Characterization of the Population of Ovarian Preantral Follicles in Juvenile Six-Banded Armadillos Infected or Not by Mycobacterium leprae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Animals
2.2. Detection of M. leprae Infection
2.3. Measurements of the Ovaries
2.4. Histological Processing
2.5. Ovarian Preantral Follicles Morphometrics
2.6. Estimation of Ovarian Preantral Follicle Population
Nº of sections observed × Mean of oocyte nuclei diameter
2.7. Morphological Analysis of Ovarian Preantral Follicles
3. Results
3.1. Overall Anatomy of the Ovaries
3.2. Histological Features of the Ovarian Tissues and Preantral Follicles
3.3. Ovarian Preantral Follicles Morphometrics and Estimation
3.4. Estimation of the Ovarian Preantral Follicle Population
3.5. Morphology of the Ovarian Preantral Follicles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, A.R.; Sousa, P.C.; Freitas, C.I.A. Assisted reproduction in six-banded armadillos (Euphractus sexcinctus): Peculiarities and challenges. Rev. Bras. Reprod. Anim. 2015, 39, 61–65. [Google Scholar]
- Balamayooran, G.; Pena, M.; Sharma, R.; Truman, R.W. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin. Dermatol. 2015, 33, 108–115. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, J.; de Carvalho, F.M.; Vidal Pessolani, M.C.; de Paula Antunes, J.M.A.; de Medeiros Oliveira, I.V.P.; Ferreira Moura, G.H.; Truman, R.W.; Peña, M.T.; Sharma, R.; Duthie, M.S.; et al. Serological and molecular detection of infection with Mycobacterium leprae in Brazilian six banded armadillos (Euphractus sexcinctus). Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101397. [Google Scholar] [CrossRef] [PubMed]
- Frota, C.C.; Lima, L.N.C.; Rocha, A.D.S.; Suffys, P.N.; Rolim, B.N.; Rodrigues, L.C.; Barreto, M.L.; Kendall, C.; Kerr, L.R.S. Mycobacterium leprae in six-banded (Euphractus sexcinctus) and nine-banded armadillos (Dasypus novemcinctus) in Northeast Brazil. Mem. Inst. Oswaldo Cruz 2012, 107 (Suppl. 1), 209–213. [Google Scholar] [CrossRef]
- Abba, A.M.; Lima, E.; Superina, M. Euphractus sexcinctus. In The IUCN Red List of Threatened Species 2014; IUCN Biodiversity Assessment & Knowledge Team: Red List Unit: Cambridge, UK, 2014; e.T8306A47441708. [Google Scholar] [CrossRef]
- Ribeiro, P.; Silveira Miranda, J.E.; Rodrigues de Araújo, D. The effect of roadkills on the persistence of xenarthran populations: The case of the Brazilian Cerrado. Edentata 2017, 18, 51–61. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; Mantellatto, A.M.B.; Superina, M.; Chiarello, A.G. Ecosystem services provided by armadillos. Biol. Rev. 2019, 95, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.; Kückelhaus, S.; Galdos-Riveros, A.; Ferreira, J.; Miglino, M. Vascularización, morfología e histología del ovario en el armadillo Euphractus sexcinctus (Linnaeus, 1758). Arch. Med. Vet. 2013, 45, 191–196. [Google Scholar] [CrossRef]
- Fernandes, D.P.; Praxedes, É.A.; Viana, J.V.D.S.; de Aquino, L.V.C.; Rodrigues, L.L.V.; Moura, Y.B.F.; de Oliveira, M.F.; Freitas, C.I.A.; Pereira, A.F. Influence of cryopreservation techniques and low concentrations of permeating cryoprotectants on the conservation of ear cartilage and skin derived from six-banded armadillos (Euphractus sexcinctus Linnaeus, 1758). Cryobiology 2023, 113, 104788. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.; Moraes-Barros, N.; Superina, M.; Abba, A.M. Tolypeutes tricinctus. In The IUCN Red List of Threatened Species 2014; IUCN Biodiversity Assessment & Knowledge Team: Red List Unit: Cambridge, UK, 2014; e.T21975A47443455. [Google Scholar] [CrossRef]
- Anacleto, T.C.S.; Miranda, F.; Medri, I.; Cuellar, E.; Abba, A.M.; Superina, M. Priodontes maximus. In The IUCN Red List of Threatened Species 2014; IUCN Biodiversity Assessment & Knowledge Team: Red List Unit: Cambridge, UK, 2014; e.T18144A47442343. [Google Scholar] [CrossRef]
- da Cunha Sousa, P.; Campos, L.B.; Silva, A.R.; Lima, G.L. The Armadillos. In Assisted Reproduction in Wild Mammals of South America; CRC Press: Boca Raton, FL, USA, 2023; pp. 291–305. [Google Scholar] [CrossRef]
- Serafim, M.K.B.; Lira, R.A.; Costa, L.L.M.; Gadelha, I.C.N.; Freitas, C.I.A.; Silva, A.R. Description of semen characteristics from six-banded armadillos (Euphractus sexcinctus) collected by electroejaculation. Anim. Reprod. Sci. 2010, 118, 362–365. [Google Scholar] [CrossRef]
- Santos, E.A.A.; Sousa, P.C.; Dias, C.E.V.; Castelo, T.S.; Peixoto, G.C.X.; Lima, G.L.; Ricarte, A.R.F.; Simão, B.R.; Freitas, C.I.A.; Silva, A.R. Assessment of sperm survival and functional membrane integrity of the six-banded armadillo (Euphractus sexcinctus). Theriogenology 2011, 76, 623–629. [Google Scholar] [CrossRef]
- Sousa, P.C.; Amorim, R.N.L.; Lima, G.L.; Paiva, A.L.C.; Paula, V.V.; Freitas, C.I.A.; Silva, A.R. Establishment of an anesthetic protocol for semen collection by electroejaculation in six-banded armadillos (Euphractus sexcinctus Linnaeus, 1758). Arq. Bras. Med. Vet. Zootec. 2016, 68, 1595–1601. [Google Scholar] [CrossRef]
- Campos, L.B.; Peixoto, G.C.X.; Lima, G.L.; Castelo, T.S.; Silva, A.R.; Freitas, C.I.A.; Silva, A.R. Monitoring the reproductive physiology of six-banded armadillos (Euphractus sexcinctus Linnaeus, 1758) through different techniques. Reprod. Domest. Anim. 2016, 51, 736–742. [Google Scholar] [CrossRef]
- Peppler, R.D.; Hossler, F.E.; Stone, S.C. Determination of reproductive maturity in the female nine-banded armadillo (Dasypus novemcinctus). Reproduction 1986, 76, 141–146. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, P.; Loughry, W.J.; Lockhart, J.M.; Inman, W.B.; Duthie, M.S.; Pena, M.T.; Marcos, L.A.; Scollard, D.M.; Cole, S.T.; et al. Zoonotic Leprosy in the Southeastern United States. Emerg. Infect. Dis. 2015, 21, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.R.F.; Lima, G.L.; Bezerra, L.G.P.; Barreto-Junior, R.A.; Oliveira, M.F.; Silva, A.R. Characterization of the ovarian preantral follicle populations and its correlation with age and nutritional status in Brazilian Northeastern donkeys (Equus assinus). Anim. Reprod. Sci. 2017, 187, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A.; Chainy, G.B. Morphometric studies of small follicles in ovaries of women at different ages. J. Reprod. Fertil. 1987, 81, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.L.; Luz, V.B.; Lima, L.F.; Rocha, R.M.P.; Castro, S.V.; Castelo, T.S.; Rodrigues, A.P.R.; Figueiredo, J.R.; Silva, A.R. Interactions between different media and follicle-stimulating hormone supplementation on in vitro culture of preantral follicles enclosed in ovarian tissue derived from collared peccaries (Pecari tajacu Linneaus, 1758). Reprod. Domest. Anim. 2018, 53, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.V.; van den Hurk, R.; Costa, S.H.F.; Andrade, E.R.; Nunes, A.P.A.; Ferreira, F.V.A.; Lôbo, R.N.B.; Figueiredo, J.R. Survival and growth of goat primordial follicles after in vitro culture of ovarian cortical slices in media containing coconut water. Anim. Reprod. Sci. 2004, 81, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Cetica, P.D.; Aldana Marcos, H.J.; Merani, M.S. Morphology of female genital tracts in Dasypodidae (Xenarthra, Mammalia): A comparative survey. Zoomorphology 2005, 124, 57–65. [Google Scholar] [CrossRef]
- Enders, A.C.; Buchanan, G.D. Some Effects of Ovariectomy and Injection of Ovarian Hormones in the Armadillo. J. Endocrinol. 1959, 19, 251–258. [Google Scholar] [CrossRef]
- Codón, S.M.; Casanave, E.B. Comparative morphology of the ovaries of three species of dasypodidae (Mammalia, Xenarthra). Rev. Chil. Anat. 2000, 18, 251–257. [Google Scholar] [CrossRef]
- Codón, S.M.; Casanave, E.B. Histology of the ovary of the armadillo Chaetophractus villosus (Mammalia, Dasypodidae). Rev. Brasil. Biol. 1996, 56, 599–604. [Google Scholar]
- Zeyl, J.; Love, O.; Higgs, D. Evaluating gonadosomatic index as an estimator of reproductive condition in the invasive round goby, Neogobius melanostomus. J. Great Lakes Res. 2014, 40, 164–171. [Google Scholar] [CrossRef]
- Codón, S.M.; Estecondo, S.G.; Galíndez, E.J.; Casanave, E.B. Ultrastructure and morphometry of ovarian follicles in the armadillo Chaetophractus villosus (Mammalia, Dasypodidae). Braz. J. Biol. 2001, 61, 485–496. [Google Scholar] [CrossRef]
- Rossi, L.F.; Solari, A.J. Large lamellar bodies and their role in the growing oocytes of the armadillo Chaetophractus villosus. J. Morphol. 2021, 282, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Superina, M.; Carreño, N.; Jahn, G.A. Characterization of seasonal reproduction patterns in female pichis Zaedyus pichiy (Xenarthra: Dasypodidae) estimated by fecal sex steroid metabolites and ovarian histology. Anim. Reprod. Sci. 2009, 116, 358–369. [Google Scholar] [CrossRef]
- Gaytán, F.; Morales, C.; Manfredi-Lozano, M.; Tena-Sempere, M. Generation of multi-oocyte follicles in the peripubertal rat ovary: Link to the invasive capacity of granulosa cells? Fertil. Steril. 2014, 101, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W. P, The occurrence of polyovular graafian follicles. J. Anat. 1924, 58, 328–334. [Google Scholar] [PubMed]
- Rossi, L.F.; Nottola, S.; Miglietta, S.; Macchiarelli, G.; Luaces, J.P.; Merico, V.; Merani, S.; Garagna, S.; Zuccotti, M. Germ cell cysts, a fetal feature in mammals, are constitutively present in the adult armadillo. Mol. Reprod. Dev. 2019, 87, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Spradling, A.C. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc. Natl. Acad. Sci. USA 2013, 110, 8585–8590. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.L.; Santos, E.A.A.; Luz, V.B.; Rodrigues, A.P.R.; Silva, A.R. Morphological Characterization of the Ovarian Preantral Follicle Population of Collared Peccaries (Tayassu tajacu Linnaeus, 1758). Anat. Histol. Embryol. 2012, 42, 304–311. [Google Scholar] [CrossRef]
- Silva-Santos, K.C.; Santos, G.M.G.; Siloto, L.S.; Hertel, M.F.; Andrade, E.R.; Rubin, M.I.B.; Sturion, L.; Melo-Sterza, F.A.; Seneda, M.M. Estimate of the population of preantral follicles in the ovaries of Bos taurus indicus and Bos taurus taurus cattle. Theriogenology 2011, 76, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Lucci, C.M.; Amorim, C.A.; Rodrigues, A.P.R.; Figueiredo, J.R.; Báo, S.N.; Silva, J.R.V.; Gonçalves, P.B.D. Study of preantral follicle population in situ and after mechanical isolation from caprine ovaries at different reproductive stages. Anim. Reprod. Sci. 1999, 56, 223–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes, G.P.; Santos, R.R.; Almeida, D.V.; Brito, A.B.; Queiroz, H.L.; Domingues, S.F.S. Population estimate and morphometry of ovarian preantral follicles from three recently recognized squirrel monkey species: A comparative study. Zygote 2017, 25, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.K.F.; Felipe, C.B.S.; Silva, G.M.L. Biologia reprodutiva de Bradypus variegatus Schinz (1825): Desafios e perspectivas/Reproduction biology in Bradypus variegatus Schinz (1825): Challenges and perspectives. Rev. Bras. Reprod. Anim. 2018, 42, 109–113. [Google Scholar]
- Hsu, S.Y.; Hsueh, A.J. Tissue-specific Bcl-2 protein partners in apoptosis, p. An ovarian paradigm. Physiol. Rev. 2000, 80, 593–614. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J.W. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed]
- Binford, C.H.; Storrs, E.E.; Walsh, G.P. Disseminated infection in the nine-banded armadillo (Dasypus novemcinctus) resulting from inoculation with M. leprae. Observations made on 15 animals studied at autopsy. Int. J. Lepr. Other Mycobact. Dis. 1976, 44, 80–83. [Google Scholar] [PubMed]
- Job, C.K.; Sanchez, R.M.; Hastings, R.C. Lepromatous placentitis and intrauterine fetal infection in lepromatous nine-banded armadillos (Dasypus novemcinctus). Lab. Investig. 1987, 56, 44–48. [Google Scholar] [PubMed]
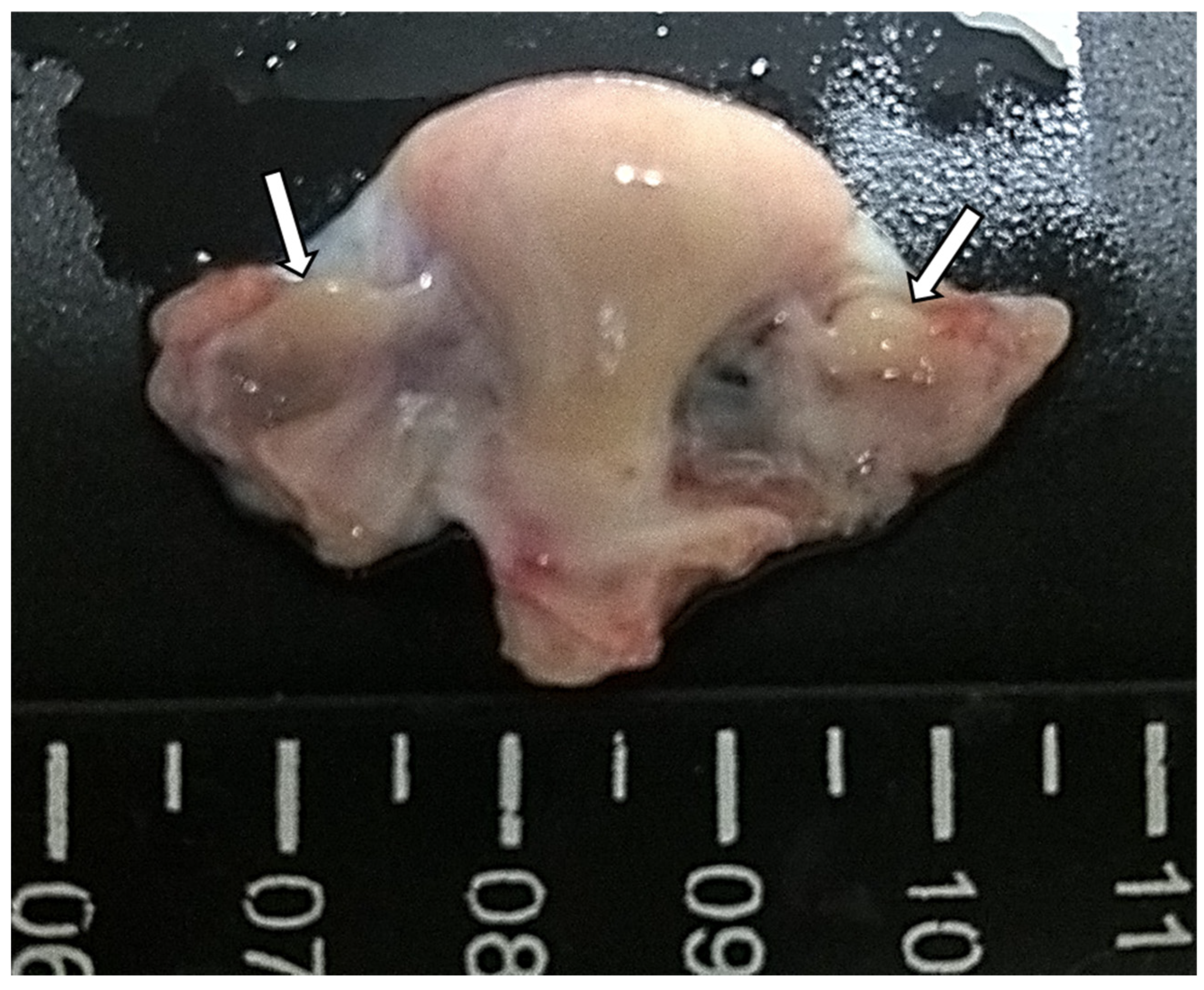
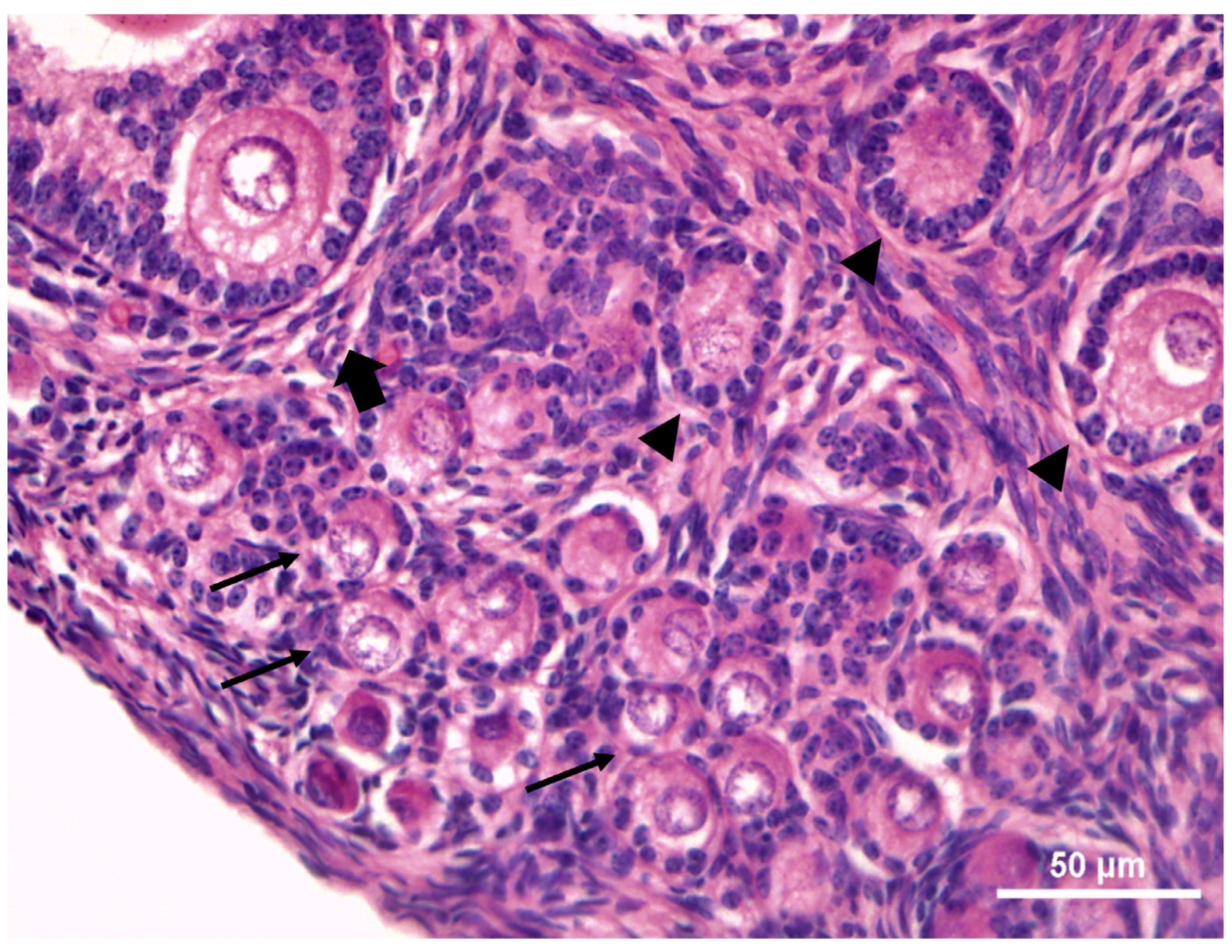
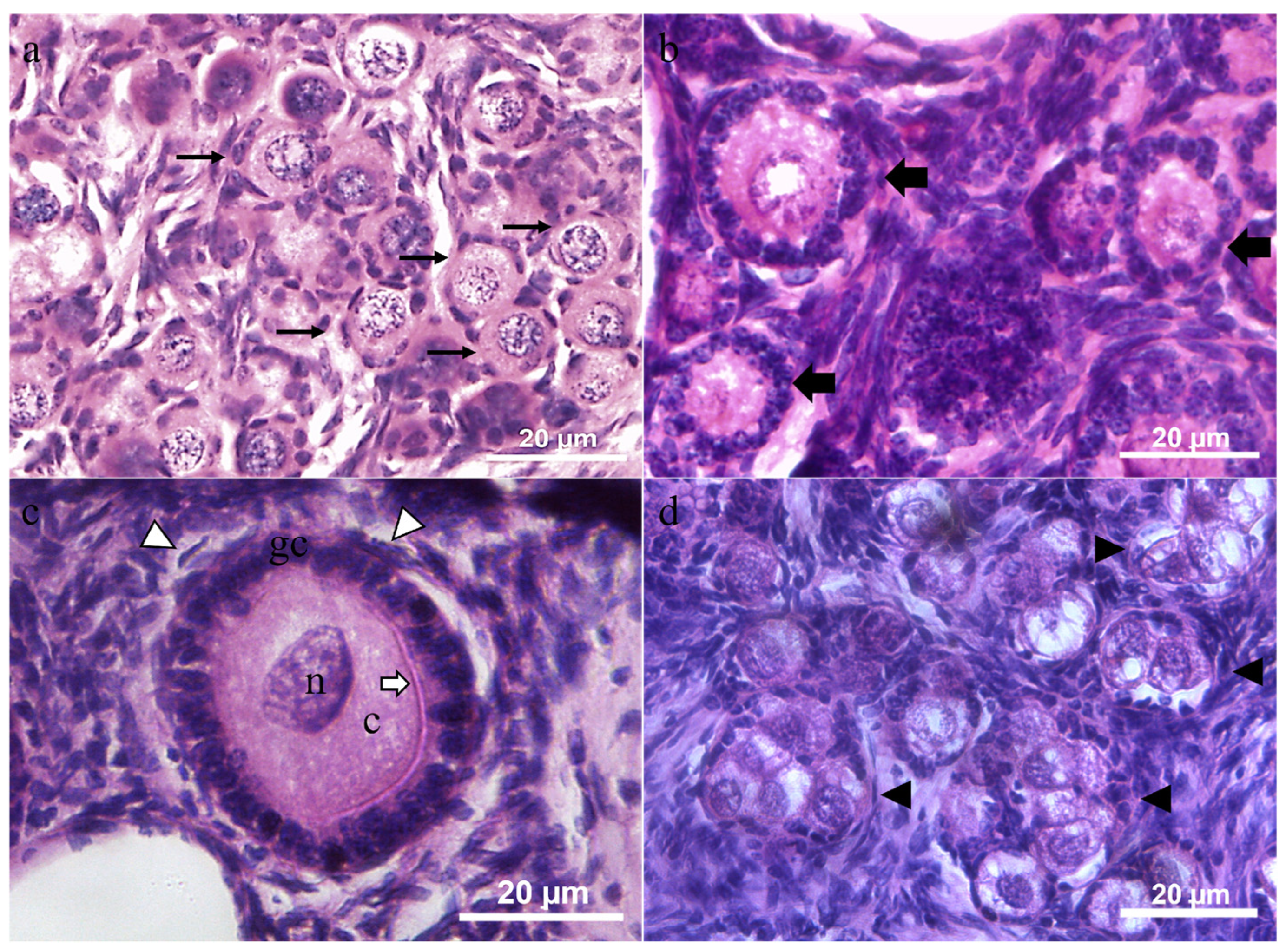
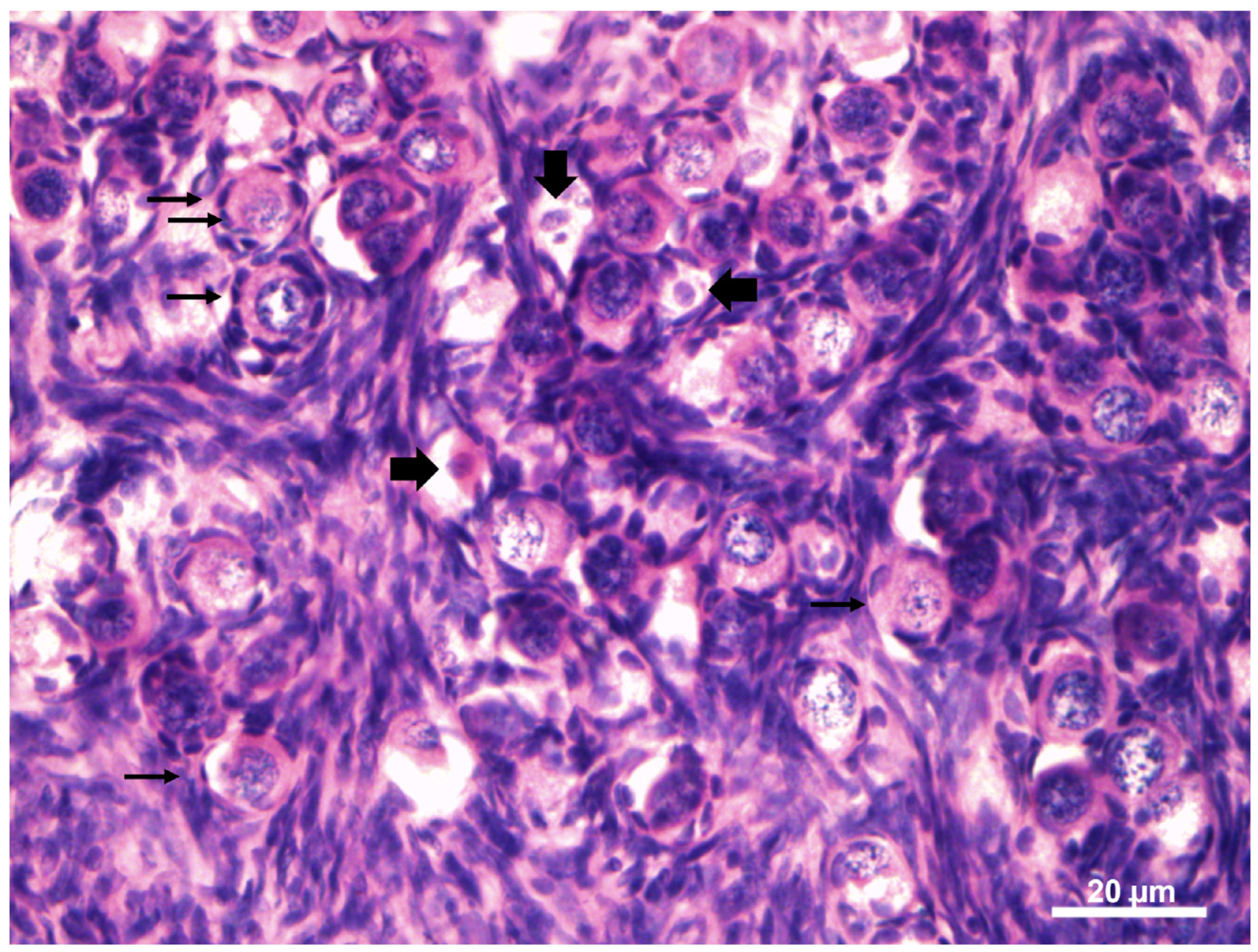
| Animals | Health Condition | Body Weight (Kg) | Right Ovary | Left Ovary | Gonadosomatic Index (%) | ||
|---|---|---|---|---|---|---|---|
| Weight (Kg) | Dimensions (mm) (Width × Length × Thickness) | Weight (Kg) | Dimensions (mm) (Width × Length × Thickness) | ||||
| A1 | Infected | 1.83 | 0.06 | 0.4 × 1 × 0.3 | 0.05 | 0.4 × 0.8 × 0.3 | 6.01 |
| A2 | Infected | 1.93 | 0.07 | 0.4 × 1 × 0.2 | 0.06 | 0.4 × 1 × 0.2 | 6.74 |
| A3 | Uninfected | 2.08 | 0.05 | 0.5 × 0.8 × 0.2 | 0.05 | 0.4 × 1 × 0.3 | 4.81 |
| A4 | Uninfected | 1.60 | 0.06 | 0.4 × 0.8 × 0.3 | 0.10 | 0.5 × 1 × 0.3 | 10.0 |
| A5 | Uninfected | 1.70 | 0.06 | 0.4 × 0.9 × 0.3 | 0.06 | 0.4 × 0.9 × 0.3 | 7.06 |
| Means ± SEM | 1.8 ± 0.1 | 0.1 ± 0.0 | 0.4 × 0.9 × 0.3 | 0.1 ± 0.0 | 0.4 × 0.9 × 0.3 | 6.9 ± 0.8 | |
| Animals | Health Condition | Right Ovary | Left Ovary | Total per Ovarian Pair |
|---|---|---|---|---|
| A1 | Infected | 7 | 6 | 13 |
| A2 | Infected | 99 | 51 | 150 |
| A3 | Non-infected | 28 | 12 | 40 |
| A4 | Non-infected | 2 | 2 | 4 |
| A5 | Non-infected | 70 | 22 | 92 |
| Means ± SEM | 37.2 ± 18.8 | 18.6 ± 8.8 | 59.8 ± 27.3 |
| Animals | Health Condition | Follicle Category | Nucleus | Oocyte | Follicle | Number of Follicles Evaluated |
|---|---|---|---|---|---|---|
| A1 | Infected | Primordial | 8.0 ± 0.3 | 13.4 ± 0.3 | 17.2 ± 0.3 | 30 |
| Primary | 9.0 ± 0.3 | 16.3 ± 0.5 | 23.4 ± 0.9 | 30 | ||
| Secondary | 12.9 ± 0.4 | 26.9 ± 1.3 | 42.9 ± 1.8 | 28 | ||
| A2 | Infected | Primordial | 7.2 ± 0.3 | 12.4 ± 0.2 | 16.1 ± 0.3 | 30 |
| Primary | 8.9 ± 0.2 | 16.3 ± 0.4 | 23.5 ± 0.4 | 30 | ||
| Secondary | 13.8 ± 1.2 | 32.5 ± 5.3 | 47.9 ± 7.3 | 10 | ||
| A3 | Non-infected | Primordial | 7.5 ± 0.1 | 10.5 ± 0.1 | 13.8 ± 0.2 | 30 |
| Primary | 7.1 ± 0.2 | 10.2 ± 0.2 | 14.3 ± 0.2 | 30 | ||
| Secondary | 8.1 ± 0.2 | 12.4 ± 0.8 | 18.7 ± 0.7 | 30 | ||
| A4 | Non-infected | Primordial | 6.9 ± 0.2 | 10.7 ± 0.2 | 14.4 ± 0.2 | 30 |
| Primary | 8.7 ± 0.2 | 17.5 ± 0.5 | 24.2 ± 0.7 | 30 | ||
| Secondary | 10.3 ± 1.3 | 24.0 ± 4.1 | 34.8 ± 7.9 | 10 | ||
| A5 | Non-infected | Primordial | 5.6 ± 0.1 | 8.5 ± 0.2 | 11.7 ± 0.2 | 30 |
| Primary | 7.1 ± 0.3 | 14.1 ± 0.7 | 21.8 ± 1.1 | 29 | ||
| Secondary | 9.3 ± 0.5 | 22.8 ± 1.4 | 38.0 ± 2.0 | 28 | ||
| Means ± SEM | Primordial | 7.0 ± 0.2 | 11.1 ± 0.2 | 14.6 ± 0.2 | 150 | |
| Primary | 8.2 ± 0.2 | 14.9 ± 0.5 | 21.4 ± 0.7 | 149 | ||
| Secondary | 10.9 ± 0.7 | 23.7 ± 2.6 | 36.5 ± 3.9 | 106 | ||
| Animals | Health Condition | Follicle Category | PF Population of Right Ovary | PF Population of Left Ovary | Total PF Population | Proportions (%) of PF Category |
|---|---|---|---|---|---|---|
| A1 | Infected | Primordial | 2929.2 | 2484.9 | 5414.1 | 35.4 |
| Primary | 5371.9 | 3697.1 | 9068.9 | 59.4 | ||
| Secondary | 315.3 | 476.9 | 792.18 | 5.2 | ||
| Total | 8616.4 | 9143.7 | 17,760.1 | 100.0 | ||
| A2 | Infected | Primordial | 7210.8 | 3708.7 | 10,919.4 | 64.4 |
| Primary | 3222.6 | 2214.8 | 5437.4 | 32.1 | ||
| Secondary | 287 | 302.9 | 589.8 | 3.5 | ||
| Total | 10,720.3 | 6226.3 | 16,946.7 | 100.0 | ||
| A3 | Uninfected | Primordial | 4559.18 | 7168.6 | 11,727.8 | 69.0 |
| Primary | 2517.8 | 2562.2 | 5082.0 | 29.9 | ||
| Secondary | 120.5 | 61.8 | 182.3 | 1.1 | ||
| Total | 7197.4 | 9792.7 | 16,990.1 | 100.0 | ||
| A4 | Uninfected | Primordial | 4598.9 | 2426.6 | 7025.6 | 66.5 |
| Primary | 1490.7 | 1908.0 | 3398.7 | 32.2 | ||
| Secondary | 57.8 | 89.7 | 147.4 | 1.3 | ||
| Total | 6147.4 | 4424.3 | 10,571.8 | 100.0 | ||
| A5 | Uninfected | Primordial | 20,368.9 | 7628.7 | 27,997.5 | 53.1 |
| Primary | 14,886.6 | 8812.7 | 23,699.3 | 44.9 | ||
| Secondary | 756.0 | 259.5 | 1015.6 | 2.0 | ||
| Total | 36,011.5 | 16,700.9 | 52,712.4 | 100.0 | ||
| Means ± SEM | 13,738.6 ± 5620.7 | 9257.6 ± 2100.6 | 22,996.2 ± 7541.6 | - | ||
| Total | Proportions (%) | ||||||
|---|---|---|---|---|---|---|---|
| Animals | Health Condition | Follicle Category | Normal | Degenerated | Total Follicle/ Category | Normal | Degenerated |
| A1 | Infected | Primordial | 1334.6 | 4079.5 | 5414.1 | 24.7 | 75.4 |
| Primary | 3120.8 | 5948.1 | 9069.0 | 34.4 | 65.6 | ||
| Secondary | 265.5 | 526.7 | 792.2 | 33.5 | 66.49 | ||
| A2 | Infected | Primordial | 4162.7 | 6756.7 | 10,919.4 | 38.1 | 61.9 |
| Primary | 2309.9 | 3127.5 | 5437.4 | 42.5 | 57.5 | ||
| Secondary | 166.0 | 423.8 | 589.8 | 28.2 | 71.9 | ||
| A3 | Uninfected | Primordial | 6190.8 | 5536.9 | 11,727.6 | 52.8 | 47.2 |
| Primary | 2748.9 | 2333.1 | 5082.0 | 54.1 | 45.9 | ||
| Secondary | 94.3 | 88 | 182.3 | 51.7 | 48.3 | ||
| A4 | Uninfected | Primordial | 3999.6 | 3026.0 | 7025.6 | 56.9 | 43.1 |
| Primary | 1789.3 | 1609.5 | 3398.7 | 52.7 | 47.4 | ||
| Secondary | 20.7 | 126.7 | 147.4 | 14.0 | 86.0 | ||
| A5 | Uninfected | Primordial | 11,482.6 | 16,514.9 | 27,997.3 | 41.0 | 59.0 |
| Primary | 11,293.9 | 12,405.4 | 23,699.3 | 47.7 | 52.3 | ||
| Secondary | 490.9 | 524.7 | 1015.6 | 48.3 | 51.7 | ||
| Means ± SEM | 3298.0 ± 965.7 | 4201.8 ± 1230.3 | 7499.9 ± 2169.6 | 41.4 ± 3.2 | 58.6 ± 3.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, G.L.; Brasil, A.V.; Silva, A.M.; Antunes, J.M.A.d.P.; Comizzoli, P.; Silva, A.R. Characterization of the Population of Ovarian Preantral Follicles in Juvenile Six-Banded Armadillos Infected or Not by Mycobacterium leprae. Vet. Sci. 2025, 12, 37. https://doi.org/10.3390/vetsci12010037
Lima GL, Brasil AV, Silva AM, Antunes JMAdP, Comizzoli P, Silva AR. Characterization of the Population of Ovarian Preantral Follicles in Juvenile Six-Banded Armadillos Infected or Not by Mycobacterium leprae. Veterinary Sciences. 2025; 12(1):37. https://doi.org/10.3390/vetsci12010037
Chicago/Turabian StyleLima, Gabriela L., Andreza V. Brasil, Andreia M. Silva, João Marcelo A. de P. Antunes, Pierre Comizzoli, and Alexandre R. Silva. 2025. "Characterization of the Population of Ovarian Preantral Follicles in Juvenile Six-Banded Armadillos Infected or Not by Mycobacterium leprae" Veterinary Sciences 12, no. 1: 37. https://doi.org/10.3390/vetsci12010037
APA StyleLima, G. L., Brasil, A. V., Silva, A. M., Antunes, J. M. A. d. P., Comizzoli, P., & Silva, A. R. (2025). Characterization of the Population of Ovarian Preantral Follicles in Juvenile Six-Banded Armadillos Infected or Not by Mycobacterium leprae. Veterinary Sciences, 12(1), 37. https://doi.org/10.3390/vetsci12010037







