Effects of Chlorogenic Acid on In Vitro Maturation and Vitrification Cryopreservation of Sheep Oocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Configuration of CGA Concentration
2.3. Configuration of Solutions
2.4. Oocyte Collection
2.5. Vitrification Freezing and Resuscitation of Mature Oocytes
2.6. Parthenogenetic Activation and Embryo Culture
2.7. Calculation of the Oocyte Maturation, Cleavage, and Blastocyst Rates
2.8. Detection of ROS and GSH Levels
2.9. Detection of Mitochondrial Membrane Potentials
2.10. Real-Time Quantitative RT-PCR
2.11. Statistical Analysis
3. Results
3.1. Effect of CGA on the Maturation of Sheep Oocytes In Vitro
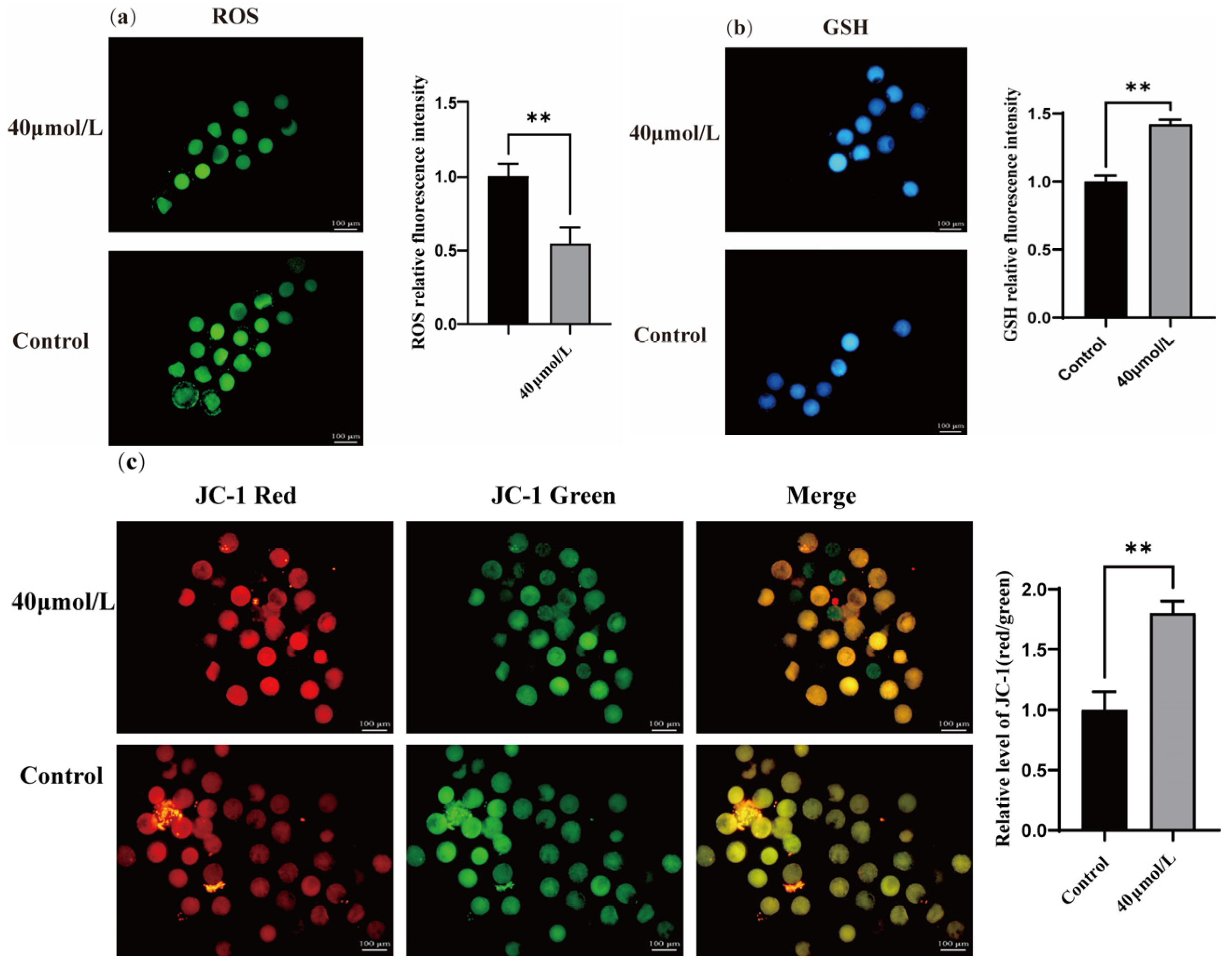
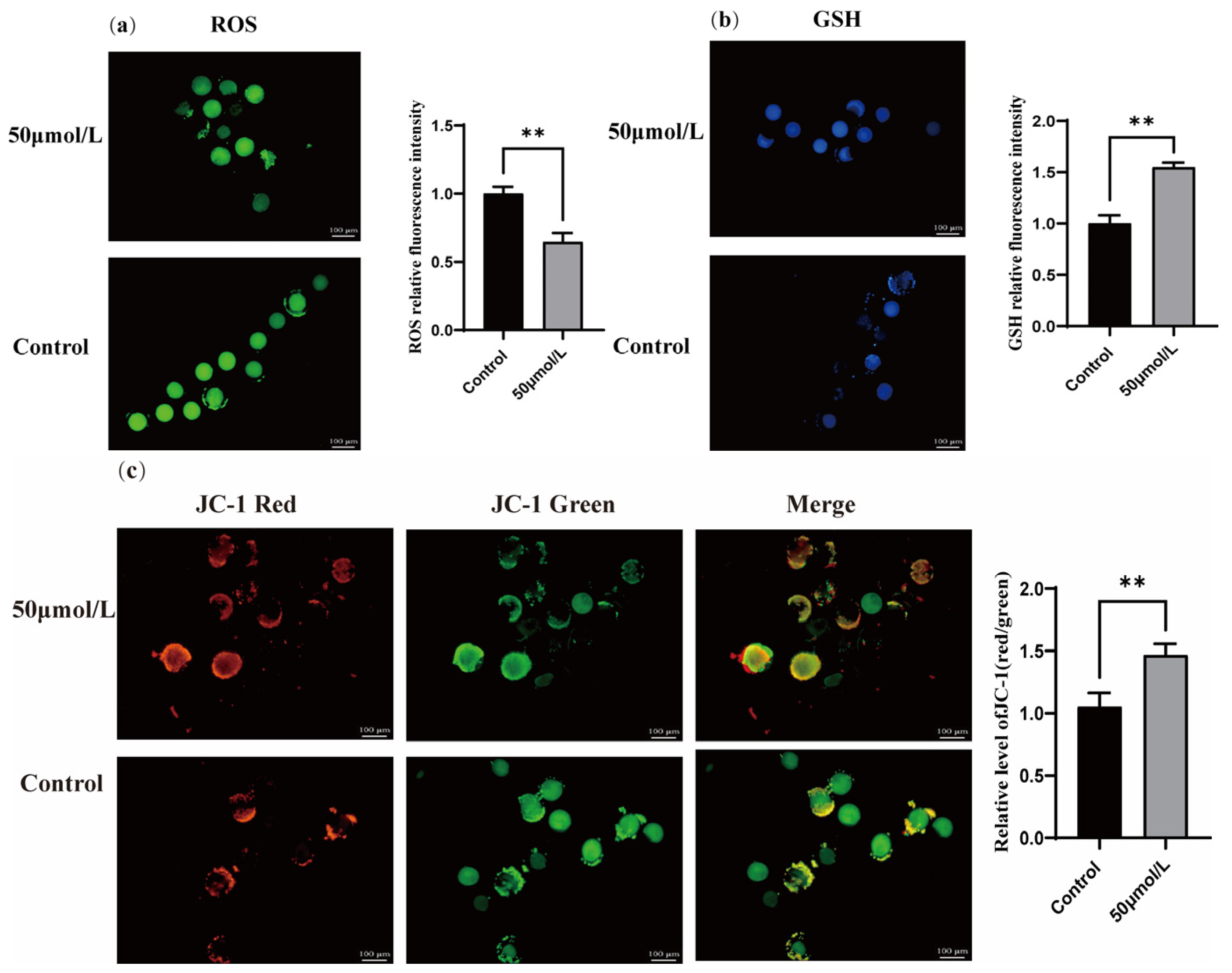
3.2. Effect of CGA on ROS, GSH, and the Mitochondrial Membrane Potential in Oocytes
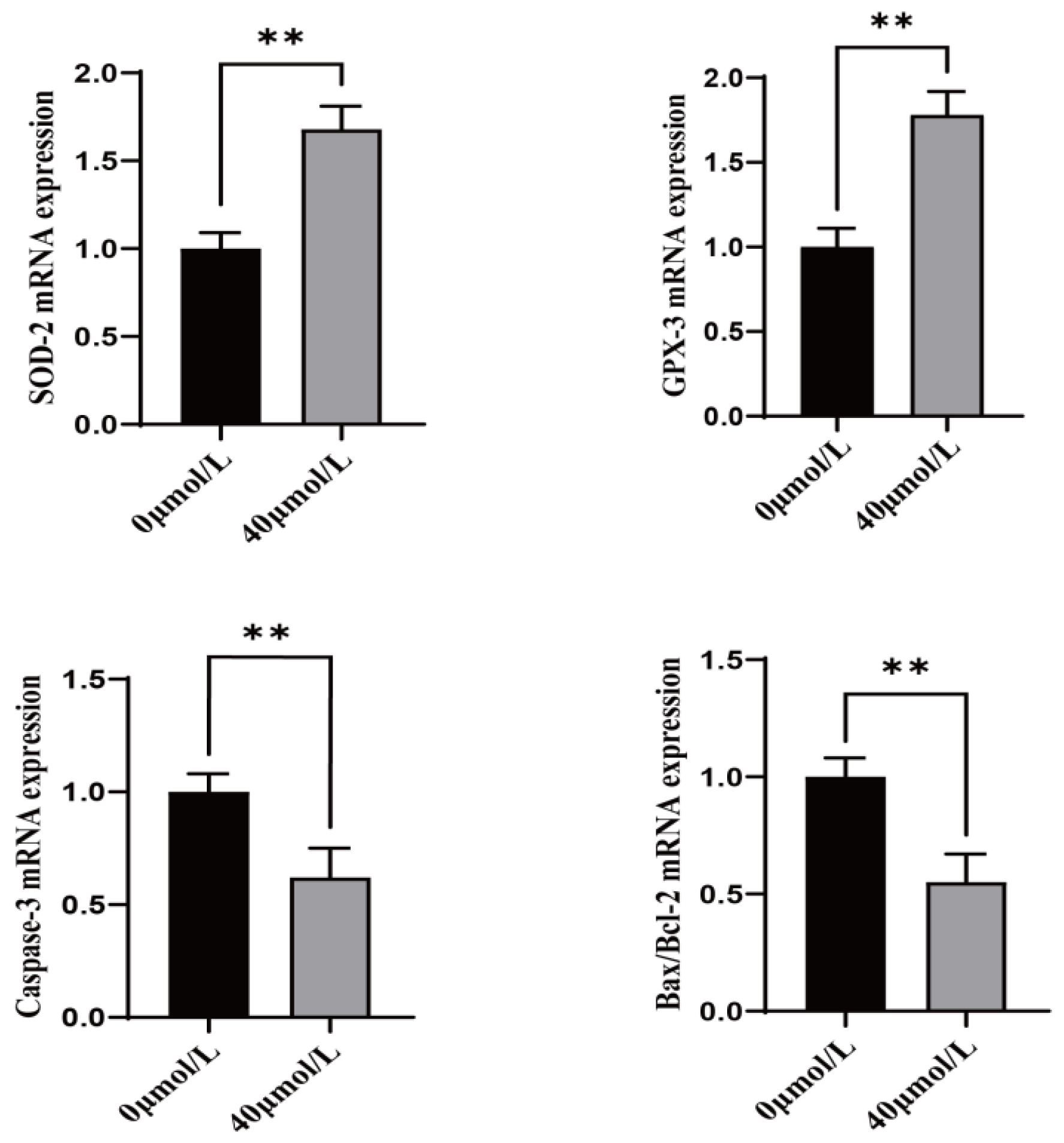
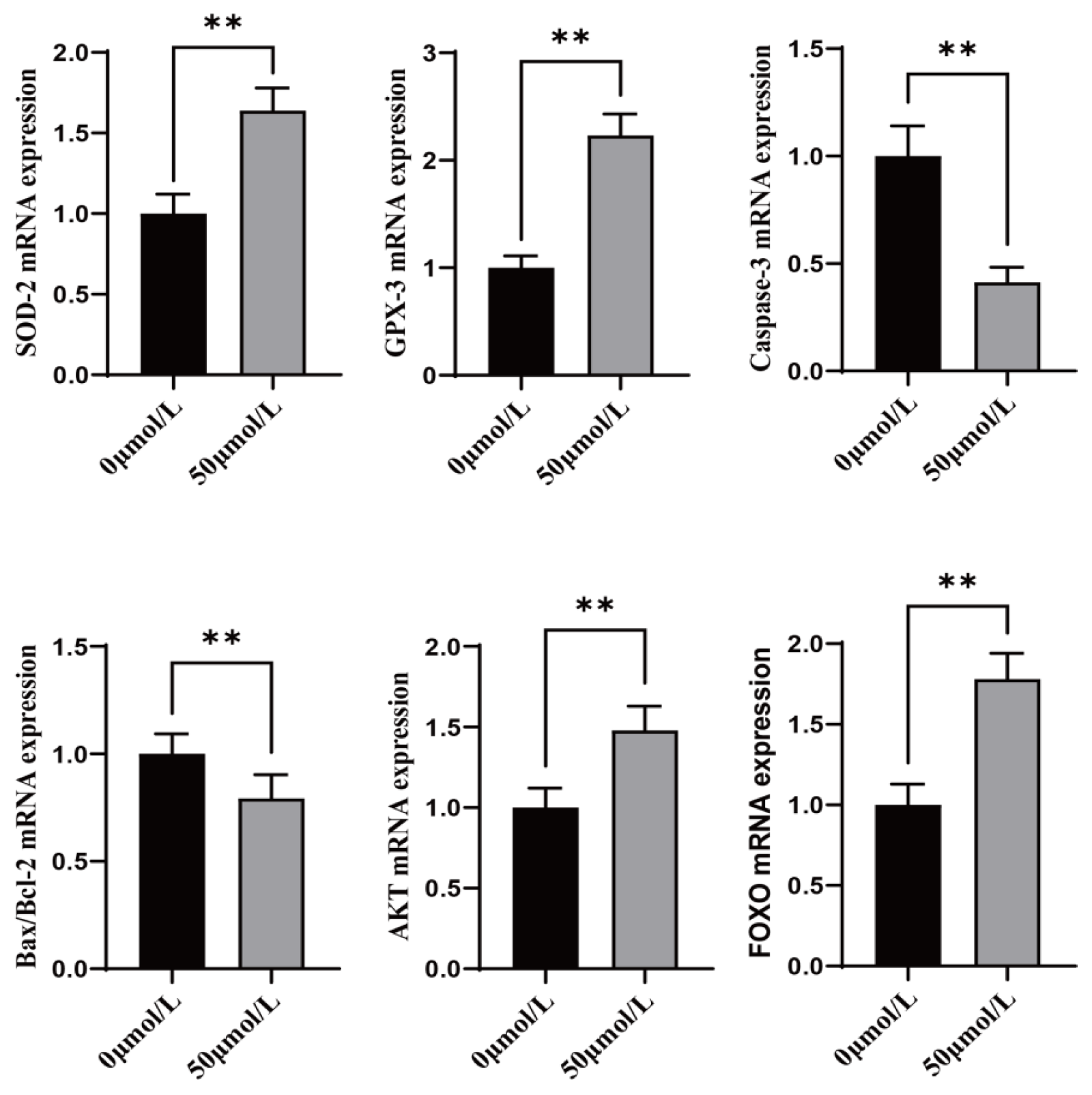
3.3. Effect of CGA on Antioxidant and Apoptosis-Related Genes in Sheep Oocytes
3.4. Effect of CGA on Vitrification Freezing of Mature Sheep Oocytes
3.5. Effect of CGA on ROS, GSH, and Mitochondrial Membrane Potential During Vitrification Freezing of Sheep Oocytes
3.6. Effect of CGA on Antioxidant, Apoptosis, and Anti-Stress Related Genes During Freezing of Sheep Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souza-Fabjan, J.M.G.; Leal, G.R.; Monteiro, C.A.S.; Batista, R.I.T.P.; Barbosa, N.O.; Freitas, V.J.F. In vitro embryo production in small ruminants: What is still missing? Anim. Reprod. 2023, 20, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Jin, Y.X.; Hao, J.D.; Huang, S.Y.; Wang, D.X.; Quan, F.S.; Ren, W.Z.; Zhang, J.B.; Zhang, M.J.; Yu, X.F. Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress. Mol. Reprod. Dev. 2022, 88, 55–66. [Google Scholar] [CrossRef]
- Huang, K.F.; Li, C.D.; Gao, F.L.; Fan, Y.S.; Zeng, F.W.; Meng, L.; Li, L.; Zhang, S.Q.; Wei, H.X. Epigallocatechin-3-gallate promotes the in vitro maturation and embryo development following IVF of porcine oocytes. Drug Des. Dev. Ther. 2021, 15, 1013–1020. [Google Scholar] [CrossRef]
- Tian, X.Z.; Wang, F.; Zhang, L.; He, C.J.; Ji, R.Y.; Wang, J.; Zhang, Z.Z.; Lv, D.Y.; Abulizi, W.; Wang, X.G.; et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 19, 4554. [Google Scholar] [CrossRef]
- Zhuan, Q.R.; Li, J.; Zhou, G.Z.; Du, X.Z.; Liu, H.Y.; Hou, Y.P.; Wan, P.C.; Fu, X.W. Procyanidin B2 protects aged oocytes against meiotic defects through cortical tension modulation. Front. Vet. Sci. 2022, 9, 795050. [Google Scholar] [CrossRef]
- Zhuan, Q.R.; Li, J.; Du, X.Z.; Zhang, L.Y.; Meng, L.; Luo, Y.W.; Zhou, D.; Liu, H.Y.; Wan, P.C.; Hou, Y.P.; et al. Antioxidant procyanidin B2 protects oocytes against cryoinjuries via mitochondria regulated cortical tension. J. Anim. Sci. Biotechnol. 2022, 13, 95. [Google Scholar] [CrossRef]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.K.; Kim, C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef]
- Kim, H.Y.; Pan, J.H.; Kim, S.H.; Lee, J.H.; Park, J.W. Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 2018, 150, 131–138. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.L.; Ruan, Z.; Mi, S.M.; Jiang, M.; Li, X.L.; Wu, X.; Deng, Z.Y.; Yin, Y.L. Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. Biosci. Biotechnol. Biochem. 2016, 80, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Bonechi, C.; Signorini, C.; Corsaro, R.; Micheli, L.; Liguori, L.; Centini, G.; Collodel, G. In Vitro Effects of Charged and Zwitterionic Liposomes on Human Spermatozoa and Supplementation with Liposomes and Chlorogenic Acid during Sperm Freezing. Cells 2024, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Namula, Z.; Hirata, M.; Wittayarat, M.; Tanihara, F.; Thi Nguyen, N.; Hirano, T.; Nii, M.; Otoi, T. Effects of chlorogenic acid and caffeic acid on the quality of frozen-thawed boar sperm. Reprod. Domest. Anim. 2018, 53, 600–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zhang, L.M.; Sohail, T.; Kang, Y.; Sun, X.M.; Li, Y. Chlorogenic acid improves quality of chilled ram sperm by mitigating oxidative stress. Animals 2022, 12, 163. [Google Scholar] [CrossRef]
- Rabelo, S.S.; Resende, C.O.; Pontelo, T.P.; Chaves, B.R.; Pereira, B.A.; Silva, W.E.D.; Peixoto, J.V.; Pereira, L.J.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen processed in Percoll. Anim. Reprod. 2020, 17, 21. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tanihara, F.; Do, L.T.K.; Sato, Y.; Taniguchi, M.; Takagi, M.; Van Nguyen, T.; Otoi, T. Chlorogenic acid supplementation during in vitro maturation improves maturation, fertilization and developmental competence of porcine oocytes. Reprod. Domest. Anim. 2017, 52, 969–975. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do, L.T.K.; Somfai, T.; Otoi, T.; Taniguchi, M.; Kikuchi, K. Presence of chlorogenic acid during in vitro maturation protects porcine oocytes from the negative effects of heat stress. Anim. Sci. J. 2019, 90, 1530–1536. [Google Scholar] [CrossRef]
- Speckhart, S.L.; Wooldridge, L.K.; Ealy, A.D. An updated protocol for in vitro bovine embryo production. STAR Protoc. 2023, 4, 101924. [Google Scholar] [CrossRef]
- Yao, X.R.; Jiang, H.; Liang, S.; Shen, X.H.; Gao, Q.S.; Xu, Y.N.; Kim, N.H. Laminarin enhances the quality of aged pig oocytes by reducing oxidative stress. J. Reprod. Dev. 2018, 64, 489–494. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhang, L.L.; Wang, J.F.; Li, X.Q.; Lv, J.T. Research progress on antitumor effects and mechanisms of chlorogenic aci. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 229–234. [Google Scholar]
- Silva, A.F.B.; Lima, L.F.; Morais, A.N.P.; Lienou, L.L.; Watanabe, Y.F.; Joaquim, D.C.; Morais, S.M.; Alves, D.R.; Pereira, A.F.; Santos, A.C.; et al. Oocyte in vitro maturation with eugenol improves the medium antioxidant capacity and total cell number per blastocyst. Theriogenology 2022, 192, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cajas, Y.N.; Cañón-Beltrán, K.; Ladrón de Guevara, M.; Millán de La Blanca, M.G.; Ramos-Ibeas, P.; Gutiérrez-Adán, A.; Rizos, D.; González, E.M. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int. J. Mol. Sci. 2020, 21, 5340. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Wang, Y.Q.; Zheng, X.M.; Sun, C.Y.; Gao, Q.S.; Fang, N.Z.; Jin, Q.G. Effects of Cepharanthine on antioxidant capacity of bovine oocytes in vitro maturation. Chin. J. Anim. Husb. 2024, 6, 245–250. [Google Scholar]
- Liu, K.X.; Zhang, L.Y.; Xu, X.L.; Xiao, L.L.; Wen, J.H.; Zhang, H.B.; Zhao, S.X.; Qiao, D.L.; Bai, J.H.; Liu, Y. The Antioxidant Salidroside Ameliorates the Quality of Postovulatory Aged Oocyte and Embryo Development in Mice. Antioxidants 2024, 13, 248. [Google Scholar] [CrossRef]
- Yang, Q.L.; Dai, S.j.; Luo, X.y.; Zhu, J.; Li, F.Y.; Liu, J.H.; Yao, G.D.; Sun, Y.P. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction 2018, 156, 81–92. [Google Scholar] [CrossRef]
- Ahmadi, E.; Shirazi, A.; Shams-Esfandabadi, N.; Nazari, H. Antioxidants and glycine can improve the developmental competence of vitrified/warmed ovine immature oocytes. Reprod. Domest. Anim. 2019, 54, 595–603. [Google Scholar] [CrossRef]
- Al-Mutary, M.; Al-Ghadi, M.; Al-Himaidi, A.; Iwamoto, D.; Al-Anazi, Y.; Ammari, A.; Ahmad, J.; Al-Khedhairy, A. Using RT-PCR and glutathione level to study the effect of follicular fluid on in vitro maturation and gene expression of sheep oocytes. Saudi J. Biol. Sci. 2019, 26, 1216–1222. [Google Scholar] [CrossRef]
- Yoshida, M.; Ishigaki, K.; Nagai, T.; Chikyu, M.; Pursel, V.G. Glutathione concentration during maturation and after fertilization in pig oocytes: Relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 1993, 49, 89–94. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidi, U.; Liu, J.; Cinar, O.; Robker, R.L.; Adhikari, D.; Carroll, J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol. Hum. Reprod. 2019, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox. Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Squirrell, J.M.; Wokosin, D.L.; White, J.G.; Bavister, B.D. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat. Biotechnol. 1999, 17, 763–767. [Google Scholar] [CrossRef]
- Orsi, N.M.; Leese, H.J. Protection against reactive oxygen species during mouse preimplantation embryo development: Role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol. Reprod. Dev. 2001, 59, 44–53. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Zhang, X.; Chen, Y.X.; Liu, X.P.; Sun, Y.X.; Xiong, B. Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ. Pollut. 2019, 225, 113194. [Google Scholar] [CrossRef]
- Zhang, H.M.; Rao, J.N.; Guo, X.; Liu, L.; Zou, T.; Turner, D.J.; Wang, J.Y. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J. Biol. Chem. 2004, 279, 22539–22547. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef]


| Gene Name | GenBank Accession | Sequence (5′–3′) | Length (bp) |
|---|---|---|---|
| SOD-2 | NM_001280703.1 | F:AGGGAGATAAAGTCGTCGTA | 165 |
| R:ACAGAGGATTAAAGTGAGGG | |||
| GPX3 | XM_015096153.3 | F:CCATTCGGTCTGGTCATT | 176 |
| R:CCCGTTCACATCGCCTTT | |||
| BCL-2 | XM_012103831.5 | F:GCCGAGTGAGCAGGAAGAC | 312 |
| R:GTTAGCCAGTGCTTGCTGAGA | |||
| BAX | XM_027978592.3 | F:CCTGGGATCTTGAAACTCTCCTT | 148 |
| R:CTGAGCCAGGCTGAAATCAAAA | |||
| Caspase-3 | XM_060406953.1 | F:AAGTTTCTTCAGAGGGGACTGTTGC | 177 |
| R:GCCATGTCGTCCTCAGAACCAC | |||
| AKT | XM_027956972.3 | F:AGTACATCAAGACCTGGCGG | 216 |
| R:GAGAAGTTGTTGAGGGGCGA | |||
| FOXO | XM_060419173.1 | F:AGTACCATTAGCGGGAGGCT | 110 |
| R: GTAGAAGCCATCTTTGCGGC | |||
| GAPDH | XM_060411593.1 | F: CGGCACAGTCAAGGCAGAGAAC | 114 |
| R: CACGTACTAGCACCAGCATCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, H.; Zhao, Y.; Zhang, Q.; Li, X.; Hu, G.; Wang, Y.; Zeng, W. Effects of Chlorogenic Acid on In Vitro Maturation and Vitrification Cryopreservation of Sheep Oocytes. Vet. Sci. 2025, 12, 62. https://doi.org/10.3390/vetsci12010062
Tao H, Zhao Y, Zhang Q, Li X, Hu G, Wang Y, Zeng W. Effects of Chlorogenic Acid on In Vitro Maturation and Vitrification Cryopreservation of Sheep Oocytes. Veterinary Sciences. 2025; 12(1):62. https://doi.org/10.3390/vetsci12010062
Chicago/Turabian StyleTao, Hong, Yukun Zhao, Qiang Zhang, Xu Li, Guangdong Hu, Yanping Wang, and Weibin Zeng. 2025. "Effects of Chlorogenic Acid on In Vitro Maturation and Vitrification Cryopreservation of Sheep Oocytes" Veterinary Sciences 12, no. 1: 62. https://doi.org/10.3390/vetsci12010062
APA StyleTao, H., Zhao, Y., Zhang, Q., Li, X., Hu, G., Wang, Y., & Zeng, W. (2025). Effects of Chlorogenic Acid on In Vitro Maturation and Vitrification Cryopreservation of Sheep Oocytes. Veterinary Sciences, 12(1), 62. https://doi.org/10.3390/vetsci12010062





