Quercetin Protects Goat Sperm Motility by Inhibiting Neutrophil Extracellular Traps and Maintaining Plasma Membrane and Acrosome Integrity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Isolation of Goat PMNs
2.3. Thawing Frozen Goat Sperm
2.4. Cell Counting Kit-8 Assay
2.5. Goat Sperm Viability Measurement
2.6. Quantification of NETs
2.7. Immunofluorescence Analysis
2.8. ROS Production Assay
2.9. Analysis of Catalase (CAT), Glutathione Peroxidase (GSH-PX), and MDA
2.10. Goat Sperm Acrosome Integrity Test
2.11. Determination of Goat Sperm Plasma Membrane Integrity
2.12. Statistical Analysis
3. Results
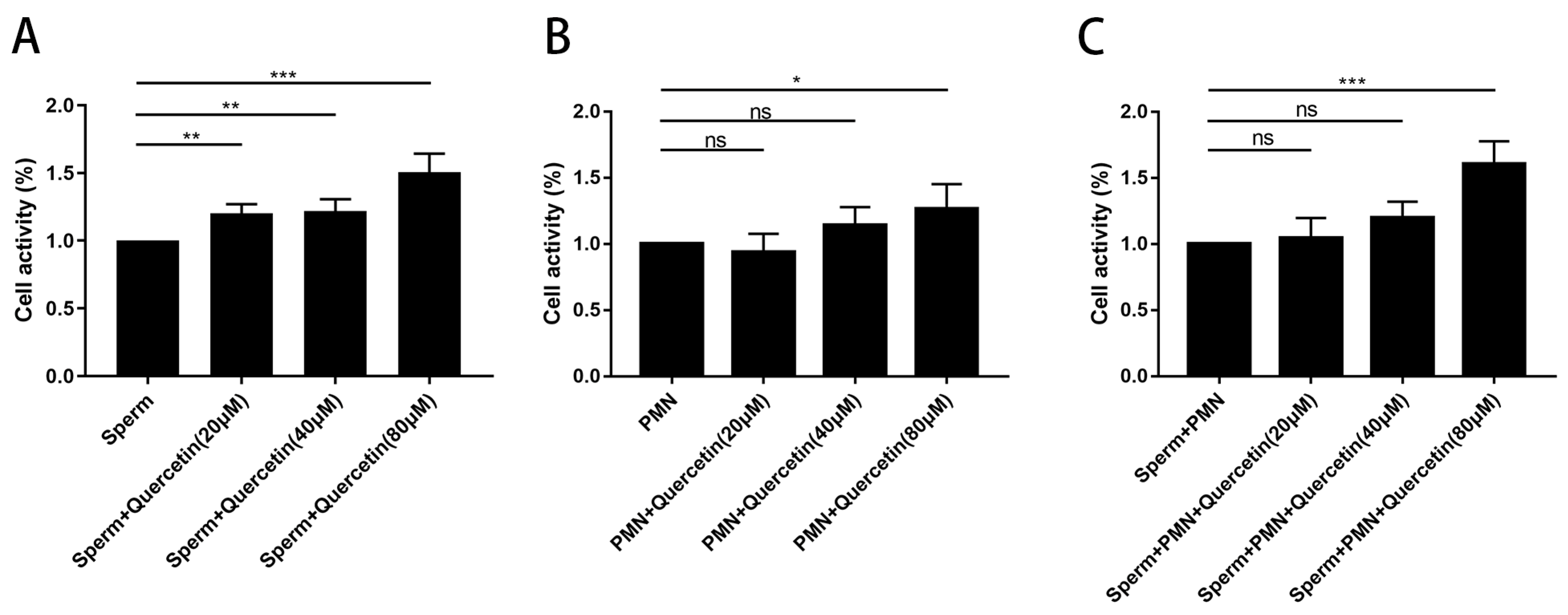
3.1. Quercetin Exhibited No Cytotoxic Effects on Goat Sperm and PMN
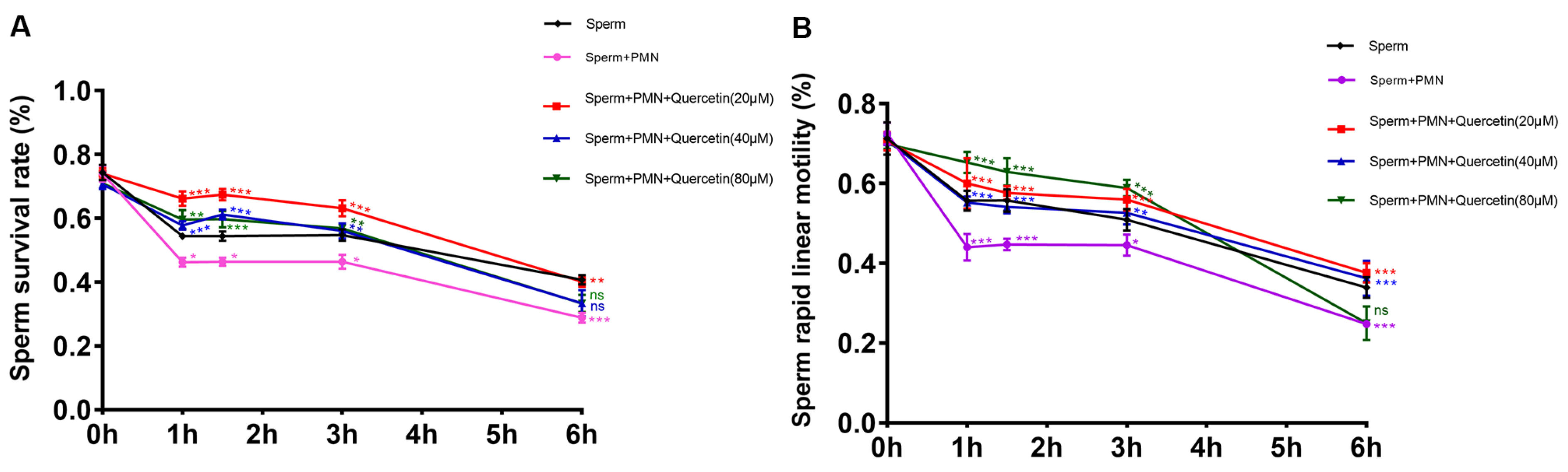
3.2. Quercetin Enhanced Goat Sperm Survival and Motility
3.3. Goat-Sperm-Induced NETs Composed of DNA, citH3, and NE
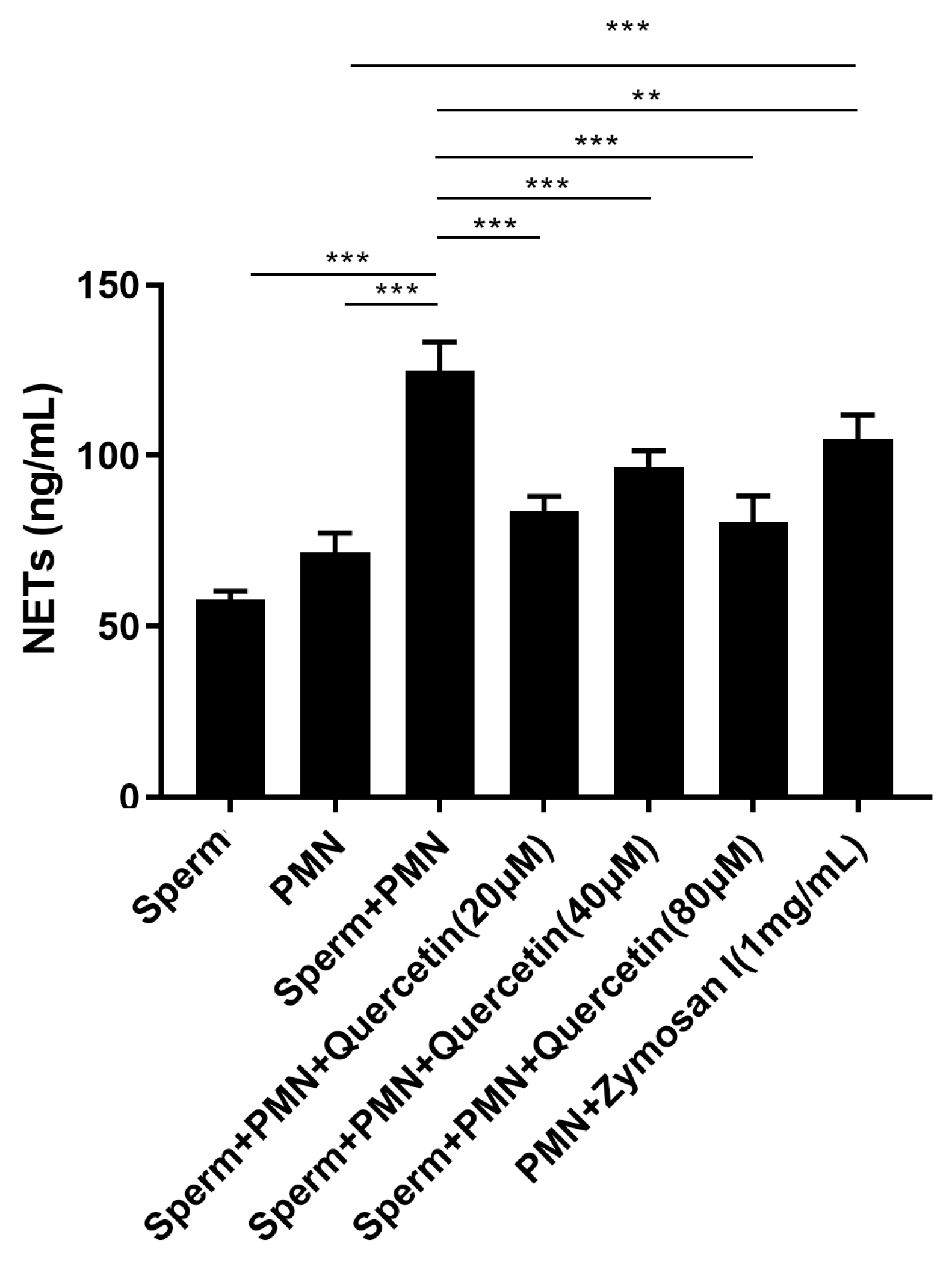
3.4. Quercetin Reduced the NETs Triggered by Goat Sperm
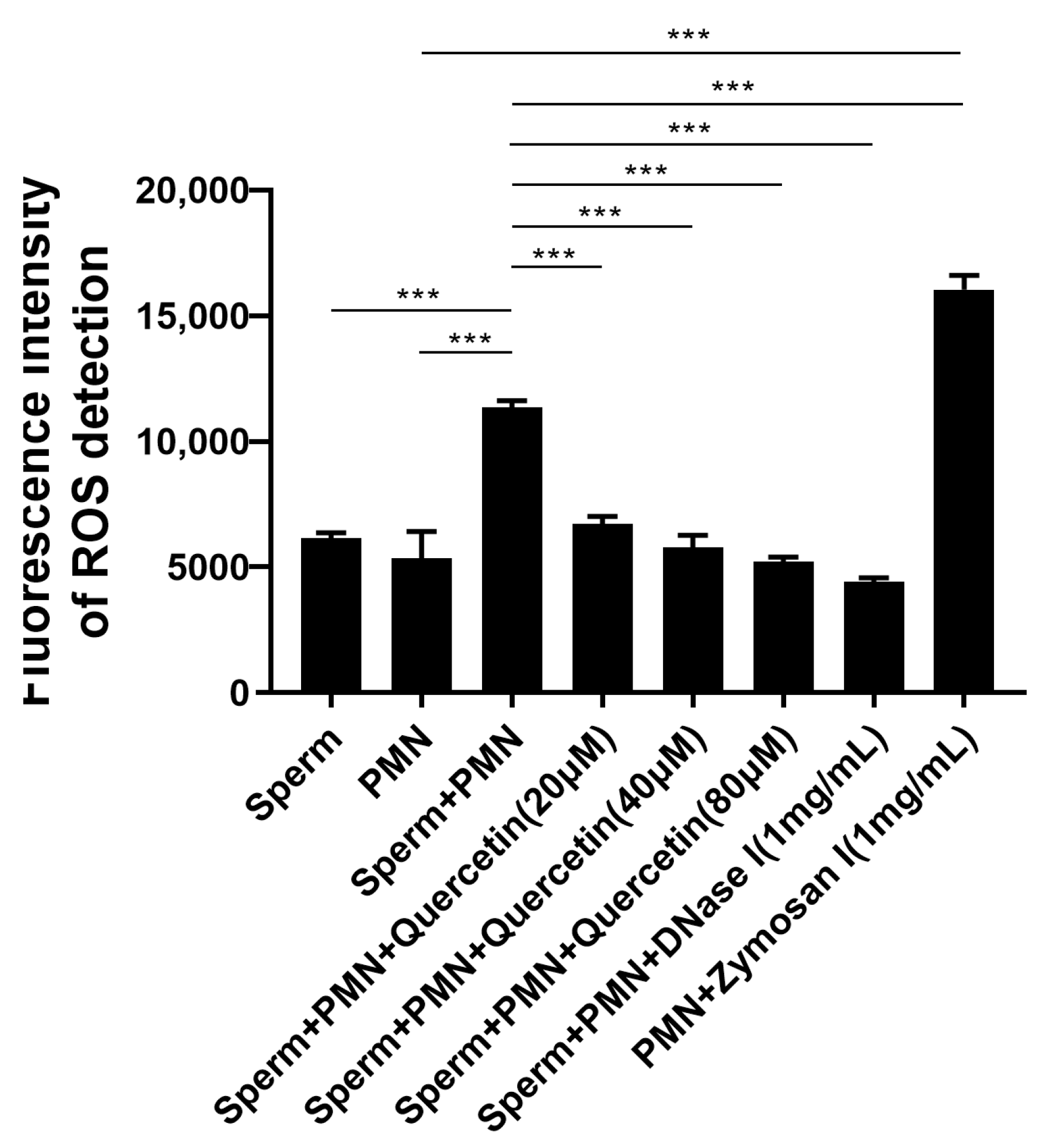
3.5. Quercetin Effectively Inhibited NETs by Attenuating ROS Production
3.6. Quercetin Protected Goat Sperm by Counteracting Oxidative Stress and Inhibiting Lipid Peroxidation
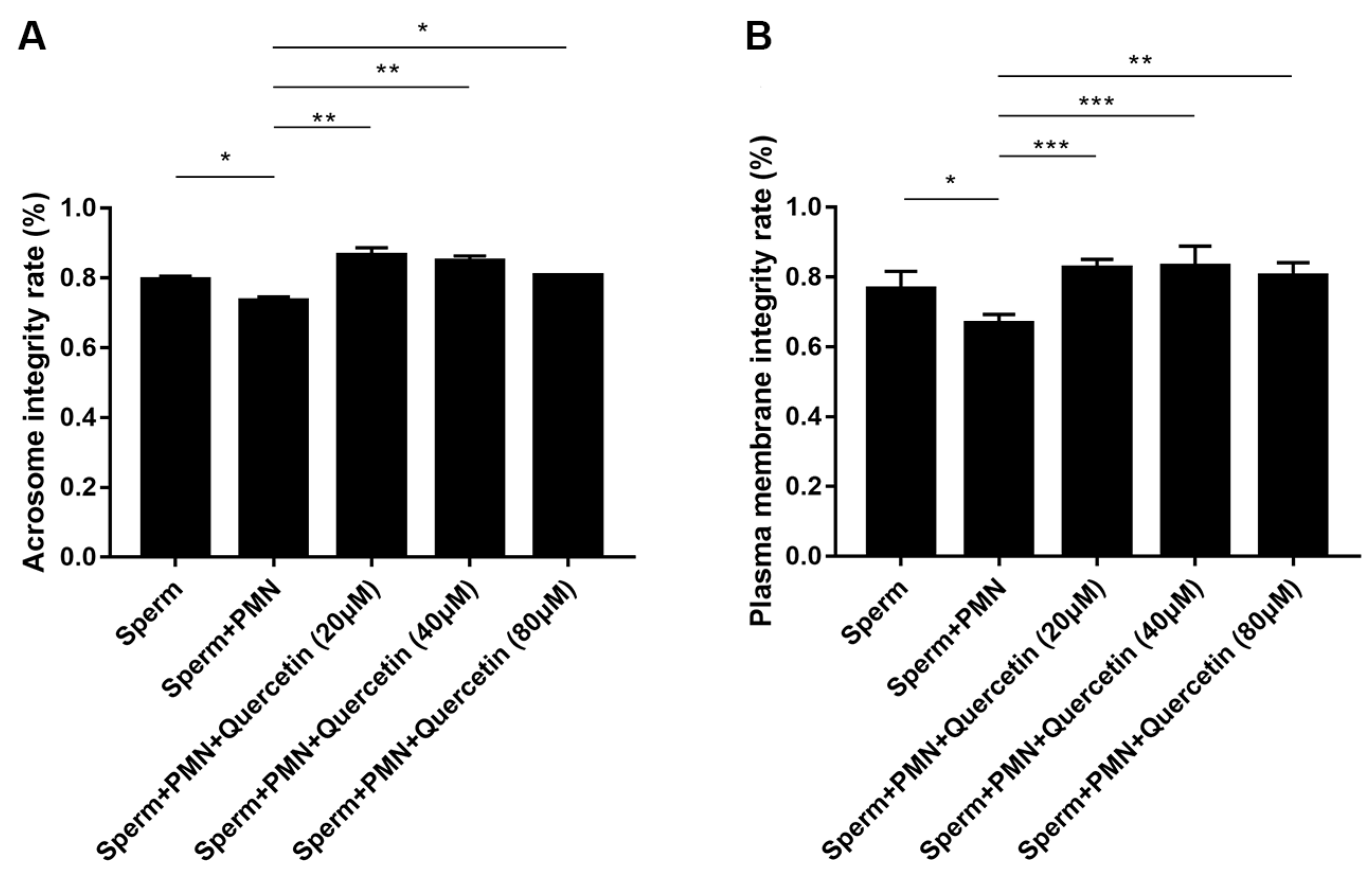
3.7. Quercetin Preserved Goat Sperm by Preserving Its Structural Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, J.J.; McKelvey, W.A.; King, M.E.; Mitchell, S.E.; Mylne, M.J.; McEvoy, T.G.; Dingwall, W.S.; Williams, L.M. Traversing the ovine cervix—A challenge for cryopreserved semen and creative science. Animal 2011, 5, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Naqvi, S.M. Effect of time and depth of insemination on fertility of Bharat Merino sheep inseminated trans-cervical with frozen-thawed semen. J. Anim. Sci. Technol. 2014, 56, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, M.E.; McKelvey, W.A.; Dingwall, W.S.; Matthews, K.P.; Gebbie, F.E.; Mylne, M.J.; Stewart, E.; Robinson, J.J. Lambing rates and litter sizes following intrauterine or cervical insemination of frozen/thawed semen with or without oxytocin administration. Theriogenology 2004, 62, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Alcay, S.; Berk Toker, M.; Gokce, E.; Ustuner, B.; Tekin Onder, N.; Sagirkaya, H.; Nur, Z.; Kemal Soylu, M. Successful ram semen cryopreservation with lyophilized egg yolk-based extender. Cryobiology 2015, 71, 329–333. [Google Scholar] [CrossRef]
- Najafi, A.; Najafi, M.H.; Zanganeh, Z.; Sharafi, M.; Martinez-Pastor, F.; Adeldust, H. Cryopreservation of ram semen in extenders containing soybean lecithin as cryoprotectant and hyaluronic acid as antioxidant. Reprod. Domest. Anim. 2014, 49, 934–940. [Google Scholar] [CrossRef]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef]
- Burkitt, M.; Walker, D.; Romano, D.M.; Fazeli, A. Using computational modeling to investigate sperm navigation and behavior in the female reproductive tract. Theriogenology 2012, 77, 703–716. [Google Scholar] [CrossRef]
- Hong, H.; Liu, Z.; Li, S.; Wu, D.; Jiang, L.; Li, P.; Wu, Z.; Xu, J.; Jiang, A.; Zhang, Y.; et al. Zinc oxide nanoparticles (ZnO-NPs) exhibit immune toxicity to crucian carp (Carassius carassius) by neutrophil extracellular traps (NETs) release and oxidative stress. Fish. Shellfish. Immunol. 2022, 129, 22–29. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, H.; Liu, W.; Jiang, L.; Xu, J.; Gao, X.; Qian, Y.; Jiang, Y.; Jin, Z.; Jin, Q.; et al. DNase I rescues goat sperm entrapped by neutrophil extracellular traps. Dev. Comp. Immunol. 2024, 153, 105107. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, T.; Wang, J.; Wang, C.; Liu, X.; Han, Z.; Zhang, X.; Zhang, Y.; Ouyang, H.; Yang, Z. Swine sperm induces neutrophil extracellular traps that entangle sperm and embryos. Reproduction 2020, 160, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kim, Y.H.; Kim, B.W.; Park, I.C.; Cheong, H.T.; Kim, J.T.; Park, C.K.; Kong, H.S.; Lee, H.K.; Yang, B.K. Ameliorative effects of melatonin against hydrogen peroxide-induced oxidative stress on boar sperm characteristics and subsequent in vitro embryo development. Reprod. Domest. Anim. 2010, 45, 943–950. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Species-specific interaction of seminal plasma on sperm-neutrophil binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef]
- Matthijs, A.; Engel, B.; Woelders, H. Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction 2003, 125, 357–367. [Google Scholar] [CrossRef]
- Doty, A.; Buhi, W.C.; Benson, S.; Scoggin, K.E.; Pozor, M.; Macpherson, M.; Mutz, M.; Troedsson, M.H. Equine CRISP3 modulates interaction between spermatozoa and polymorphonuclear neutrophils. Biol. Reprod. 2011, 85, 157–164. [Google Scholar] [CrossRef]
- Woelders, H.; Matthijs, A. Phagocytosis of boar spermatozoa in vitro and in vivo. Reprod. Suppl. 2001, 58, 113–127. [Google Scholar]
- Du, X.M.; Kohinata, K.; Kawasaki, T.; Guo, Y.T.; Miyahara, K. Components of the ether-insoluble resin glycoside-like fraction from Cuscuta chinensis. Phytochemistry 1998, 48, 843–850. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Z.; Fang, J.; Li, X. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002, 68, 237–243. [Google Scholar] [CrossRef]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.H.; Donkor, P.O.; Gao, X.M.; Chang, Y.X. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.; Gan, H.; Tian, F.; Cai, X.; Zhen, W.; Song, X.; Duan, Y.G. In vitro antioxidation effect of Quercetin on sperm function from the infertile patients with leukocytospermia. Am. J. Reprod. Immunol. 2019, 82, e13155. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Chen, M.; Jin, Z.; Jiang, Y.; Hong, H.; Qian, Y.; Liu, W.; Gao, X.; Jiang, L.; Xu, J.; et al. Quercetin alleviates gliotoxin-induced duckling tissue injury by inhibiting oxidative stress, inflammation and increasing heterophil extracellular traps release. Food Chem. Toxicol. 2023, 176, 113748. [Google Scholar] [CrossRef]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.; Maleki, N.A.; Khamnei, H.J.; Ahmadi, P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother. Res. 2010, 24, 1285–1291. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Ben Abdallah, F.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing-thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef]
- Johinke, D.; de Graaf, S.P.; Bathgate, R. Quercetin reduces the in vitro production of H2O2 during chilled storage of rabbit spermatozoa. Anim. Reprod. Sci. 2014, 151, 208–219. [Google Scholar] [CrossRef]
- El-Khawagah, A.R.M.; Kandiel, M.M.M.; Samir, H. Effect of Quercetin Supplementation in Extender on Sperm Kinematics, Extracellular Enzymes Release, and Oxidative Stress of Egyptian Buffalo Bulls Frozen-Thawed Semen. Front. Vet. Sci. 2020, 7, 604460. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Lu, G.; Gu, J. Pharmacological Activity of Flavonoid Quercetin and Its Therapeutic Potential in Testicular Injury. Nutrients 2023, 15, 2231. [Google Scholar] [CrossRef]
- Bharti, S.; Misro, M.M.; Rai, U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell Endocrinol. 2014, 383, 10–20. [Google Scholar] [CrossRef]
- Kujoana, T.C.; Sehlabela, L.D.; Mabelebele, M.; Sebola, N.A. The potential significance of antioxidants in livestock reproduction: Sperm viability and cryopreservation. Anim. Reprod. Sci. 2024, 267, 107512. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Carrau, T.; Gärtner, U.; Seipp, A.; Taubert, A.; Felmer, R.; Sanchez, R.; Hermosilla, C. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil. Steril. 2016, 106, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Jin, Q.; Chen, M.; Liu, W.; Hong, H.; Jiang, Y.; Gao, X.; Qian, Y.; Wang, Z.; Liu, Q.; et al. Toxoplasma gondii-induced neutrophil extracellular traps are relevant to glycolysis, TLR2, and TLR4 MAPK signaling pathway in goats. Parasitol. Res. 2023, 123, 34. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, Z.; Liu, X.; Wang, C.; Han, Z.; Wu, D.; Zhang, Y.; Zhang, X.; Yang, Z.; Liu, Q. Toxoplasma gondii Triggers Neutrophil Extracellular Traps Release in Dogs. Front. Cell Infect. Microbiol. 2020, 10, 429. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Hong, H.; Liu, W.; He, K.; Wang, J.; Guo, X.; Zhang, D.; Li, Q.; Yang, Z. Quercetin Protects Goat Sperm Motility by Inhibiting Neutrophil Extracellular Traps and Maintaining Plasma Membrane and Acrosome Integrity. Vet. Sci. 2024, 11, 553. https://doi.org/10.3390/vetsci11110553
Wei Z, Hong H, Liu W, He K, Wang J, Guo X, Zhang D, Li Q, Yang Z. Quercetin Protects Goat Sperm Motility by Inhibiting Neutrophil Extracellular Traps and Maintaining Plasma Membrane and Acrosome Integrity. Veterinary Sciences. 2024; 11(11):553. https://doi.org/10.3390/vetsci11110553
Chicago/Turabian StyleWei, Zhengkai, Hongrong Hong, Wei Liu, Kaifeng He, Jiaxuan Wang, Xin Guo, Dezhi Zhang, Qianyong Li, and Zhengtao Yang. 2024. "Quercetin Protects Goat Sperm Motility by Inhibiting Neutrophil Extracellular Traps and Maintaining Plasma Membrane and Acrosome Integrity" Veterinary Sciences 11, no. 11: 553. https://doi.org/10.3390/vetsci11110553
APA StyleWei, Z., Hong, H., Liu, W., He, K., Wang, J., Guo, X., Zhang, D., Li, Q., & Yang, Z. (2024). Quercetin Protects Goat Sperm Motility by Inhibiting Neutrophil Extracellular Traps and Maintaining Plasma Membrane and Acrosome Integrity. Veterinary Sciences, 11(11), 553. https://doi.org/10.3390/vetsci11110553



