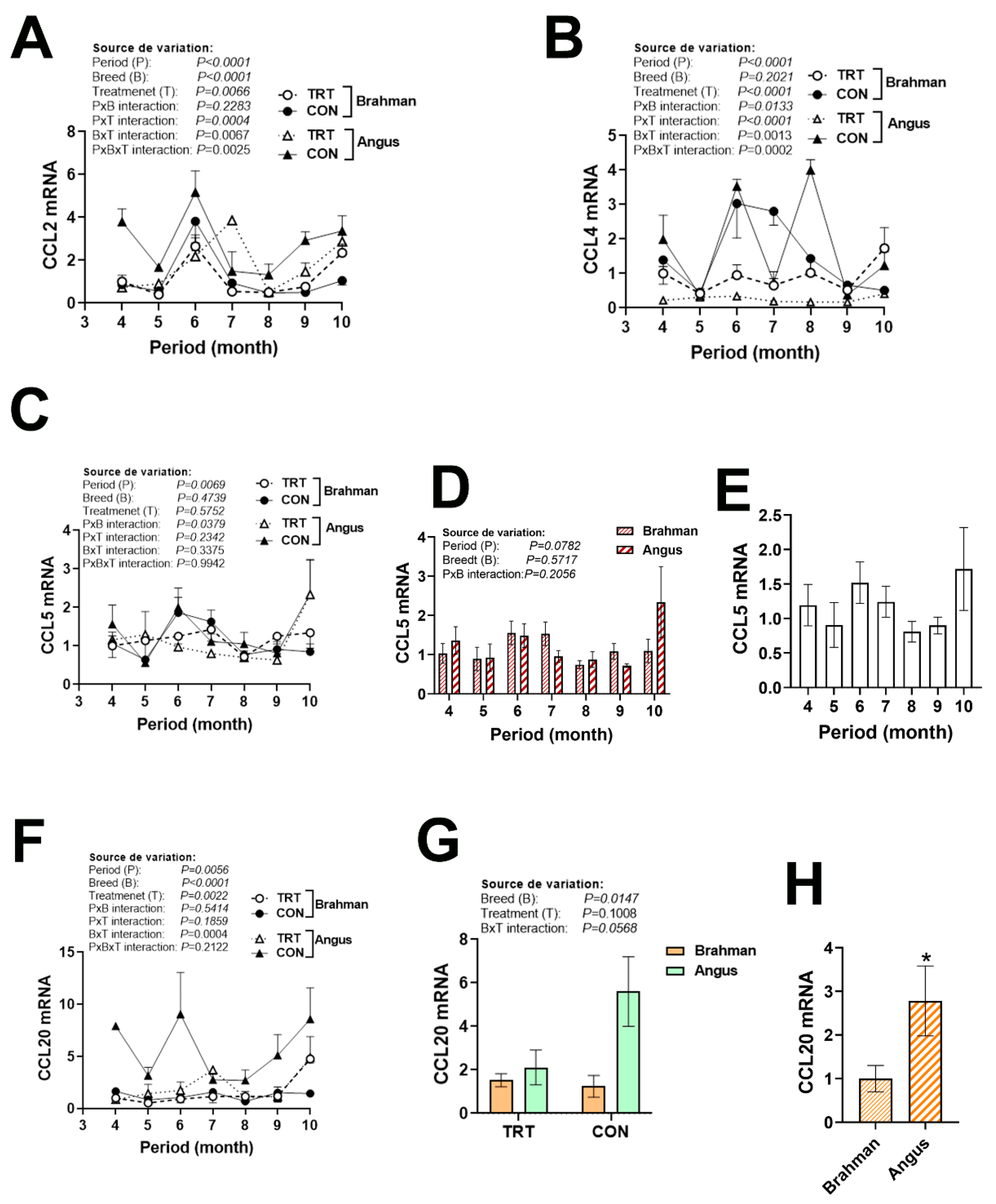
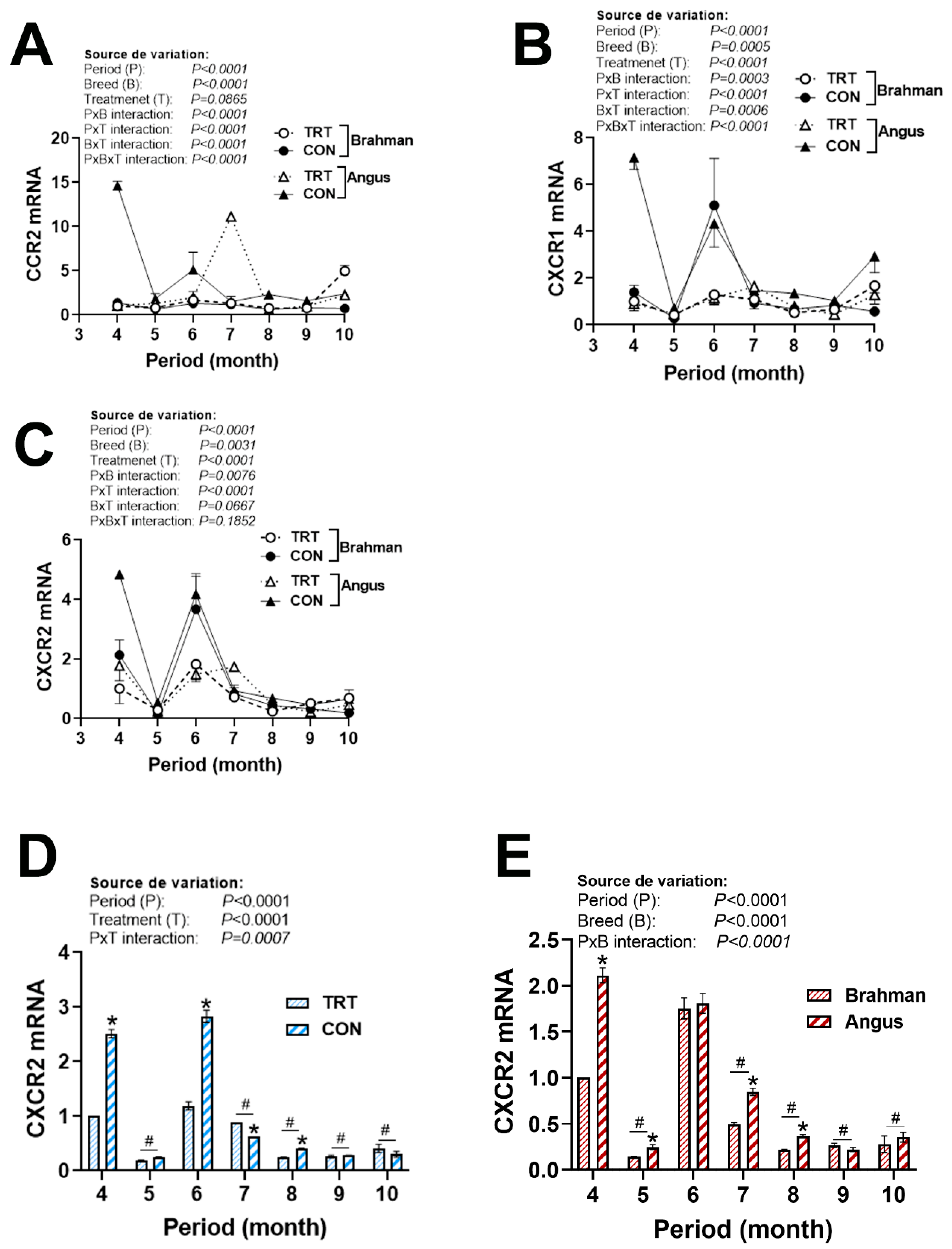
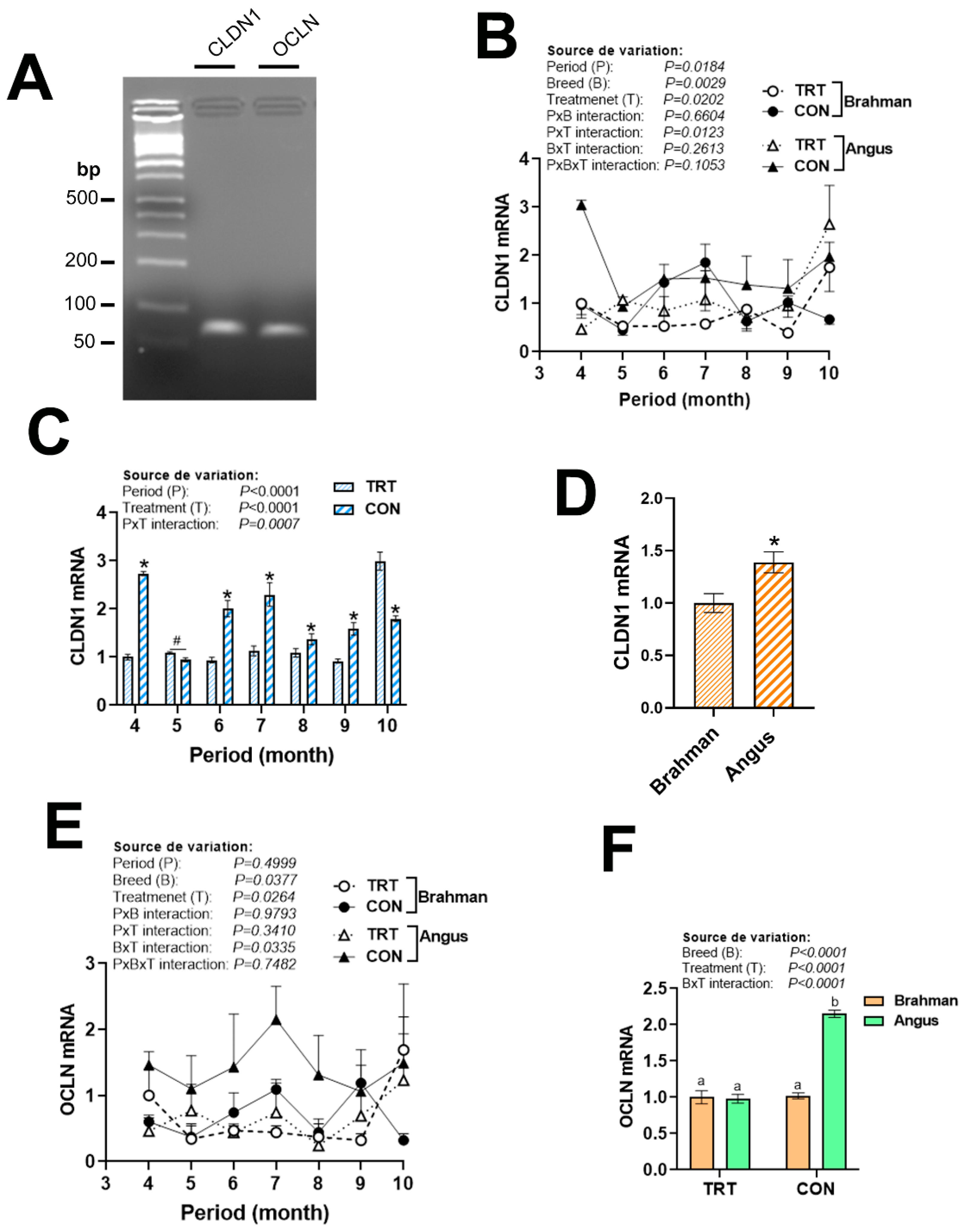
| Gene 1 | Accession Number 2 | Primer Sequence (5′→3′) | Orientation | Product Size (bp) |
|---|---|---|---|---|
| HSP60 | NM_001166609 | CGCGGAAATGCTTCGATTAC | Forward | 63 |
| GCCAGTGCCCTGGACACT | Reverse | |||
| HSPA1A | NM_203322 | GAGCTTCACGTCGTTGATCCT | Forward | 59 |
| CGGCTCCGAGATAAGCTTCA | Reverse | |||
| HSP90 | NM_001012670 | GCAAGATCGAACCCTCACCAT | Forward | 59 |
| TCAAATCGGCCTTGGTCATC | Reverse | |||
| IL6 | NM_173923 | GCCCTCCAGGAACAGCTATG | Forward | 62 |
| GGAGACAGCGAATGGAGTGAA | Reverse | |||
| IL10 | NM_174088 | GGCGGTGGAGAAGGTGAA | Forward | 61 |
| GGCTTTGTAGACACCCCTCTCTT | Reverse | |||
| IL18 | NM_174091 | ACAGTTCTGCTCTCCAATGCTTT | Forward | 61 |
| GCCCCTTCAGCAGCAGAAG | Reverse | |||
| IL-1β | NM_174093 | GAGCCTGTCATCTTCGAAACG | Forward | 55 |
| GCACGGGTGCGTCACA | Reverse | |||
| TNFα | NM_173966 | CGCATTGCAGTCTCCTACCA | Forward | 56 |
| GGGCTCTTGATGGCAGACA | Reverse | |||
| CRP | NM_001144097 | TGGACATGAGTTTGAGCAAGCT | Forward | 60 |
| CAGCACGCCAGGCTTTTC | Reverse | |||
| CCL2 | NM_174006 | CCAAAGCCTTGAGCACTCACT | Forward | 64 |
| AAGCCGGAAGAACACAAATTGT | Reverse | |||
| CCL4 | NM_001075147 | TGCTCATGGCTGCCTTCTG | Forward | 57 |
| GAGGGTCTGAGCCCATTGGT | Reverse | |||
| CCL5 | NM_175827 | TTGCTTCTCGCTCTTGTCCTAA | Forward | 59 |
| TGGGAGGAGGGCATTGC | Reverse | |||
| CCL20 | NM_174263 | CCCAGTATTCTTGTGGGCTTCA | Forward | 59 |
| GCATTGATGTCACAGGCTTCA | Reverse | |||
| XCL1 | NM_175716 | AGCCAGGCCAAGCCTACAG | Forward | 60 |
| CCCAGTCAGGGTCACAGTTGT | Reverse | |||
| CXCL12 | NM_001113174 | AGATGCCCTTGCCGATTCT | Forward | 56 |
| AGGTGCTTGACGTTGGCTTT | Reverse | |||
| CXCL14 | NM_001034410 | CCGCTACAGCGACGTGAA | Forward | 56 |
| CCTCGCAGTGCGGGTACTT | Reverse | |||
| CXCR1 | NM_174360 | CCACCGTACTCCGACCTAGTCT | Forward | 61 |
| TCCGCCATTTCGTTGTATTG | Reverse | |||
| CXCR2 | NM_001101285 | CCGCCGCCCTTTCTTC | Forward | 53 |
| TGTGGGACACCTCCAGGAA | Reverse | |||
| CCR2 | NM_001194959 | CCACGTTCTTCCGAAAGCATA | Forward | 62 |
| CCCATAGAAAACTGGGCATTG | Reverse | |||
| CLDN1 | NM_001001854 | GCTCCTGTCCCCGGAAAA | Forward | 61 |
| GGTGCTGGCTTGGGATAGG | Reverse | |||
| OCLN | NM_001082433 | GACTTCCGGCAGCCTCATTA | Forward | 64 |
| CGGGAGCCCTTTTTGAAAG | Reverse | |||
| r18S | NR_036642 | CCGCGGTTCTATTTTGTTGGT | Forward | 57 |
| CGGCCGCCCCTCTTAA | Reverse |
| Breed (B) | Brahman (Bos indicus) | Angus (Bos taurus) | ||||||
|---|---|---|---|---|---|---|---|---|
| Period (P) 1/System (S) 2 | TRT | CON | TRT | CON | Three-Way ANOVA 3 | |||
| S. of Variation | MS | F (DFn, DFd) | p | |||||
| April (Initial) | 346 ± 13 aα | 351 ± 16 aα | 338 ± 12 aα | 345 ± 6 aα | P | 179,786 | 86.22 | <0.0001 |
| May | 383 ± 14 aα | 388 ± 18 aα | 391 ± 14 aα | 390 ± 8 aα | B | 56,831 | 27.25 | <0.0001 |
| June | 412 ± 18 aα | 425 ± 21 aα | 462 ± 13 aβ | 443 ± 17 aβ | S | 10,006 | 4.798 | 0.0302 |
| July | 448 ± 19 aβ | 456 ± 26 aα | 508 ± 14 aβ | 493 ± 18 aβ | P × B | 4688 | 2.248 | 0.0424 |
| August | 483 ± 21 aβ | 460 ± 33 aα | 553 ± 17 aβ | 514 ± 18 aβ | P × S | 2602 | 1.248 | 0.2860 |
| September | 520 ± 22 aβ | 514 ± 34 aβ | 609 ± 21 aβ | 549 ± 17 aβ | B × S | 8482 | 4.068 | 0.0457 |
| October (Final) | 580 ± 23 aβ | 569 ± 38 aβ | 686 ± 18 bβ | 595 ± 23 aβ | P × B × S | 1116 | 0.5350 | 0.7808 |
| Residual | 2085 | |||||||
| Breed (B) | Brahman (Bos indicus) | Angus (Bos taurus) | ||||||
|---|---|---|---|---|---|---|---|---|
| Period (P) 1/System (S) 2 | TRT | CON | TRT | CON | Three-Way ANOVA 3 | |||
| S. of Variation | MS | F (DFn, DFd) | p | |||||
| May | 1.31 ± 0.1 aα | 1.29 ± 0.1 aα | 1.99 ± 0.1 aα | 1.68 ± 0.08 aα | P | 3.120 | 10.09 | <0.0001 |
| June | 1.06 ± 0.1 aα | 1.24 ± 0.1 aα | 2.65 ± 0.1 bα | 2.05 ± 0.3 bα | B | 10.75 | 27.11 | <0.0001 |
| July | 1.27 ± 0.09 aα | 1.16 ± 0.2 aα | 1.68 ± 0.07 aα | 1.86 ± 0.1 aα | S | 4.432 | 65.75 | <0.0001 |
| August | 1.23 ± 0.1 aα | 0.29 ± 0.4 bβ | 1.57 ± 0.1 aα | 0.70 ± 0.2 cβ | P × B | 0.6586 | 4.458 | 0.0009 |
| September | 1.33 ± 0.1 aα | 1.71 ± 0.2 aα | 2.18 ± 0.2 bα | 1.35 ± 0.1 aα | P × S | 0.7288 | 4.029 | 0.0021 |
| October | 2.15 ± 0.06 aβ | 1.55 ± 0.1 bα | 2.63 ± 0.1 aα | 1.90 ± 0.3 abα | B × S | 1.021 | 6.248 | 0.0138 |
| P × B × S | 0.4556 | 2.787 | 0.0203 | |||||
| Residual | 0.1635 | |||||||
| Breed (B) | Brahman (Bos indicus) | Angus (Bos taurus) | Two-Way ANOVA (p Value) 3 | ||||
|---|---|---|---|---|---|---|---|
| Parameter 1/System (S) 2 | TRT | CON | TRT | CON | B | S | B × S |
| HCW (Kg) | 368.8 ± 12.11 | 356.6 ± 9.18 | 430.9 ± 16.20 | 390.8 ± 8.87 | 0.0004 | 0.0314 | 0.2313 |
| Dressing (%) 4 | 63.63 ± 0.92 | 64.83 ± 2.12 | 62.19 ± 3.13 | 63.13 ± 4.17 | 0.5880 | 0.7114 | 0.9641 |
| Main Effect (Breed) | |||||||
| Brahman (Bos indicus) | Angus (Bos taurus) | t-test (p value) | |||||
| HCW (Kg) | 362.7 ± 10.64 | 410.85 ± 12.53 | 0.0078 | ||||
| Main Effect (Production System) | |||||||
| TRT | CON | p value | |||||
| HCW (Kg) | 399.85 ± 14.15 | 373.70 ± 9.02 | 0.1334 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branine, M.; Schilling-Hazlett, A.K.; Carvalho, P.H.V.; Stackhouse-Lawson, K.R.; Martins, E.C.; da Silva, J.T.; Amundson, L.; Ashworth, C.; Socha, M.; Dridi, S. Effects of Production System With or Without Growth-Promoting Technologies on Growth and Blood Expression of (Cyto)Chemokines and Heat Shock and Tight Junction Proteins in Bos taurus and indicus Breeds During Summer Season. Vet. Sci. 2025, 12, 65. https://doi.org/10.3390/vetsci12010065
Branine M, Schilling-Hazlett AK, Carvalho PHV, Stackhouse-Lawson KR, Martins EC, da Silva JT, Amundson L, Ashworth C, Socha M, Dridi S. Effects of Production System With or Without Growth-Promoting Technologies on Growth and Blood Expression of (Cyto)Chemokines and Heat Shock and Tight Junction Proteins in Bos taurus and indicus Breeds During Summer Season. Veterinary Sciences. 2025; 12(1):65. https://doi.org/10.3390/vetsci12010065
Chicago/Turabian StyleBranine, Mark, Ashley K. Schilling-Hazlett, Pedro H. V. Carvalho, Kim R. Stackhouse-Lawson, Edilane C. Martins, Julia T. da Silva, Laura Amundson, Chris Ashworth, Mike Socha, and Sami Dridi. 2025. "Effects of Production System With or Without Growth-Promoting Technologies on Growth and Blood Expression of (Cyto)Chemokines and Heat Shock and Tight Junction Proteins in Bos taurus and indicus Breeds During Summer Season" Veterinary Sciences 12, no. 1: 65. https://doi.org/10.3390/vetsci12010065
APA StyleBranine, M., Schilling-Hazlett, A. K., Carvalho, P. H. V., Stackhouse-Lawson, K. R., Martins, E. C., da Silva, J. T., Amundson, L., Ashworth, C., Socha, M., & Dridi, S. (2025). Effects of Production System With or Without Growth-Promoting Technologies on Growth and Blood Expression of (Cyto)Chemokines and Heat Shock and Tight Junction Proteins in Bos taurus and indicus Breeds During Summer Season. Veterinary Sciences, 12(1), 65. https://doi.org/10.3390/vetsci12010065









