β-Tocotrienol and δ-Tocotrienol as Additional Inhibitors of the Main Protease of Feline Infectious Peritonitis Virus: An In Silico Analysis
Abstract
Simple Summary
Abstract
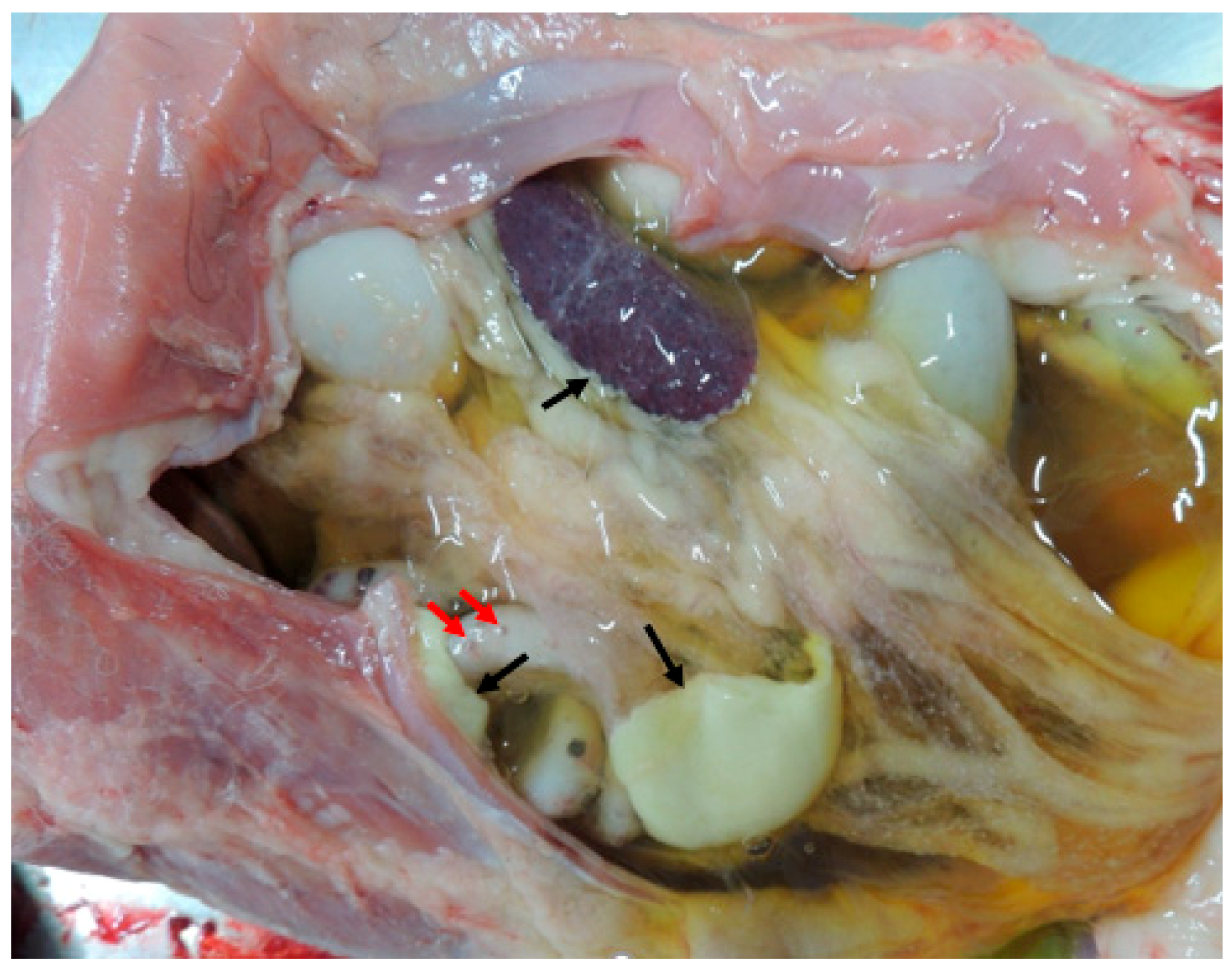
1. Introduction
2. Materials and Methods
2.1. Computational Tools
2.2. Computational Methods
2.2.1. Protein Structure Preparation
2.2.2. Database Preparation
2.2.3. Structure-Based Virtual Screening
2.2.4. Molecular Docking by iGEMDOCK
2.2.5. Binding Free Energy Estimation
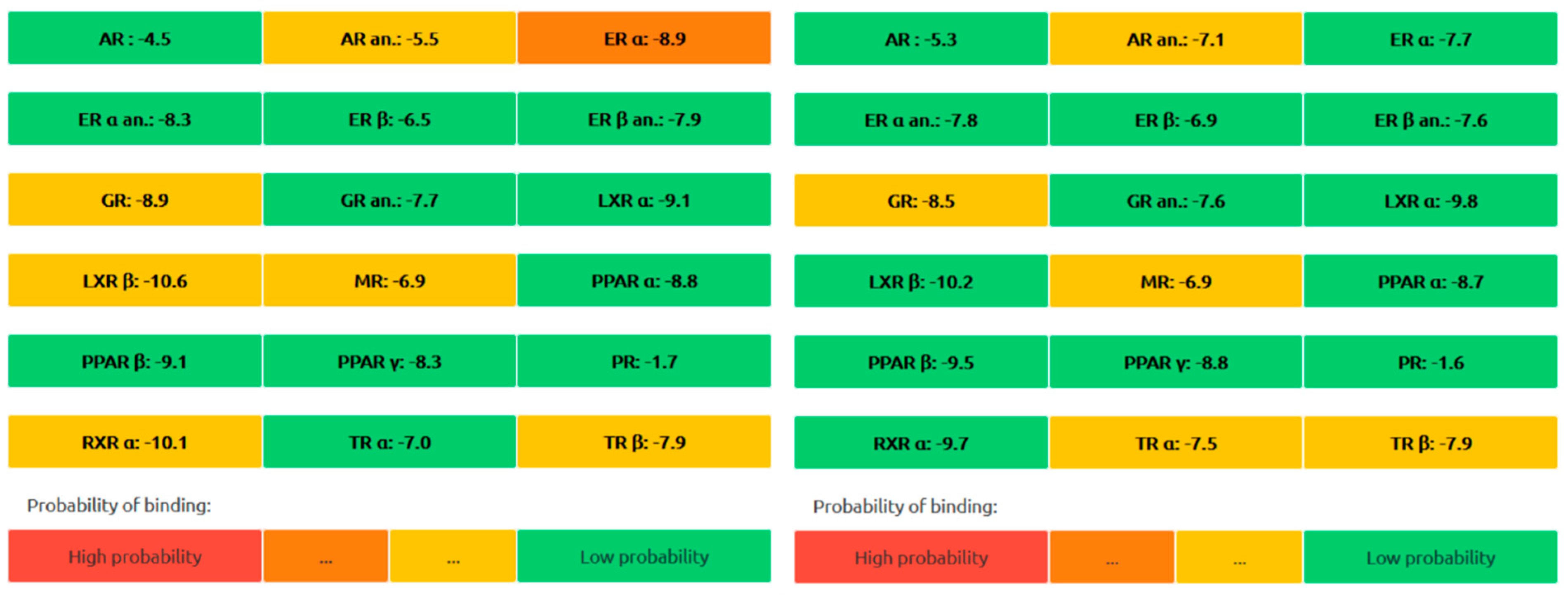
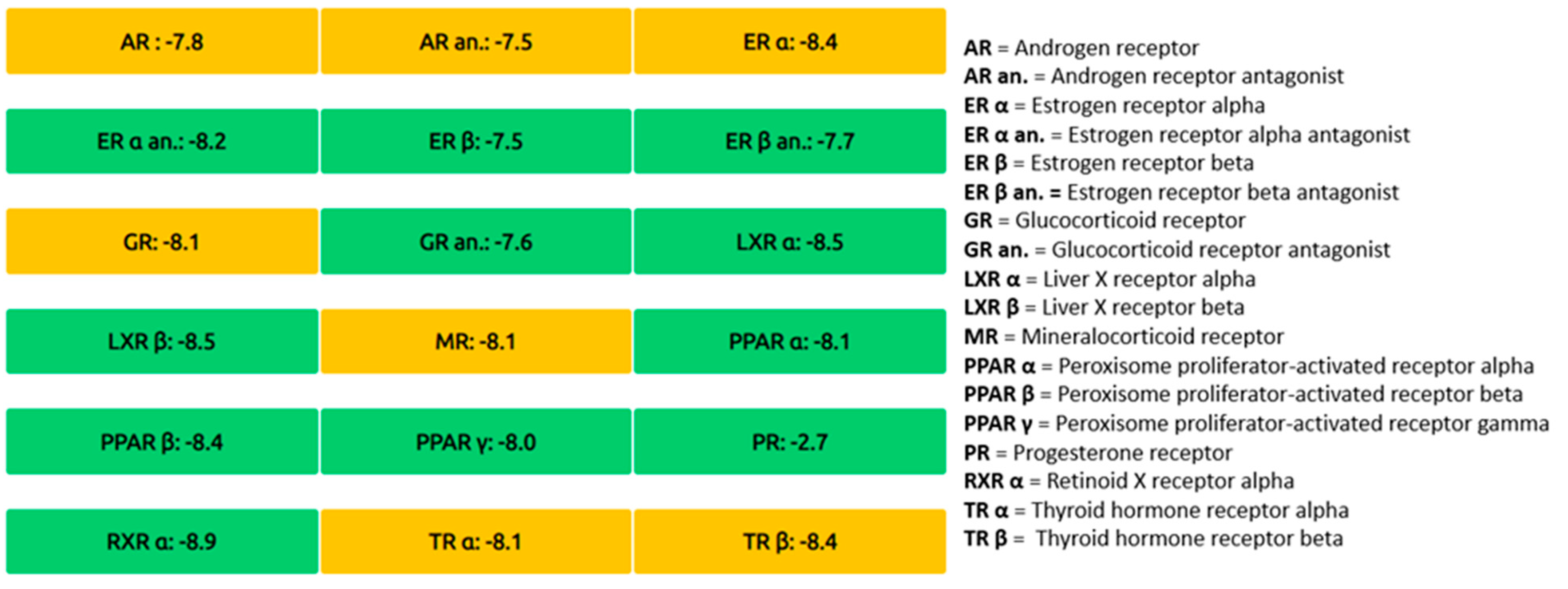
2.2.6. In Silico ADMET Studies
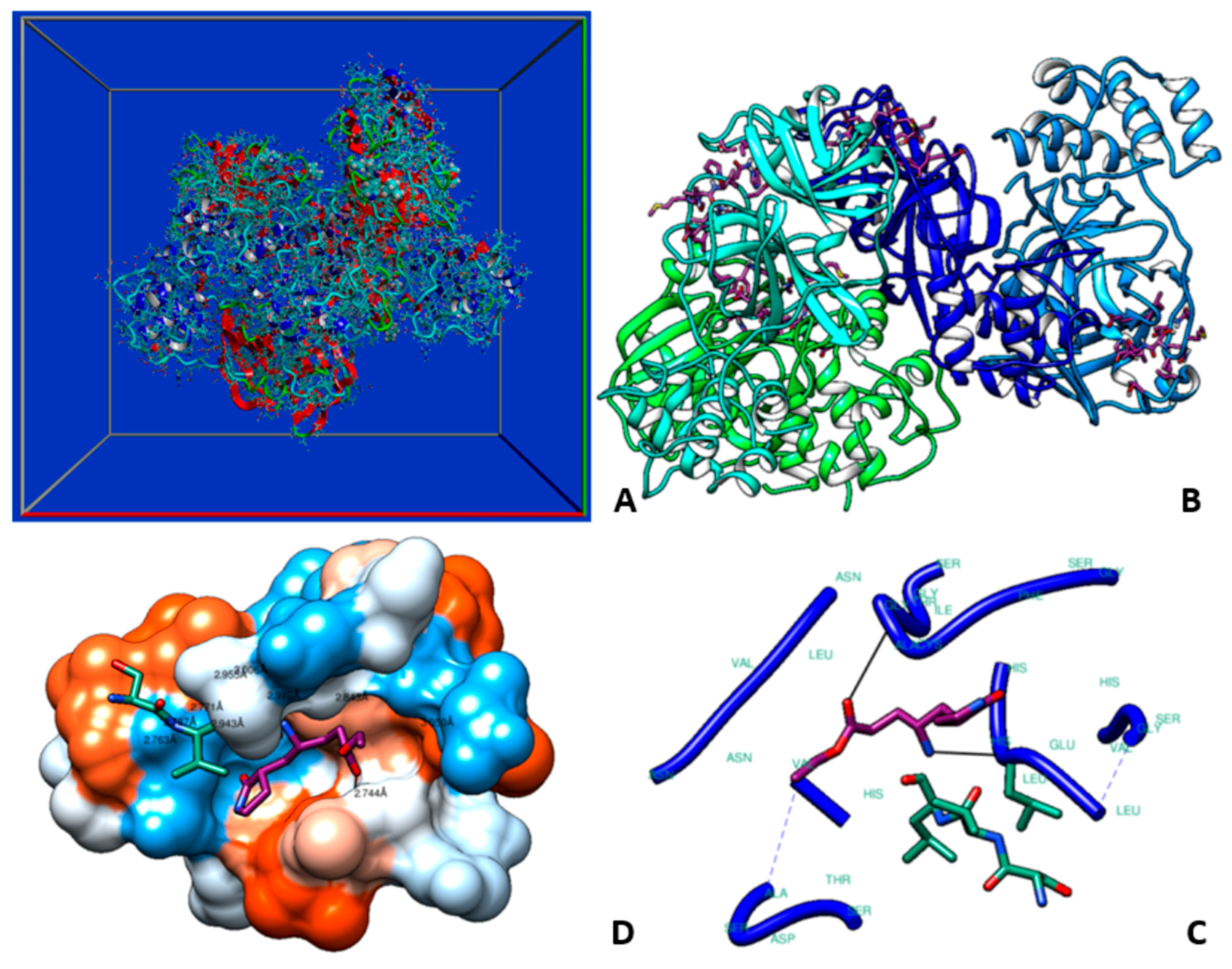
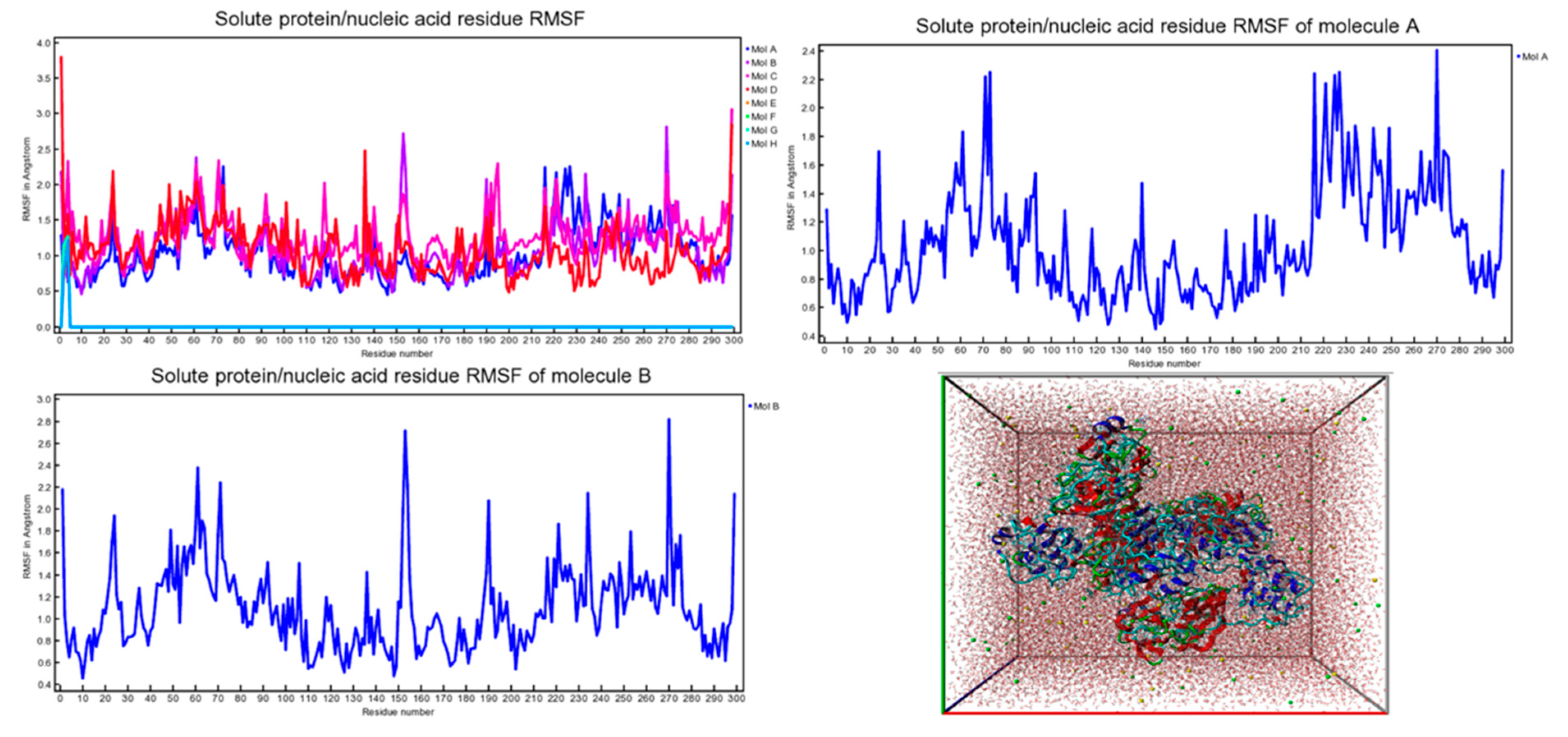
2.3. Molecular Dynamics (MD) Studies
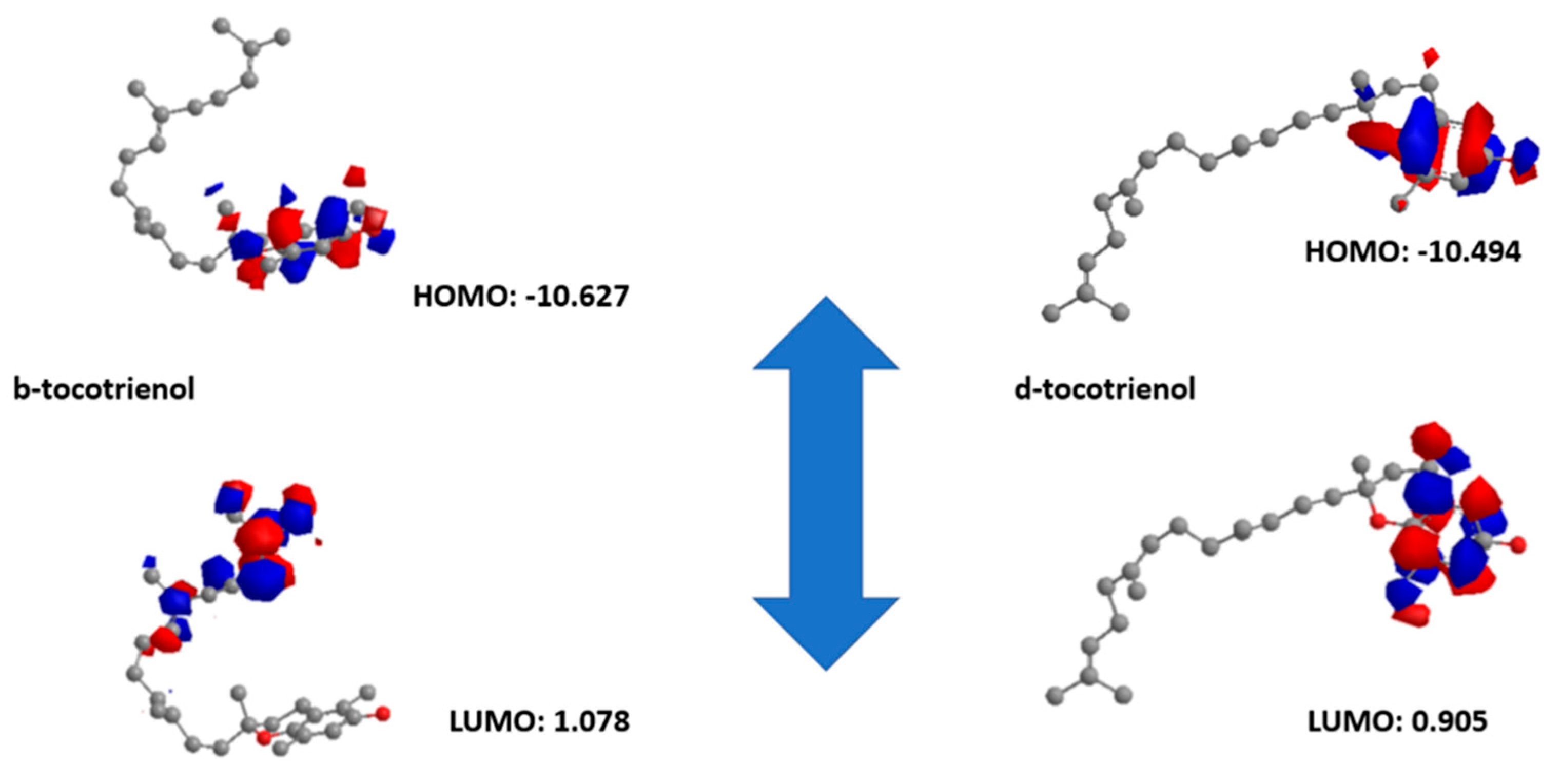
2.4. Density Functional Theory (DFT) Studies
3. Results
4. Discussion
4.1. Virtual Screening and Molecular Docking
4.2. ADMET and Molecular Dynamics (MD) Studies
4.3. Comprehensive Analysis and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Z.; Yan, Y.; Chen, Y.; Wang, G.; Wang, X.; Li, L.; Yang, M.; Hu, X.; Guo, Y.; Shi, Y.; et al. Adaptive Mutation in the Main Protease Cleavage Site of Feline Coronavirus Renders the Virus More Resistant to Main Protease Inhibitors. J. Virol. 2022, 96, e00907-22. [Google Scholar] [CrossRef] [PubMed]
- Sherding, R.G. Feline Infectious Peritonitis (Feline Coronavirus). Saunders Man. Small Anim. Pract. 2020, 21, 1–9. [Google Scholar]
- Vojtkovská, V.; Lukešová, G.; Voslářová, E.; Konvalinová, J.; Večerek, V.; Lobová, D. Direct Detection of Feline Coronavirus by Three Rapid Antigen Immunochromatographic Tests and by Real-Time PCR in Cat Shelters. Vet. Sci. 2022, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Colina, S.E.; Serena, M.S.; Echeverría, M.G.; Metz, G.E. Clinical and molecular aspects of veterinary coronaviruses. Virus Res. 2021, 297, 198382. [Google Scholar] [CrossRef]
- Cook, S.; Wittenburg, L.; Yan, V.C.; Theil, J.H.; Castillo, D.; Reagan, K.L.; Williams, S.; Pham, C.D.; Li, C.; Muller, F.L.; et al. An Optimized Bioassay for Screening Combined Anticoronaviral Compounds for Efficacy against Feline Infectious Peritonitis Virus with Pharmacokinetic Analyses of GS-441524, Remdesivir, and Molnupiravir in Cats. Viruses 2022, 14, 2429. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.; Liu, X.; Yang, K.; Xu, X.; Yang, H. Crystal Structure of Feline Infectious Peritonitis Virus Main Protease in Complex with Synergetic Dual Inhibitors. J. Virol. 2016, 90, 1910–1917. [Google Scholar] [CrossRef]
- Dunbar, D.; Babayan, S.A.; Krumrie, S.; Haining, H.; Hosie, M.J.; Weir, W. Assessing the feasibility of applying machine learning to diagnosing non-effusive feline infectious peritonitis. Sci. Rep. 2024, 14, 2517. [Google Scholar] [CrossRef]
- Lu, J.; Chen, S.A.; Khan, M.B.; Brassard, R.; Arutyunova, E.; Lamer, T.; Vuong, W.; Fischer, C.; Young, H.S.; Vederas, J.C.; et al. Crystallization of Feline Coronavirus Mpro With GC376 Reveals Mechanism of Inhibition. Front. Inchemistry 2022, 10, 852210. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Liao, G.R.; Lien, A.; Hsu, W.L. Current concepts in the development of therapeutics against human and animal coronavirus diseases by targeting NP. Comput. Struct. Biotechnol. J. 2021, 19, 1072–1080. [Google Scholar] [CrossRef]
- St. John, S.E.; Therkelsen, M.D.; Nyalapatla, P.R.; Osswald, H.L.; Ghosh, A.K.; Mesecar, A.D. X-ray structure and inhibition of the feline infectious peritonitis virus 3C-like protease: Structural implications for drug design. Bioorganic Med. Chem. Lett. 2015, 25, 5072–5077. [Google Scholar] [CrossRef]
- Zhou, M.; Han, Y.; Li, M.; Ye, G.; Peng, G. Two Inhibitors Against the 3C-Like Proteases of Swine Coronavirus and Feline Coronavirus. Virol. Sin. 2021, 36, 14211430. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Lanave, G.; Catella, C.; Lucente, M.S.; Sposato, A.; Mari, V.; Tempesta, M.; Martella, V.; Buonavoglia, A. ERDRP-0519 inhibits feline coronavirus in vitro. BMC Vet. Res. 2022, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, J.; Liu, X.; Wang, M.; Wang, X.; Jiang, P. Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet. Res. 2020, 51, 136. [Google Scholar] [CrossRef] [PubMed]
- Furbish, A.; Allinder, M.; Austin, G.; Tynan, B.; Byrd, E.; Gomez, I.P.; Peterson, Y. First analytical confirmation of drug-induced crystal nephropathy in felines caused by GS-441524, the active metabolite of Remdesivir. J. Pharm. Biomed. Anal. 2024, 247, 116248. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; De Franco, F.; Torquato, P.; Marinelli, R.; Cerra, B.; Ronchetti, R.; Schon, A.; Fallarino, F.; De Luca, A.; Bellezza, G.; et al. Garcinoic Acid Is a Natural and Selective Agonist of Pregnane X Receptor. J. Med. Chem. 2020, 63, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Arutyunova, E.; Khan, M.B.; Fischer, C.; Lu, J.; Lamer, T.; Vuong, W.; van Belkum, M.J.; McKay, R.T.; Tyrrell, D.L.; Vederas, J.C.; et al. N-Terminal Finger Stabilizes the S1 Pocket for the Reversible Feline Drug GC376 in the SARS-CoV-2 Mpro Dimer. J. Mol. Biol. 2021, 433, 167003. [Google Scholar] [CrossRef]
- Guarnieri, C.; Bertola, L.; Ferrari, L.; Quintavalla, C.; Corradi, A.; Di Lecce, R. Myocarditis in an FIP-Diseased Cat with FCoV M1058L Mutation: Clinical and Pathological Changes. Animals 2024, 14, 1673. [Google Scholar] [CrossRef]
- Theerawatanasirikul, S.; Kuo, C.J.; Phetcharat, N.; Lekcharoensuk, P. In silico and in vitro analysis of small molecules and natural compounds targeting the 3CL protease of feline infectious peritonitis virus. Antivir. Res. 2020, 174, 104697. [Google Scholar] [CrossRef]
- Takano, T.; Endoh, M.; Fukatsu, H.; Sakurada, H.; Doki, T.; Hohdatsu, T. The cholesterol transport inhibitor U18666A inhibits type I feline coronavirus infection. Antivir. Res. 2017, 145, 96–102. [Google Scholar] [CrossRef]
- Jose, S.; Devi, S.S.; Sajeev, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Alshammari, A.; Sethi, G.; Kunnumakkara, A.B. Repurposing FDA-approved drugs as FXR agonists: A structure based in silico pharmacological study. Biosci. Rep. 2023, 43, BSR20212791. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Zhong, X.; Hu, W.; Lin, Z.; Zhang, S.; Deng, H.; Lin, W. Prevalence of natural feline coronavirus infection in domestic cats in Fujian, China. Virol. J. 2024, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Boorla, V.S.; Maranas, C.D. Computational biophysical characterization of the SARS-CoV-2 spike protein binding with the ACE2 receptor and implications for infectivity. Comput. Struct. Biotechnol. J. 2020, 18, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Fath, M.K.; Naderi, M.; Hamzavi, H.; Ganji, M.; Shabani, S.; Noorabad ghahroodi, F.; Khalesi, B.; Pourzardosht, N.; Hashemi, Z.S.; Khalili, S. Molecular mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J. Trace Elem. Med. Biol. 2022, 73, 127044. [Google Scholar] [CrossRef] [PubMed]
- August, J.R. Feline Infectious Peritonitis: An Immune-Mediated Coronaviral Vasculitis. Vet. Clin. N. Am. Small Anim. Pract. 1984, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L. Feline Infectious Peritonitis: Still an Enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef]
- Attipa, C.; Gunn-Moore, D.; Mazeri, S.; Epaminondas, D.; Lyraki, M.; Hardas, A.; Loukaidou, S.; Gentil, M. Concerning feline infectious peritonitis outbreak in Cyprus. Vet. Rec. 2023, 192, 449–450. [Google Scholar] [CrossRef]
- Attipa, C.; Warr, A.; Epaminondas, D.; O’Shea, M.; Fletcher, S.; Malbon, A.; Lyraki, M.; Hammond, R.; Hardas, A.; Zanti, A.; et al. Emergence and spread of feline infection peritonitis due to a highly. Biorxiv 2023, 1–23. Available online: https://www.biorxiv.org/content/10.1101/2023.11.08.566182v1.full.pdf (accessed on 7 July 2024).
- Mavra, A.; Petrou, C.C.; Vlasiou, M.C. Ligand and Structure-Based Virtual Screening in Combination, to Evaluate Small Organic Molecules as Inhibitors for the XIAP Anti-Apoptotic Protein: The Xanthohumol Hypothesis. Molecules 2022, 27, 4825. [Google Scholar] [CrossRef]
- Verbrugghe, A.; Janssens, G.P.J.; Van de Velde, H.; Cox, E.; De Smet, S.; Vlaeminck, B.; Hesta, M. Failure of a dietary model to affect markers of inflammation in domestic cats. BMC Vet. Res. 2014, 10, 104. [Google Scholar] [CrossRef][Green Version]
- Candellone, A.; Badino, P.; Gianella, P.; Girolami, F.; Raviri, G.; Saettone, V.; Meineri, G. Evaluation of antioxidant supplementation on redox unbalance in hyperthyroid cats treated with methimazole: A blinded randomized controlled trial. Antioxidants 2020, 9, 15. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Haghjooy Javanmard, S.; Dana, N.; Rafiee, L.; Rostami, M. Novel Tocopherol Succinate-Polyoxomolybdate Bioconjugate as Potential Anti-Cancer Agent. J. Inorg. Organomet. Polym. 2021, 31, 3183–3195. [Google Scholar] [CrossRef]
- Broniatowski, M.; Flasiński, M.; Hąc-Wydro, K. Antagonistic effects of α-tocopherol and ursolic acid on model bacterial membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848 Pt A, 2154–2162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salih, M.; Omolo, C.A.; Devnarain, N.; Elrashedy, A.A.; Mocktar, C.; Soliman, M.E.S.; Govender, T. Supramolecular self-assembled drug delivery system (SADDs) of vancomycin and tocopherol succinate as an antibacterial agent: In vitro, in silico and in vivo evaluations. Pharm. Dev. Technol. 2020, 25, 1090–1108. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, A.S.; Al Masoudi, L.M.; Hamdi, H.; Abu Zaid, A. Chemical Composition and Antioxidant, Antiviral, Antifungal, Antibacterial and Anticancer Potentials of Opuntia ficus-indica Seed Oil. Molecules 2022, 27, 5453. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.-S.; Kang, H.W. Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: Investigation on anticancer, wound healing, and antibacterial effects. Colloids Surf. B Biointerfaces 2018, 172, 170–179. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar] [PubMed] [PubMed Central]
- Azzi, A. Molecular mechanism of α-tocopherol action. Free. Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef]
- Azzi, A.; Ricciarelli, R.; Zingg, J.-M. Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Kajiyama, T.; Arai, Y. Antioxidant activities of tocopherols. Polym. Degrad. Stab. 2001, 72, 303–311. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Saari Csallany, A. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Timmons, R.M.; Webb, C.B. Vitamin E supplementation fails to impact measures of oxidative stress or the anaemia of feline chronic kidney disease: A randomised, double-blinded placebo control study. Vet. Med. Sci. 2016, 2, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vlasiou, M.C.; Petrou, C.C.; Sarigiannis, Y.; Pafiti, K.S. Density functional theory studies and molecular docking on xanthohumol, 8-prenylnaringenin and their symmetric substitute diethanolamine derivatives as inhibitors for colon cancer-related proteins. Symmetry 2021, 13, 948. [Google Scholar] [CrossRef]
- Vlasiou, M.C.; Pati, K.S. Screening Possible Drug Molecules for COVID-19. The Example of Vanadium (III/IV/V) Complex Molecules with Computational Chemistry and Molecular Docking. Comput. Toxicol. 2021, 18, 100157. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, K.; Vlasiou, M.C. Metal-based complexes against SARS-CoV-2. BioMetals 2022, 35, 639–652. [Google Scholar] [CrossRef]
- Vlasiou, M.C. Computer-aided Drug Discovery Methods for Zoonoses. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2023, 22, 131–132. [Google Scholar] [CrossRef]
- El-Assaad, A.M.; Hamieh, T. SARS-CoV-2: Prediction of critical ionic amino acid mutations. Comput. Biol. Med. 2024, 178, 108688. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Y.; Qin, S.; Yang, Z. Exploring the mechanism of 6-Methoxydihydrosanguinarine in the treatment of lung adenocarcinoma based on network pharmacology, molecular docking and experimental investigation. BMC Complement. Med. Ther. 2024, 24, 202. [Google Scholar] [CrossRef]
- Zhou, C.; Yan, L.; Xu, J.; Hamezah, H.S.; Wang, T.; Du, F.; Tong, X.; Han, R. Phillyrin: An adipose triglyceride lipase inhibitor supported by molecular docking, dynamics simulation, and pharmacological validation. J. Mol. Model. 2024, 30, 68. [Google Scholar] [CrossRef]
- Mattson, N.M.; Chan, A.K.N.; Miyashita, K.; Mukhaleva, E.; Chang, W.H.; Yang, L.; Ma, N.; Wang, Y.; Pokharel, S.P.; Li, M.; et al. A novel class of inhibitors that disrupts the stability of integrin heterodimers identified by CRISPR-tiling-instructed genetic screens. Nat. Struct. Mol. Biol. 2024, 31, 465–475. [Google Scholar] [CrossRef]
- Ludwig, V.; da Costa Ludwig, Z.M.; Modesto, M.d.A.; Rocha, A.A. Binding energies and hydrogen bonds effects on DNA-cisplatin interactions: A DFT-xTB study. J. Mol. Model. 2024, 30, 187. [Google Scholar] [CrossRef]
- Khachatryan, H.; Matevosyan, M.; Harutyunyan, V.; Gevorgyan, S.; Shavina, A.; Tirosyan, I.; Gabrielyan, Y.; Ayvazyan, M.; Bozdaganyan, M.; Fakhar, Z.; et al. Computational evaluation and benchmark study of 342 crystallographic holo-structures of SARS-CoV-2 Mpro enzyme. Sci. Rep. 2024, 14, 14255. [Google Scholar] [CrossRef]
- Tendongmo, H.; Kogge, B.F.; Tamafo Fouegue, A.D.; Tasheh, S.N.; Tessa, C.B.N.; Ghogomu, J.N. Theoretical screening of N-[5′-methyl-3′-isoxasolyl]-N-[(E)-1-(-2-thiophene)] methylidene]amine and its isoxazole based derivatives as donor materials for bulk heterojunction organic solar cells: DFT and TD-DFT investigation. J. Mol. Model. 2024, 30, 176. [Google Scholar] [CrossRef] [PubMed]
- Vlasiou, M. In Silico Techniques Used for the Evaluation of Anticancer Metal-Based Compounds. Anti-Cancer Agents Med. Chem. 2022, 23, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Vlasiou, M. In Search of Antiviral Metal-Based Drugs. Open Med. Chem. J. 2021, 15, 30–31. [Google Scholar] [CrossRef]
- Vlasiou, M.C. Structural characterization of two novel, biological active chalcone derivatives, using density functional theory studies. Biointerface Res. Appl. Chem. 2021, 11, 15051–15057. [Google Scholar] [CrossRef]
- Vlasiou, M.C.; Pafiti, K.S. Spectroscopic evaluation of Zn (II) complexes with drug analogues: Interactions with BSA and the pH effect on the drug-Zn (II) system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118641. [Google Scholar] [CrossRef]
- Piacham, T.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Synthesis and computational investigation of molecularly imprinted nanospheres for selective recognition of alpha-tocopherol succinate. EXCLI J. 2013, 12, 701–718. [Google Scholar] [PubMed] [PubMed Central]
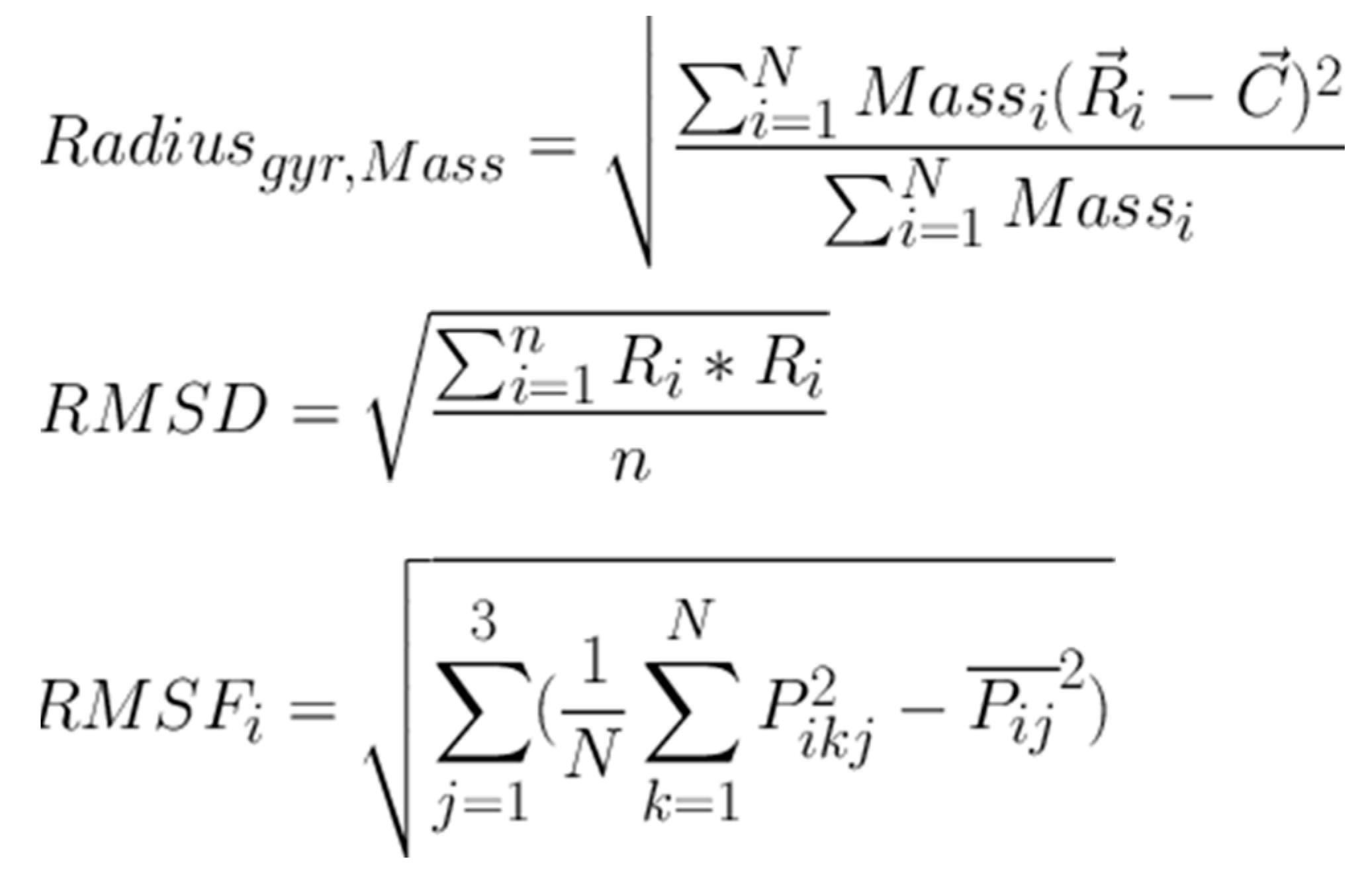
| Complex | Total Energy (KJ/mole) | Energy VDW (KJ/mole) | Energy Hbond (KJ/mole) | Amino Acid Residue Hbonds | Amino Acid Residue VDW Interactions |
|---|---|---|---|---|---|
| β-tocotrienol-FIPV-3CLpro | −94.92 | −91.42 | −3.5 | PHE 111 | MET 6, GLN 8, GLU 291, ARG 294 |
| δ-tocotrienol-FIPV-3CLpro | −98.23 | −86.32 | −11.9 | PHE 111, VAL 127 | GLY 122, GLN 8, PHE 111, ASN 112, GLU 291, ARG 294, VAL 299 |
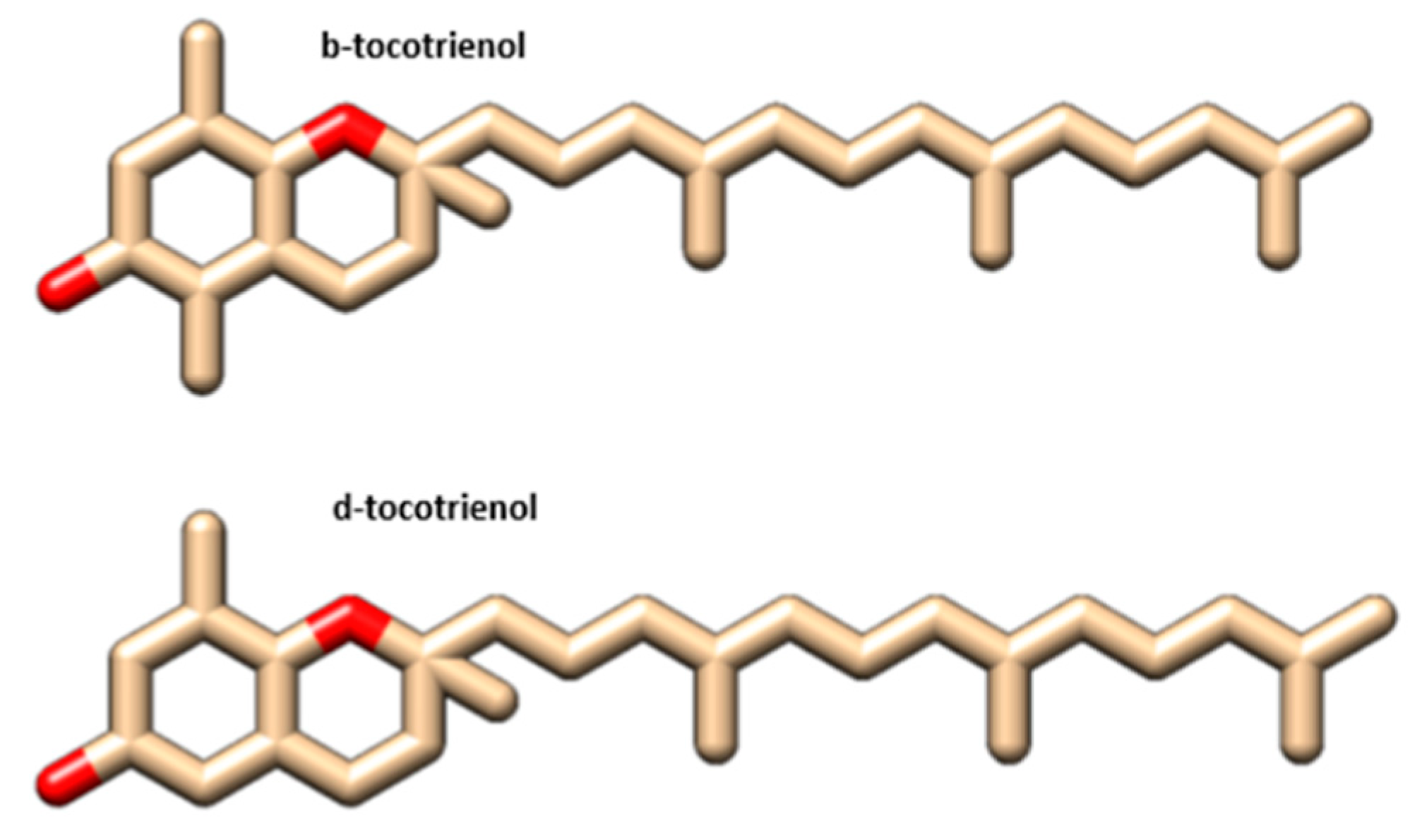
| Formula | C28H42O2 | C27H40O2 | Formula | C28H42O2 | C27H40O2 |
|---|---|---|---|---|---|
| Molecular weight | 410.63 g/mol | 396.61 g/mol | Total clearance | 0.814 log mL/min/kg | 0.847 log ml/min/kg |
| Num. of heavy atoms | 30 | 29 | Renal OCT2 substrate | No | No |
| Num. of aromatic heavy atoms | 6 | 6 | AMES toxicity | No | No |
| Num. of rotatable bonds | 9 | 9 | Max. tolerated dose | 0.45 log mg/kg/day | 0.729 log mg/kg/day |
| Num. of H-bond acceptors | 2 | 2 | hERG I Inhibitor | No | No |
| Num. of H-bond donors | 1 | 1 | hERG II Inhibitor | Yes | Yes |
| Molar refractivity | 132.88 | 127.2 | Oral rat acute toxicity (LD50) | 2.18 mol/kg | 1.945 mol/kg |
| Log Po/w | 5.14 | 5.35 | Oral rat chronic toxicity (LOAEL) | 2.967 log mg/kg_bw/day | 2.956 log mg/kg_bw/day |
| Log S | −7.57 | −7.26 | Hepatotoxicity | No | No |
| GI absorption | Low | Low | Skin sensitisation | No | No |
| BBB permeant | No | No | T. pyriformis toxicity | 1.127 log ug/L | 1.182 log ug/L |
| P-gp substrate | Yes | Yes | Minnow toxicity | −2.5 mM | −4.247 mM |
| CYP1A2 inhibitor | No | No | No | No | |
| CYP2C19 inhibitor | No | No | |||
| CYP2C9 inhibitor | No | No | |||
| CYP2D6 inhibitor | No | No | |||
| CYP3A4 inhibitor | No | Yes | |||
| Log Kp (skin permeation) | −2.46 cm/s | −2.63 cm/s | |||
| Lipinski | Yes, 1 violation: MLOGP > 4.15 | Yes, 1 violation: MLOGP > 4.15 | |||
| Bioavailability Score | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasiou, M.C.; Nikolaou, G.; Spanoudes, K.; Mavrides, D.E. β-Tocotrienol and δ-Tocotrienol as Additional Inhibitors of the Main Protease of Feline Infectious Peritonitis Virus: An In Silico Analysis. Vet. Sci. 2024, 11, 424. https://doi.org/10.3390/vetsci11090424
Vlasiou MC, Nikolaou G, Spanoudes K, Mavrides DE. β-Tocotrienol and δ-Tocotrienol as Additional Inhibitors of the Main Protease of Feline Infectious Peritonitis Virus: An In Silico Analysis. Veterinary Sciences. 2024; 11(9):424. https://doi.org/10.3390/vetsci11090424
Chicago/Turabian StyleVlasiou, Manos C., Georgios Nikolaou, Kyriakos Spanoudes, and Daphne E. Mavrides. 2024. "β-Tocotrienol and δ-Tocotrienol as Additional Inhibitors of the Main Protease of Feline Infectious Peritonitis Virus: An In Silico Analysis" Veterinary Sciences 11, no. 9: 424. https://doi.org/10.3390/vetsci11090424
APA StyleVlasiou, M. C., Nikolaou, G., Spanoudes, K., & Mavrides, D. E. (2024). β-Tocotrienol and δ-Tocotrienol as Additional Inhibitors of the Main Protease of Feline Infectious Peritonitis Virus: An In Silico Analysis. Veterinary Sciences, 11(9), 424. https://doi.org/10.3390/vetsci11090424







