Assessing the Relationship between proAKAP4 Level and Longevity of Sexed Sperm Quality after Thawing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sperm Samples
2.2. Sperm Motility and Kinetic Parameters
2.3. Flow Cytometry
2.3.1. Plasma Membrane and Acrosome Integrity (PMAI)
2.3.2. Mitochondrial Membrane Potential
2.4. ProAKAP4 Assay with ELISA
2.5. Statistical Analysis
3. Results
3.1. ProAKAP4 Levels of Sexed Semen Samples
3.2. Evaluation of Sperm Motility, PMAI, and HMMP after Thawing and Incubation for 3 h
3.3. Sperm Kinetic Parameters of the proAKAP4 Groups
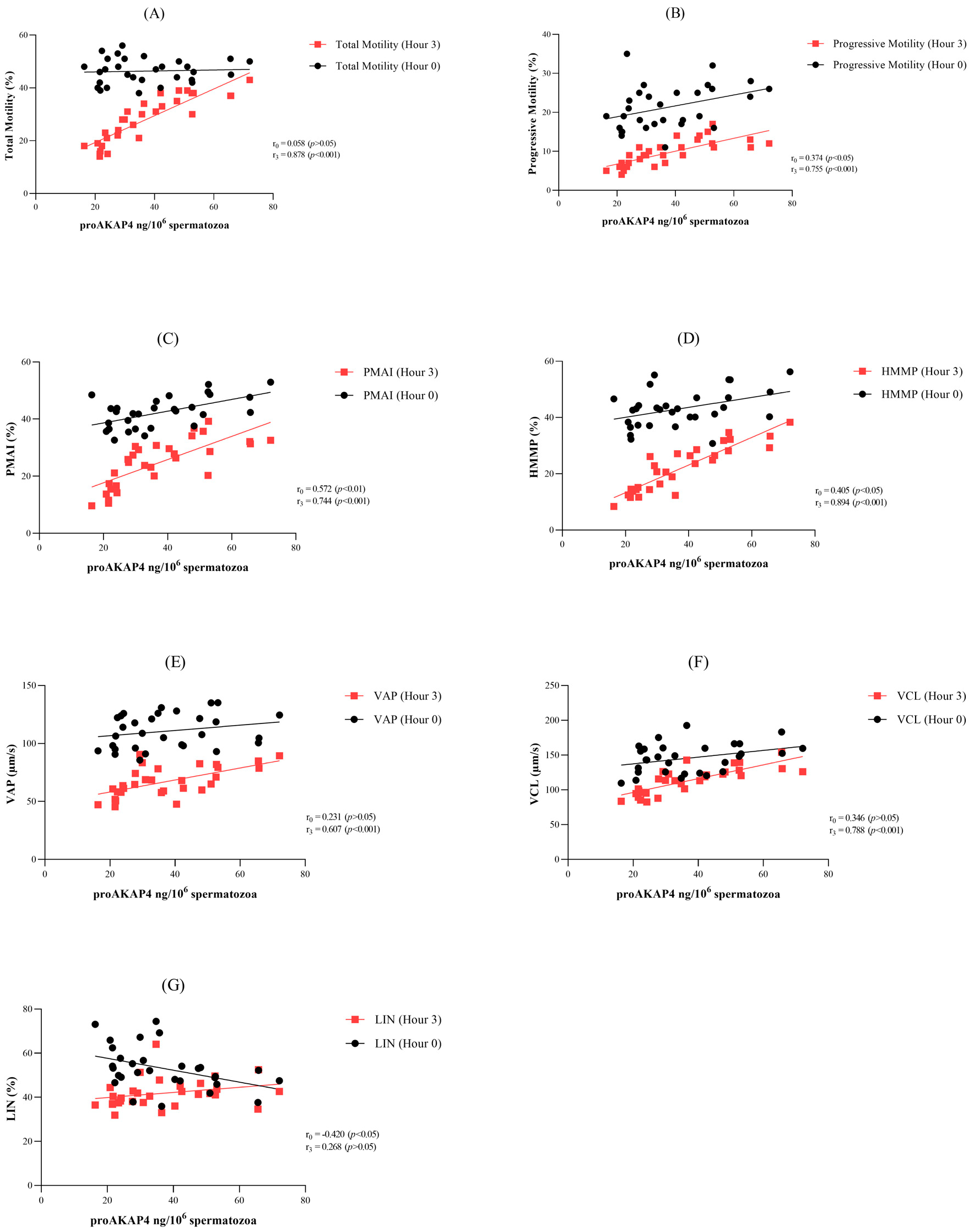
3.4. Correlation between proAKAP4 and Sperm Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, S.A.; Butler, S.T. Applications and benefits of sexed semen in dairy and beef herds. Animal 2018, 12, s97–s103. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Seidel, G.E., Jr. History of commercializing sexed semen for cattle. Theriogenology 2008, 69, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Moreno, J.F. Semen sexing–current state of the art with emphasis on bovine species. Animal 2018, 12, s85–s96. [Google Scholar] [CrossRef] [PubMed]
- Gogol, P.; Trzcińska, M. Relationship between quality parameters and fertilizing ability of cryopreserved sexed bull sperm. Ann. Anim. Sci. 2022, 22, 1257–1263. [Google Scholar] [CrossRef]
- Boneya, G. Sexed semen and major factors affecting its conception rate in dairy cattle. Int. J. Adv. Res. Biol. Sci 2021, 8, 99–107. [Google Scholar]
- Guo, Y.; Fan, Z.; Zhao, F.; Ge, S.; Chu, H.; Wei, Z.; Khan, R.; Faisal, M.; Ayari-Akkari, A.; Yassin, H.M.; et al. Assessment of semen quality and anti-oxidative enzyme activity between bovine sex-sorted and non-sex-sorted frozen–thawed semen. Reprod. Domest. Anim. 2023, 58, 657–661. [Google Scholar] [CrossRef]
- Steele, H.; Makri, D.; Maalouf, W.E.; Reese, S.; Kölle, S.J.S.R. Bovine sperm sexing alters sperm morphokinetics and subsequent early embryonic development. Sci. Rep. 2020, 10, 6255. [Google Scholar] [CrossRef]
- Brogliatti, G.; Barreiro, G.; Larraburu, G.; Laborde, A. 10 CASA evaluation of sexed and non-sexed frozen bull semen. Reprod. Fertil. Dev. 2003, 16, 127–128. [Google Scholar] [CrossRef]
- Seyoum, K. Advanced Semen Evaluation Techniques: Computer Assisted Sperm Analysis (CASA) and Flow Cytometery. GSJ 2020, 8, 2303–2335. [Google Scholar]
- Fallon, L.; Diaz-Miranda, E.; Hamilton, L.; Sutovsky, P.; Zigo, M.; Spencer, T.E.; Ortega, M.S. The development of new biomarkers of spermatozoa quality in cattle. Front. Vet. Sci. 2023, 10, 1258295. [Google Scholar] [CrossRef]
- Jodar, M.; Soler-Ventura, A.; Oliva, R. Semen proteomics and male infertility. J. Proteom. 2017, 162, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Peddinti, D.; Nanduri, B.; Kaya, A.; Feugang, J.M.; Burgess, S.C.; Memili, E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst. Biol. 2008, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, O.; Frenette, G.; Bourassa, S.; Calvo, E.; Blondin, P.; Sullivan, R. Proteomic markers of functional sperm population in bovines: Comparison of low-and high-density spermatozoa following cryopreservation. J. Proteome Res. 2018, 17, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Zhang, Y. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm cryo-tolerance in farm animals. Front. Vet. Sci. 2021, 8, 609180. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Arangasamy, A.; Kastelic, J.P. Sperm and seminal plasma proteomics of high-versus low-fertility Holstein bulls. Theriogenology 2019, 126, 41–48. [Google Scholar] [CrossRef]
- Kaya, A.; Memili, E. Sperm macromolecules associated with bull fertility. Anim. Reprod. Sci. 2016, 169, 88–94. [Google Scholar] [CrossRef]
- Souto, P.L.; Carmouy, L.S.T.; Santos, C.; Martins, E.; Martins, V.; Hatamoto-Zervoudakis, L.K.; Ramos, A.F. Seasonal differences in seminal plasma proteins from two bovine breeds adapted to a subtropical climate. Trop. Anim. Health Prod. 2021, 53, 61. [Google Scholar] [CrossRef]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Zhang, G.; Li, D.; Tu, C.; Meng, L.; Tan, Y.; Ji, Z.; Cheng, J.; Lu, G.; Lin, G.; Zhang, H.; et al. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum. Mol. Genet. 2022, 31, 219–231. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Serteyn, D. Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine semen: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef]
- Carracedo, S.; Briand-Amirat, L.; Dordas-Perpinyà, M.; Escuredo, Y.R.; Delcombel, R.; Sergeant, N.; Delehedde, M. ProAKAP4 protein marker: Towards a functional approach to male fertility. Anim. Reprod. Sci. 2022, 247, 107074. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Sergeant, N.; Yánez-Ortiz, I.; Mevel, V.; Catalán, J.; Bruyas, J.F.; Briand-Amirat, L.; Miró, J. ProAKAP4 as a motility long-lasting marker in Catalan donkey spermatozoa. Anim. Reprod. Sci. 2024, 262, 107427. [Google Scholar] [CrossRef] [PubMed]
- Rahamim Ben-Navi, L.; Almog, T.; Yao, Z.; Seger, R.; Naor, Z. A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 2016, 6, 37922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, X.H.; Wu, J.; Wang, N.; Li, G.; Hao, G.M.; Cao, J.F. Decreased AKAP4/PKA signaling pathway in high DFI sperm affects sperm capacitation. Asian J. Androl. 2024, 26, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Jumeau, F.; Sigala, J.; Dossou-Gbete, F.; Frimat, K.; Barbotin, A.L.; Buee, L.; Mitchell, V. A-kinase anchor protein 4 precursor (pro-AKAP4) in human spermatozoa. Andrology 2018, 6, 854–859. [Google Scholar] [CrossRef]
- Almeida, A.B.M.; Hidalgo, M.M.T.; de Moraes, F.L.Z.; Trautwein, L.G.C.; de Fátima Schnitzer, J.; dos Santos Silva, L.A.; Rizzoto, G.; Ferreira, J.C.P.; Martins, M.I.M. The proAKAP4 concentrations in Nelore bull sperm and their relation to FTAI conception rate results. Anim. Reprod. Sci. 2022, 247, 107156. [Google Scholar] [CrossRef]
- Le Couazer, D.; Bencharif, D. Are AKAP4 and proAKAP4 Found in Canine Semen. Preliminary Results. Asp. J. Nanotechnol. 2021, 3, 70–75. [Google Scholar]
- Malo, C.; Carracedo, S.; Delehedde, M.; Sergeant, N.; Skidmore, J.A. Identification of proAKAP4 concentration variations in dromedary sperm and their correlation with monthly semen parameters. Reprod. Fertil. 2021, 2, 268–279. [Google Scholar] [CrossRef]
- Riesco, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; Anel, L. ProAKAP4 as novel molecular marker of sperm quality in ram: An integrative study in fresh, cooled and cryopreserved sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef]
- Bastan, I.; Akcay, E. Quality assessment of frozen bull semen with the precursor A-kinase anchor protein 4 biomarker. Andrologia 2021, 53, e14164. [Google Scholar] [CrossRef]
- Ruelle, I.; Charreaux, F.; Bencharif, D.; Thorin, C.; Michaud, S.; Schmitt, E.; Delehedde, M. Assessment of the sperm specific protein proAKAP4 as a marker to evaluate sperm quality and fertility in Holstein bulls. Rev. Bras. Reprod. Anim. 2019, 43, 472. [Google Scholar]
- Brown, P.R.; Miki, K.; Harper, D.B.; Eddy, E.M. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol. Reprod. 2003, 68, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Janta, A.; Ciereszko, A. Proteomic comparison of non-sexed and sexed (X-bearing) cryopreserved bull semen. Anim. Reprod. Sci. 2020, 221, 106552. [Google Scholar] [CrossRef] [PubMed]
- Şahin, D.; Baştan, İ.; Çil, B.; Tekin, K.; Akçay, E.; Daşkın, A.; Stelletta, C. The number of false mounting affects the quality of semen in bulls. Lalahan Hay. Araşt. Enst. Derg. 2020, 60, 9–14. [Google Scholar] [CrossRef]
- Korkmaz, F.; Baştan, İ.; Şahin, D.; Şimşek, S.; Kaya, U.; Satılmış, M. Reaction time as a libido indicator and its relation to pre-freeze and post-thaw sperm quality in bulls. Reprod. Domest. Anim. 2023, 58, 965–971. [Google Scholar] [CrossRef]
- Korkmaz, F.; Malama, E.; Siuda, M.; Leiding, C.; Bollwein, H. Effects of sodium pyruvate on viability, synthesis of reactive oxygen species, lipid peroxidation and DNA integrity of cryopreserved bovine sperm. Anim. Reprod. Sci. 2017, 185, 18–27. [Google Scholar] [CrossRef]
- Kanno, C.; Sakamoto, K.Q.; Kang, S.S.; Yanagawa, Y.; Katagiri, S.; Nagano, M. The Difference in Subpopulation Structures in Sex-Sorted and Non-Sorted Semen by Discriminant Analysis of Bull Sperm Motility Data Collected by a Computer-Assisted Sperm Analysis System. Andrologia 2023, 2023, 9944344. [Google Scholar] [CrossRef]
- Szczykutowicz, J.; Kałuża, A.; Kaźmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The potential role of seminal plasma in the fertilization outcomes. Biomed. Res. Int. 2019, 2019, 5397804. [Google Scholar] [CrossRef]
- Moce, E.; Graham, J.K.; Schenk, J.L. Effect of sex-sorting on the ability of fresh and cryopreserved bull sperm to undergo an acrosome reaction. Theriogenology 2006, 66, 929–936. [Google Scholar] [CrossRef]
- Carvalho, J.O.; Sartori, R.; Dode, M.A.N. Different ways to evaluate bovine sexed sperm in vitro. Anim. Reprod. 2018, 11, 199–206. [Google Scholar]
- Guner, B.; Erturk, M.; Yilmazbas-Mecitoglu, G.; Keskin, A.; Karakaya-Bilen, E.; Cakircali, R.; Gumen, A. Effect of delaying the time of insemination with sex-sorted semen on pregnancy rate in Holstein heifers. Reproduction in Domestic. Animal 2020, 55, 1411–1417. [Google Scholar]
- Tippenhauer, C.M.; Plenio, J.L.; Madureira, A.; Heuwieser, W.; Borchardt, S. Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers. Animal 2023, 13, 2994. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.; Dervisevic, E.; Devendran, C.; Cadarso, V.J.; O’Bryan, M.K.; Nosrati, R.; Neild, A. High-Frequency Ultrasound Boosts Bull and Human Sperm Motility. Adv. Sci. 2022, 9, 2104362. [Google Scholar] [CrossRef] [PubMed]
- Zilli, L.; Schiavone, R.; Storelli, C.; Vilella, S. Molecular mechanisms determining sperm motility initiation in two sparids (Sparus aurata and Lithognathus mormyrus). Biol. Reprod. 2008, 79, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Frintrop, L.; Wiesehöfer, C.; Stoskus, A.; Hilken, G.; Dubicanac, M.; von Ostau, N.E.; Wennemuth, G. cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement. Int. J. Mol. Sci 2022, 23, 10607. [Google Scholar] [CrossRef]
- Bajpai, M.; Fiedler, S.E.; Huang, Z.; Vijayaraghavan, S.; Olson, G.E.; Livera, G.; Carr, D.W. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol. Reprod. 2006, 74, 109–118. [Google Scholar] [CrossRef]
- Peter, A.T.; Brito, L.; Althouse, G.; Aurich, C.; Chenoweth, P.; Fraser, N.; Waberski, D. Andrology laboratory review: Evaluation of sperm motility. Clin. Theriogenol. 2021, 13, 24–36. [Google Scholar] [CrossRef]
- Mortimer, S.T. CASA—Practical aspects. J. Androl. 2000, 21, 515–524. [Google Scholar] [CrossRef]
- Kathiravan, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective sperm motion analysis to assess dairy bull fertility using computer-aided system–a review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef]
- Yániz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in mammals: An update. Reprod. Fertil. Dev. 2018, 30, 799–809. [Google Scholar] [CrossRef]
- Dordas-Perpinyà, M.; Sergeant, N.; Ruelle, I.; Bruyas, J.F.; Charreaux, F.; Michaud, S.; Carracedo, S.; Catalán, J.; Miró, J.; Delehedde, M.; et al. ProAKAP4 semen concentrations as a valuable marker protein of post-thawed semen quality and bull fertility: A retrospective study. Vet. Sci. 2022, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Yánez-Ortiz, I.; Sergeant, N.; Mevel, V.; Catalán, J.; Bruyas, J.F.; Miró, J.; Briand-Amirat, L. ProAKAP4 as Indicator of Long-Lasting Motility Marker in Post-Thaw Conditions in Stallions. Animals 2024, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.R.; Guerreiro, D.D.; Moura, A.A.; Memili, E. Identification of biomarkers for bull fertility using functional genomics. Anim. Reprod. 2022, 19, e20220004. [Google Scholar] [CrossRef] [PubMed]
| Parameters | proAKAP4 Groups | Time | Main Effect of Group | p | |||
|---|---|---|---|---|---|---|---|
| Hour 0 | Hour 3 | Group Effect | Time Effect | Group × Time Interaction | |||
| Total motility (%) | HC | 46.17 ± 1.00 a | 36.50 ± 1.06 b, A | 41.33 ± 1.23 | <0.001 | <0.001 | <0.001 |
| MC | 47.78 ± 1.91 a | 27.11 ± 1.43 b, B | 37.44 ± 2.76 | ||||
| LC | 45.22 ± 1.77 a | 17.67 ± 1.00 b, C | 31.44 ± 3.48 | ||||
| Overall mean | 46.37 ± 0.87 | 28.03 ± 1.59 | |||||
| Progressive motility (%) | HC | 23.58 ± 1.43 | 12.67 ± 0.62 | 18.13 ± 1.37 A | 0.003 | <0.001 | 0.401 |
| MC | 19.78 ± 1.70 | 8.89 ± 0.56 | 14.33 ± 1.58 B | ||||
| LC | 19.67 ± 2.17 | 6.11 ± 0.48 | 12.89 ± 1.97 B | ||||
| Overall mean | 21.27 ± 1.03 a | 9.57 ± 0.60 b | |||||
| PMAI (%) | HC | 45.90 ± 1.34 a, A | 31.20 ± 1.48 b, A | 38.55 ± 1.82 | <0.001 | <0.001 | <0.001 |
| MC | 39.59 ± 1.38 a, B | 26.16 ± 1.20 b, B | 32.88 ± 1.85 | ||||
| LC | 39.83 ± 1.69 a, B | 14.47 ± 1.22 b, C | 27.15 ± 3.24 | ||||
| Overall mean | 42.18 ± 0.99 | 24.67 ± 1.51 | |||||
| HMMP (%) | HC | 45.21 ± 2.10 a, A | 29.83 ± 1.26 b, A | 37.52 ± 2.00 | <0.001 | <0.001 | <0.001 |
| MC | 44.03 ± 2.01 a, AB | 19.95 ± 1.68 b, B | 31.99 ± 3.18 | ||||
| LC | 39.43 ± 1.65 a, B | 12.70 ± 0.69 b, C | 26.07 ± 3.36 | ||||
| Overall mean | 43.12 ± 1.20 | 21.73 ± 1.51 | |||||
| proAKAP4 Groups | Time | Main Effect of Group | p | ||||
|---|---|---|---|---|---|---|---|
| Hour 0 | Hour 3 | Group Effect | Time Effect | Group × Time Interaction | |||
| VAP (µm/s) | HC | 113.83 ± 4.35 | 72.51 ± 3.57 | 93.17 ± 5.11 A | 0.018 | <0.001 | 0.200 |
| MC | 109.18 ± 5.33 | 71.69 ± 3.66 | 90.43 ± 5.52 AB | ||||
| LC | 107.80 ± 4.69 | 55.22 ± 2.21 | 81.51 ± 6.85 B | ||||
| Overall mean | 110.63 ± 2.70 a | 67.08 ± 2.35 b | |||||
| VCL (µm/s) | HC | 149.87 ± 5.55 a | 128.29 ± 3.22 b, A | 139.08 ± 3.86 | 0.004 | <0.001 | 0.010 |
| MC | 147.66 ± 8.40 a | 114.78 ± 5.15 b, A | 131.22 ± 6.22 | ||||
| LC | 138.27 ± 6.46 a | 91.94 ± 2.28 b, B | 115.11 ± 6.53 | ||||
| Overall mean | 145.72 ± 3.85 | 113.33 ± 3.47 | |||||
| VSL (µm/s) | HC | 71.82 ± 1.83 a | 59.52 ± 2.61 b | 65.67 ± 2.02 | 0.825 | <0.001 | 0.008 |
| MC | 79.17 ± 2.36 a | 55.42 ± 3.11 b | 67.29 ± 3.45 | ||||
| LC | 77.42 ± 1.85 a | 57.46 ± 3.85 b | 67.44 ± 3.19 | ||||
| Overall mean | 75.70 ± 1.27 | 57.67 ± 1.78 | |||||
| ALH (µm/s) | HC | 8.33 ± 0.24 | 6.94 ± 0.19 | 7.63 ± 0.21 | 0.838 | <0.001 | 0.394 |
| MC | 8.23 ± 0.32 | 6.81 ± 0.18 | 7.52 ± 0.25 | ||||
| LC | 8.12 ± 0.22 | 7.22 ± 0.19 | 7.67 ± 0.18 | ||||
| Overall mean | 8.24 ± 0.15 a | 6.99 ± 0.11 b | |||||
| STR (%) | HC | 64.38 ± 3.55 a | 55.57 ± 3.79 b | 59.97 ± 2.70 | 0.206 | <0.001 | 0.013 |
| MC | 73.57 ± 3.66 a | 62.24 ± 1.53 b | 67.91 ± 2.37 | ||||
| LC | 72.93 ± 3.70 a | 53.09 ± 2.39 b | 63.01 ± 3.22 | ||||
| Overall mean | 69.70 ± 2.19 | 56.83 ± 1.82 | |||||
| LIN (%) | HC | 48.30 ± 1.41 a, B | 43.08 ± 1.45 b | 45.69 ± 1.13 | 0.420 | <0.001 | 0.001 |
| MC | 55.53 ± 4.42 a, AB | 44.11 ± 3.08 b | 49.82 ± 2.96 | ||||
| LC | 56.89 ± 2.91 a, A | 38.04 ± 1.13 b | 47.47 ± 2.74 | ||||
| Overall mean | 53.05 ± 1.78 | 41.88 ± 1.20 | |||||
| BCF (Hz) | HC | 33.06 ± 0.66 | 28.44 ± 0.53 | 30.75 ± 0.63 A | <0.001 | <0.001 | 0.161 |
| MC | 28.50 ± 0.65 | 26.33 ± 0.48 | 27.42 ± 0.47 B | ||||
| LC | 32.27 ± 1.21 | 27.53 ± 0.71 | 29.90 ± 0.89 A | ||||
| Overall mean | 31.45 ± 0.59 a | 27.54 ± 0.36 b | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastan, İ.; Korkmaz, F.; Şahin, D.; Şimşek, S.; Kaya, U. Assessing the Relationship between proAKAP4 Level and Longevity of Sexed Sperm Quality after Thawing. Vet. Sci. 2024, 11, 444. https://doi.org/10.3390/vetsci11090444
Bastan İ, Korkmaz F, Şahin D, Şimşek S, Kaya U. Assessing the Relationship between proAKAP4 Level and Longevity of Sexed Sperm Quality after Thawing. Veterinary Sciences. 2024; 11(9):444. https://doi.org/10.3390/vetsci11090444
Chicago/Turabian StyleBastan, İlktan, Fırat Korkmaz, Derya Şahin, Seher Şimşek, and Ufuk Kaya. 2024. "Assessing the Relationship between proAKAP4 Level and Longevity of Sexed Sperm Quality after Thawing" Veterinary Sciences 11, no. 9: 444. https://doi.org/10.3390/vetsci11090444
APA StyleBastan, İ., Korkmaz, F., Şahin, D., Şimşek, S., & Kaya, U. (2024). Assessing the Relationship between proAKAP4 Level and Longevity of Sexed Sperm Quality after Thawing. Veterinary Sciences, 11(9), 444. https://doi.org/10.3390/vetsci11090444





