Extracted Plasma Cell-Free DNA Concentrations Are Elevated in Colic Patients with Systemic Inflammation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. DNA Extraction
2.3. Cell-Free DNA Measurement
2.4. Clinical Data Collection and Characterization
2.5. Statistical Analyses
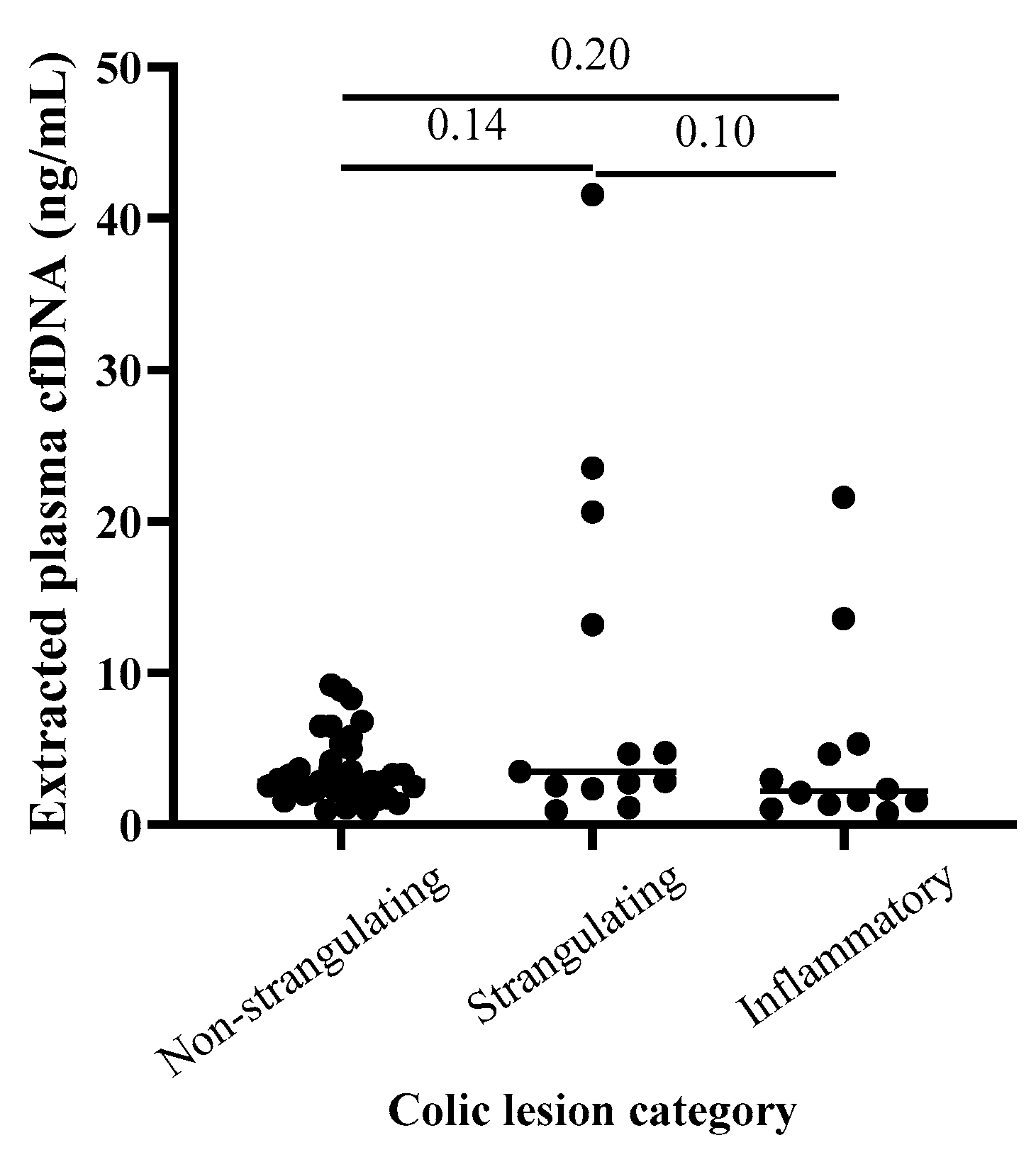
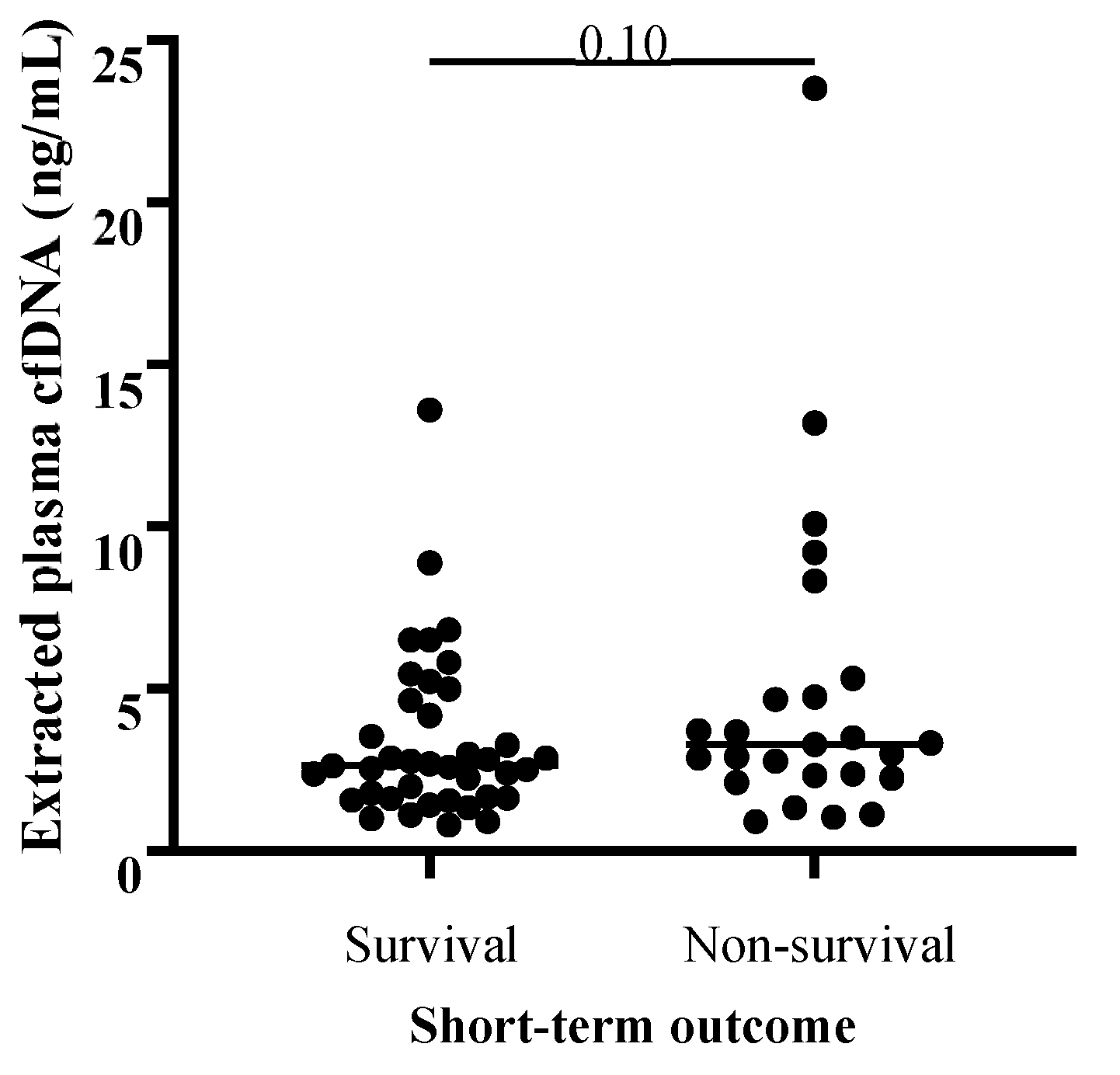
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Equine Mortality in the United States; Veterinary Services, Center for Epidemiology and Animal Health, Animal and Plant Inspection Service, United States Department of Agriculture: Fort Collins, CO, USA, 2017.
- Mair, T.S.; Smith, L.J. Survival and Complication Rates in 300 Horses Undergoing Surgical Treatment of Colic. Part 1: Short-Term Survival Following a Single Laparotomy. Equine Vet. J. 2005, 37, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, M.T.; Dupont, N.; Berg-Sørensen, K.S.; Konnerup, C.; Pihl, T.H.; Andersen, P.H. Short-Term Survival and Mortality Rates in a Retrospective Study of Colic in 1588 Danish Horses. Acta Vet. Scand. 2014, 56, 20. [Google Scholar] [CrossRef] [PubMed]
- Mair, T.; Smith, L. Survival and Complication Rates in 300 Horses Undergoing Surgical Treatment of Colic. Part 4: Early (Acute) Relaparotomy. Equine Vet. J. 2005, 37, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.J.; Curtis, C.R.; Salman, M.D.; Stashak, T.S.; Reif, J.S. Multivariable Prediction Model for the Need for Surgery in Horses with Colic. Am. J. Vet. Res. 1991, 51, 1093–1907. [Google Scholar] [CrossRef]
- Bishop, R.C.; Gutierrez-Nibeyro, S.D.; Stewart, M.C.; McCoy, A.M. Performance of Predictive Models of Survival in Horses Undergoing Emergency Exploratory Laparotomy for Colic. Vet. Surg. 2022, 51, 891–902. [Google Scholar] [CrossRef]
- Farrell, A.; Kersh, K.; Liepman, R.; Dembek, K.A. Development of a Colic Scoring System to Predict Outcome in Horses. Front. Vet. Sci. 2021, 8, 697589. [Google Scholar] [CrossRef]
- Cummings, C.O.; Krucik, D.D.R.; Price, E. Clinical Predictive Models in Equine Medicine: A Systematic Review. Equine Vet. J. 2023, 55, 573–583. [Google Scholar] [CrossRef]
- Furr, M.O.; Lessard, P.; White, N.A. Development of a Colic Severity Score for Predicting the Outcome of Equine Colic. Vet. Surg. 1995, 24, 97–101. [Google Scholar] [CrossRef]
- Spadari, A.; Gialletti, R.; Gandini, M.; Valle, E.; Cerullo, A.; Cavallini, D.; Bertoletti, A.; Rinnovati, R.; Forni, G.; Scilimati, N.; et al. Short-Term Survival and Postoperative Complications Rates in Horses Undergoing Colic Surgery: A Multicentre Study. Animals 2023, 13, 1107. [Google Scholar] [CrossRef]
- Ludwig, E.K.; Hobbs, K.J.; McKinney-Aguirre, C.A.; Gonzalez, L.M. Biomarkers of Intestinal Injury in Colic. Animals 2023, 13, 227. [Google Scholar] [CrossRef]
- Radcliffe, R.M.; Divers, T.J.; Fletcher, D.J.; Mohammed, H.; Kraus, M.S. Evaluation of L-Lactate and Cardiac Troponin I in Horses Undergoing Emergency Abdominal Surgery. J. Vet. Emerg. Crit. Care 2012, 22, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Holcombe, S.J.; Hauptman, J.G. Plasma Lactate as a Predictor of Colonic Viability and Survival after 360° Volvulus of the Ascending Colon in Horses. Vet. Surg. 2007, 36, 563–567. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M.; Hackett, E.S.; Wagner, A.E.; Mama, K.R.; Hendrickson, D.A. Pulmonary Gas Exchange and Plasma Lactate in Horses with Gastrointestinal Disease Undergoing Emergency Exploratory Laparotomy: A Comparison with an Elective Surgery Horse Population. Vet. Surg. 2011, 40, 601–609. [Google Scholar] [CrossRef]
- Delesalle, C.; Dewulf, J.; Lefebvre, R.; Schuurkes, J.; Proot, J.; Lefere, L.; Deprez, P. Determination of Lactate Concentrations in Blood Plasma and Peritoneal Fluid in Horses with Colic by an Accusport Analyzer. J. Vet. Intern. Med. 2007, 21, 293–301. [Google Scholar] [CrossRef]
- Westerman, T.L.; Foster, C.M.; Tornquist, S.J.; Poulsen, K.P. Evaluation of Serum Amyloid A and Haptoglobin Concentrations as Prognostic Indicators for Horses with Colic. J. Am. Vet. Med. Assoc. 2016, 248, 935–940. [Google Scholar] [CrossRef]
- Krueger, C.R.; Ruple-Czerniak, A.; Hackett, E.S. Evaluation of Plasma Muscle Enzyme Activity as an Indicator of Lesion Characteristics and Prognosis in Horses Undergoing Celiotomy for Acute Gastrointestinal Pain. BMC Vet. Res. 2014, 10, S7. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Predictive Value of Plasma and Peritoneal Creatine Kinase in Horses with Strangulating Intestinal Lesions. Vet. Surg. 2019, 48, 152–158. [Google Scholar] [CrossRef]
- Nocera, I.; Bonelli, F.; Vitale, V.; Meucci, V.; Conte, G.; Jose-Cunilleras, E.; Gracia-Calvo, L.A.; Sgorbini, M. Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals 2021, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Diagnostic Value of Plasma and Peritoneal Fluid Procalcitonin Concentrations in Horses with Strangulating Intestinal Lesions. J. Am. Vet. Med. Assoc. 2020, 256, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.; Nir, E.; Eyngor, M.; Eldar, A.; Bdolah-Abram, T.; Kelmer, E.; Steinman, A.; Bruchim, Y. Dynamic of Cytokine Gene Transcription (TNF-α, IL-1β, IL-6, IL-8) in Surgically Treated Colic Horses by Use of Real-Time PCR (RT-PCR). Isr. J. Vet. Med. 2016, 71, 24–30. [Google Scholar]
- Barton, M.H.; Collatos, C. Tumor Necrosis Factor and Interleukin-6 Activity and Endotoxin Concentration in Peritoneal Fluid and Blood of Horses with Acute Abdominal Disease. J. Vet. Intern. Med. 1999, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Steverink, P.J.G.M.; Sturk, A.; Rutten, V.P.M.G.; Wagenaar-Hilbers, J.P.A.; Klein, W.R.; Van Der Velden, M.A.; Németh, F.; Nemeth, F. Endotoxin, Interleukin-6 and Tumor Necrosis Factor Concentrations in Equine Acute Abdominal Disease: Relation to Clinical Outcome. J. Endotoxin Res. 1995, 2, 289–299. [Google Scholar] [CrossRef]
- Latson, K.M.; Nieto, J.E.; Beldomenico, P.M.; Snyder, J.R. Evaluation of Peritoneal Fluid Lactate as a Marker of Intestinal Ischaemia in Equine Colic. Equine Vet. J. 2005, 37, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Peloso, J.G.; Cohen, N.D. Use of Serial Measurements of Peritoneal Fluid Lactate Concentration to Identify Strangulating Intestinal Lesions in Referred Horses with Signs of Colic. J. Am. Vet. Med. Assoc. 2012, 240, 1208–1217. [Google Scholar] [CrossRef]
- Vandenplas, M.L.; Moore, J.N.; Barton, M.H.; Ruossel, A.J.; Cohen, N.D. Concentrations of Serum Amyloid A and Lipopolysaccharide-Binding Protein in Horses with Colic. Am. J. Vet. Res. 2005, 66, 1509–1516. [Google Scholar] [CrossRef]
- Copas, V.E.N.; Durham, A.E.; Stratford, C.H.; McGorum, B.C.; Waggett, B.; Pirie, R.S. In Equine Grass Sickness, Serum Amyloid A and Fibrinogen Are Elevated, and Can Aid Differential Diagnosis from Non-Inflammatory Causes of Colic. Vet. Rec. 2013, 172, 395. [Google Scholar] [CrossRef]
- Daniel, A.J.; Leise, B.S.; Burgess, B.A.; Morley, P.S.; Cloninger, M.; Hassel, D.M. Concentrations of Serum Amyloid A and Plasma Fibrinogen in Horses Undergoing Emergency Abdominal Surgery. J. Vet. Emerg. Crit. Care 2016, 26, 344–351. [Google Scholar] [CrossRef]
- Dondi, F.; Lukacs, R.M.; Gentilini, F.; Rinnovati, R.; Spadari, A.; Romagnoli, N. Serum Amyloid A, Haptoglobin, and Ferritin in Horses with Colic: Association with Common Clinicopathological Variables and Short-Term Outcome. Vet. J. 2015, 205, 50–55. [Google Scholar] [CrossRef]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.S.; Pretorius, P.J. The Diverse Origins of Circulating Cell-Free DNA in the Human Body: A Critical Re-Evaluation of the Literature. Biol. Rev. 2018, 93, 1649–1683. [Google Scholar] [CrossRef]
- Yu, D.; Tong, Y.; Guo, X.; Feng, L.; Jiang, Z.; Ying, S.; Jia, J.; Fang, Y.; Yu, M.; Xia, H.; et al. Diagnostic Value of Concentration of Circulating Cell-Free DNA in Breast Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 95. [Google Scholar] [CrossRef]
- Okajima, W.; Komatsu, S.; Ichikawa, D.; Miyamae, M.; Ohashi, T.; Imamura, T.; Kiuchi, J.; Nishibeppu, K.; Arita, T.; Konishi, H.; et al. Liquid Biopsy in Patients with Hepatocellular Carcinoma: Circulating Tumor Cells and Cell-Free Nucleic Acids. World J. Gastroenterol. 2017, 23, 5650–5668. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; Horswell, S.; Mitter, R.; Sakarya, O.; Constantin, T.; Salari, R.; Kirkizlar, E.; Sigurjonsson, S.; Pelham, R.; et al. Detection of Ubiquitous and Heterogeneous Mutations in Cell-Free DNA from Patients with Early-Stage Non-Small-Cell Lung Cancer. Ann. Oncol. 2016, 27, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of Cell-Free DNA in Maternal Blood in Screening for Aneuploidies: Updated Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Burnham, P.; Khush, K.; De Vlaminck, I. Myriad Applications of Circulating Cell-Free DNA in Precision Organ Transplant Monitoring. Ann. Am. Thorac. Soc. 2017, 14, S237–S241. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Schütz, E.; Beck, J.; Kanzow, P.; Plowman, P.; Weiss, G.; Walson, P. Using Circulating Cell-Free DNA to Monitor Personalized Cancer Therapy. Crit. Rev. Clin. Lab. Sci. 2017, 54, 205–218. [Google Scholar] [CrossRef]
- Naumann, D.N.; Hazeldine, J.; Dinsdale, R.J.; Bishop, J.R.; Midwinter, M.J.; Harrison, P.; Hutchings, S.D.; Lord, J.M. Endotheliopathy Is Associated with Higher Levels of Cell-Free DNA Following Major Trauma: A Prospective Observational Study. PLoS ONE 2017, 12, e0189870. [Google Scholar] [CrossRef]
- Avriel, A.; Wiessman, M.P.; Almog, Y.; Perl, Y.; Novack, V.; Galante, O.; Klein, M.; Pencina, M.J.; Douvdevani, A. Admission Cell Free DNA Levels Predict 28-Day Mortality in Patients with Severe Sepsis in Intensive Care. PLoS ONE 2014, 9, e100514. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.J.; Toltl, L.J.; Swystun, L.L.; Pogue, J.; Liaw, K.L.; Weitz, J.I.; Cook, D.J.; Fox-Robichaud, A.E.; Liaw, P.C. Prognostic Utility and Characterization of Cell-Free DNA in Patients with Severe Sepsis. Crit. Care 2012, 16, R151. [Google Scholar] [CrossRef]
- Rainer, T.H.; Chan, A.K.C.; Lee, L.L.Y.; Yim, V.W.T.; Lam, N.Y.L.; Yeung, S.W.; Graham, C.A.; Lo, D.Y.M. Use of Plasma DNA to Predict Mortality and Need for Intensive Care in Patients with Abdominal Pain. Clin. Chim. Acta 2008, 398, 113–117. [Google Scholar] [CrossRef]
- Arnalich, F.; Maldifassi, M.C.; Ciria, E.; Quesada, A.; Codoceo, R.; Herruzo, R.; Garcia-Cerrada, C.; Montoya, F.; Vazquez, J.J.; López-Collazo, E.; et al. Association of Cell-Free Plasma DNA with Perioperative Mortality in Patients with Suspected Acute Mesenteric Ischemia. Clin. Chim. Acta 2010, 411, 1269–1274. [Google Scholar] [CrossRef]
- Letendre, J.-A.; Goggs, R. Measurement of Plasma Cell-Free DNA Concentrations in Dogs with Sepsis, Trauma, and Neoplasia. J. Vet. Emerg. Crit. Care 2017, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Letendre, J.-A.; Goggs, R. Determining Prognosis in Canine Sepsis by Bedside Measurement of Cell-Free DNA and Nucleosomes. J. Vet. Emerg. Crit. Care 2018, 28, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Letendre, J.-A.; Goggs, R. Concentrations of Plasma Nucleosomes but Not Cell-Free DNA Are Prognostic in Dogs Following Trauma. Front. Vet. Sci. 2018, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, U.; Ruterbories, L.; Hanel, R.; LeVine, D.N. Cell-Free DNA and DNase Activity in Dogs with Immune-Mediated Hemolytic Anemia. J. Vet. Intern. Med. 2017, 31, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Troia, R.; Giunti, M.; Calipa, S.; Goggs, R. Cell-Free DNA, High-Mobility Group Box-1, and Procalcitonin Concentrations in Dogs with Gastric Dilatation–Volvulus Syndrome. Front. Vet. Sci. 2018, 5, 67. [Google Scholar] [CrossRef]
- Fingerhut, L.; Ohnesorge, B.; von Borstel, M.; Schumski, A.; Strutzberg-Minder, K.; Mörgelin, M.; Deeg, C.A.; Haagsman, H.P.; Beineke, A.; von Köckritz-Blickwede, M.; et al. Neutrophil Extracellular Traps in the Pathogenesis of Equine Recurrent Uveitis (ERU). Cells 2019, 8, 1528. [Google Scholar] [CrossRef]
- Colmer, S.F.; Luethy, D.; Abraham, M.; Stefanovski, D.; Hurcombe, S.D. Utility of Cell-Free DNA Concentrations and Illness Severity Scores to Predict Survival in Critically Ill Neonatal Foals. PLoS ONE 2021, 16, e0242635. [Google Scholar] [CrossRef]
- Panizzi, L.; Dittmer, K.E.; Vignes, M.; Doucet, J.S.; Gedye, K.; Waterland, M.R.; Rogers, C.W.; Sano, H.; McIlwraith, C.W.; Riley, C.B. Plasma and Synovial Fluid Cell-Free DNA Concentrations Following Induction of Osteoarthritis in Horses. Animals 2023, 13, 1053. [Google Scholar] [CrossRef]
- Bayless, R.L.; Cooper, B.L.; Sheats, M.K. Investigation of Plasma Cell-Free DNA as a Potential Biomarker in Horses. J. Vet. Diagn. Investig. 2022, 34, 402–406. [Google Scholar] [CrossRef]
- Burnett, D.L.; Cave, N.J.; Gedye, K.R.; Bridges, J.P. Investigation of Cell-Free DNA in Canine Plasma and Its Relation to Disease. Vet. Q. 2016, 36, 122–129. [Google Scholar] [CrossRef]
- Forsblom, E.; Aittoniemi, J.; Ruotsalainen, E.; Helmijoki, V.; Huttunen, R.; Jylhävä, J.; Hurme, M.; Järvinen, A. High Cell-Free DNA Predicts Fatal Outcome among Staphylococcus Aureus Bacteraemia Patients with Intensive Care Unit Treatment. PLoS ONE 2014, 9, e87741. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R. Effect of Sample Type on Plasma Concentrations of Cell-Free DNA and Nucleosomes in Dogs. Vet. Rec. Open 2019, 6, e000357. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.J.; Burchell, R.K.; Worth, A.J.; Burton, S.E.; Gedye, K.R.; Clark, K.J.; Crosse, K.R.; Jack, M.; Odom, T.F.; De Grey, S.J.; et al. Kinetics of Plasma Cell-Free DNA and Creatine Kinase in a Canine Model of Tissue Injury. J. Vet. Intern. Med. 2018, 32, 157–164. [Google Scholar] [CrossRef]
- Huttunen, R.; Kuparinen, T.; Jylhävä, J.; Aittoniemi, J.; Vuento, R.; Huhtala, H.; Laine, J.; Syrjänen, J.; Hurme, M. Fatal Outcome in Bacteremia Is Characterized by High Plasma Cell Free DNA Concentration and Apoptotic DNA Fragmentation: A Prospective Cohort Study. PLoS ONE 2011, 6, e21700. [Google Scholar] [CrossRef]
- Hobbs, K.J.; Cooper, B.L.; Dembek, K.; Sheats, M.K. Investigation of Extracted Plasma Cell-Free DNA as a Biomarker in Foals with Sepsis. Vet. Sci. 2024, 11, 346. [Google Scholar] [CrossRef]
- Saukkonen, K.; Lakkisto, P.; Pettilä, V.; Varpula, M.; Karlsson, S.; Ruokonen, E.; Pulkki, K. Cell-Free Plasma DNA as a Predictor of Outcome in Severe Sepsis and Septic Shock. Clin. Chem. 2008, 54, 1000–1007. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Y.; Chang, J.; Zhou, X.; Qi, Q.; Tian, X.; Li, M.; Zeng, X.; Xu, M.; Zhang, W.; et al. High Levels of Circulating Cell-Free DNA Are a Biomarker of Active SLE. Eur. J. Clin. Investig. 2018, 48, e13015. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.-F.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic Value and Development of a Scoring System in Horses with Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef]
- Barrett, A.N.; Thadani, H.A.; Laureano-Asibal, C.; Ponnusamy, S.; Choolani, M. Stability of Cell-Free DNA from Maternal Plasma Isolated Following a Single Centrifugation Step. Prenat. Diagn. 2014, 34, 1283–1288. [Google Scholar] [CrossRef]
- Jung, M.; Klotzek, S.; Lewandowski, M.; Fleischhacker, M.; Jung, K. Changes in Concentration of DNA in Serum and Plasma during Storage of Blood Samples. Clin. Chem. 2003, 49, 1028–1029. [Google Scholar] [CrossRef]
- Sato, A.; Nakashima, C.; Abe, T.; Kato, J.; Hirai, M.; Nakamura, T.; Komiya, K.; Kimura, S.; Sueoka, E.; Sueoka-Aragane, N. Investigation of Appropriate Pre-Analytical Procedure for Circulating Free DNA from Liquid Biopsy. Oncotarget 2018, 9, 31904–31914. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Lawson, C.M.; McMichael, M.A.; Jung, K.; O’Brien, M.; Achiel, R. Evaluation of Assays for Quantification of DNA in Canine Plasma as an Indirect Marker of NETosis. Vet. Clin. Pathol. 2017, 46, 278–286. [Google Scholar] [CrossRef]
- Rather, R.A.; Saha, S.C.; Dhawan, V. The Most Favourable Procedure for the Isolation of Cell-Free DNA from the Plasma of Iso-Immunized RHD-Negative Pregnant Women. J. Circ. Biomark. 2015, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Kloten, V.; Rüchel, N.; Brüchle, N.O.; Gasthaus, J.; Freudenmacher, N.; Steib, F.; Mijnes, J.; Eschenbruch, J.; Binnebösel, M.; Knüchel, R.; et al. Liquid Biopsy in Colon Cancer: Comparison of Different Circulating DNA Extraction Systems Following Absolute Quantification of KRAS Mutations Using Intplex Allele-Specific PCR. Oncotarget 2017, 8, 86253–86263. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Manfredini, M.; Kaleci, S.; Mandrioli, M.; Pellacani, G.; Ozben, T.; Depenni, R.; Bianchi, G.; Pirola, G.M.; et al. The Value of Fluorimetry (Qubit) and Spectrophotometry (NanoDrop) in the Quantification of Cell-Free DNA (CfDNA) in Malignant Melanoma and Prostate Cancer Patients. Clin. Chim. Acta 2018, 479, 14–19. [Google Scholar] [CrossRef]
- Parackal, S.; Zou, D.; Day, R.; Black, M.; Guilford, P. Comparison of Roche Cell-Free DNA Collection Tubes® to Streck Cell-Free DNA BCT®s for Sample Stability Using Healthy Volunteers. Pract. Lab. Med. 2019, 16, e00125. [Google Scholar] [CrossRef]
- Nikolaev, S.; Lemmens, L.; Koessler, T.; Blouin, J.L.; Nouspikel, T. Circulating Tumoral DNA: Preanalytical Validation and Quality Control in a Diagnostic Laboratory. Anal. Biochem. 2018, 542, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Barrios, C.; Nieto-Alcolado, I.; Torrente, M.; Jiménez-Sánchez, C.; Calvo, V.; Gutierrez-Sanz, L.; Palka, M.; Donoso-Navarro, E.; Provencio, M.; Romero, A. Comparison of Methods for Circulating Cell-Free DNA Isolation Using Blood from Cancer Patients: Impact on Biomarker Testing. Transl. Lung Cancer Res. 2016, 5, 665–672. [Google Scholar] [CrossRef]
- Jackson Chornenki, N.L.; Coke, R.; Kwong, A.C.; Dwivedi, D.J.; Xu, M.K.; McDonald, E.; Marshall, J.C.; Fox-Robichaud, A.E.; Charbonney, E.; Liaw, P.C. Comparison of the Source and Prognostic Utility of CfDNA in Trauma and Sepsis. Intensive Care Med. Exp. 2019, 7, 29. [Google Scholar] [CrossRef]
- Fridlich, O.; Peretz, A.; Fox-Fisher, I.; Pyanzin, S.; Dadon, Z.; Shcolnik, E.; Sadeh, R.; Fialkoff, G.; Sharkia, I.; Moss, J.; et al. Elevated CfDNA after Exercise Is Derived Primarily from Mature Polymorphonuclear Neutrophils, with a Minor Contribution of Cardiomyocytes. Cell Rep. Med. 2023, 4, 101074. [Google Scholar] [CrossRef]
- Juškevičiūtė, E.; Neuberger, E.; Eimantas, N.; Heinkel, K.; Simon, P.; Brazaitis, M. Cell-Free DNA Kinetics in Response to Muscle-Damaging Exercise: A Drop Jump Study. Exp. Physiol. 2024, 109, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.V.; Curty, V.M.; Coutinho, J.V.S.; Santos, M.Â.A.; Vassallo, P.F.; de Sousa, N.F.; Barauna, V.G. Cell-Free DNA as an Earlier Predictor of Exercise-Induced Performance Decrement Related to Muscle Damage. Int. J. Sports Physiol. Perform. 2018, 13, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Cave, N.; Bridges, J.; Gedye, K.; Hill, K. Plasma NT-ProBNP and Cell-Free DNA Concentrations after Prolonged Strenuous Exercise in Working Farm Dogs. J. Vet. Intern. Med. 2018, 32, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Devall, V.C.; Goggs, R.; Hansen, C.; Frye, C.W.; Letendre, J.A.; Wakshlag, J.J. Serum Myoglobin, Creatine Kinase, and Cell-Free DNA in Endurance Sled Dogs and Sled Dogs with Clinical Rhabdomyolysis. J. Vet. Emerg. Crit. Care 2018, 28, 310–316. [Google Scholar] [CrossRef]

| Variable | Colic Patients (N = 67) * |
|---|---|
| Age (median, range) | 12 years (2–35 years) |
| Sex (number of horses) | 28 Mare |
| 39 Gelding | |
| 0 Stallion | |
| Breed (number of horses) | 14 Quarter Horse and Related Breed † |
| 14 Warmblood | |
| 13 Thoroughbred | |
| 6 Arabian | |
| 5 Draft Horse | |
| 4 Pony | |
| 2 Andalusian | |
| 2 Friesian/Friesian Cross | |
| 2 Paso Fino | |
| 1 Miniature Horse | |
| 1 Morgan | |
| 1 Mule | |
| 1 Norwegian Fjord | |
| 1 Saddlebred | |
| Lesion category | 38 Non-strangulating |
| 13 Strangulating | |
| 12 Inflammatory | |
| 4 n/a # | |
| SIRS status | 40 Non-SIRS |
| 22 SIRS | |
| 5 n/a # | |
| Short-term outcome | 41 Survival |
| (survival to discharge) | 26 Non-survival |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayless, R.L.; Cooper, B.L.; Sheats, M.K. Extracted Plasma Cell-Free DNA Concentrations Are Elevated in Colic Patients with Systemic Inflammation. Vet. Sci. 2024, 11, 427. https://doi.org/10.3390/vetsci11090427
Bayless RL, Cooper BL, Sheats MK. Extracted Plasma Cell-Free DNA Concentrations Are Elevated in Colic Patients with Systemic Inflammation. Veterinary Sciences. 2024; 11(9):427. https://doi.org/10.3390/vetsci11090427
Chicago/Turabian StyleBayless, Rosemary L., Bethanie L. Cooper, and M. Katie Sheats. 2024. "Extracted Plasma Cell-Free DNA Concentrations Are Elevated in Colic Patients with Systemic Inflammation" Veterinary Sciences 11, no. 9: 427. https://doi.org/10.3390/vetsci11090427
APA StyleBayless, R. L., Cooper, B. L., & Sheats, M. K. (2024). Extracted Plasma Cell-Free DNA Concentrations Are Elevated in Colic Patients with Systemic Inflammation. Veterinary Sciences, 11(9), 427. https://doi.org/10.3390/vetsci11090427







