Evaluation of Blood C Reactive Protein (CRP) and Neutrophil-to-Lymphocyte Ratio (NLR) Utility in Canine Epilepsy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Grouping
2.2. NLR Measurement
2.3. Serum CRP Measurment
2.4. Data Collection and Analysis
3. Results
3.1. Study Population
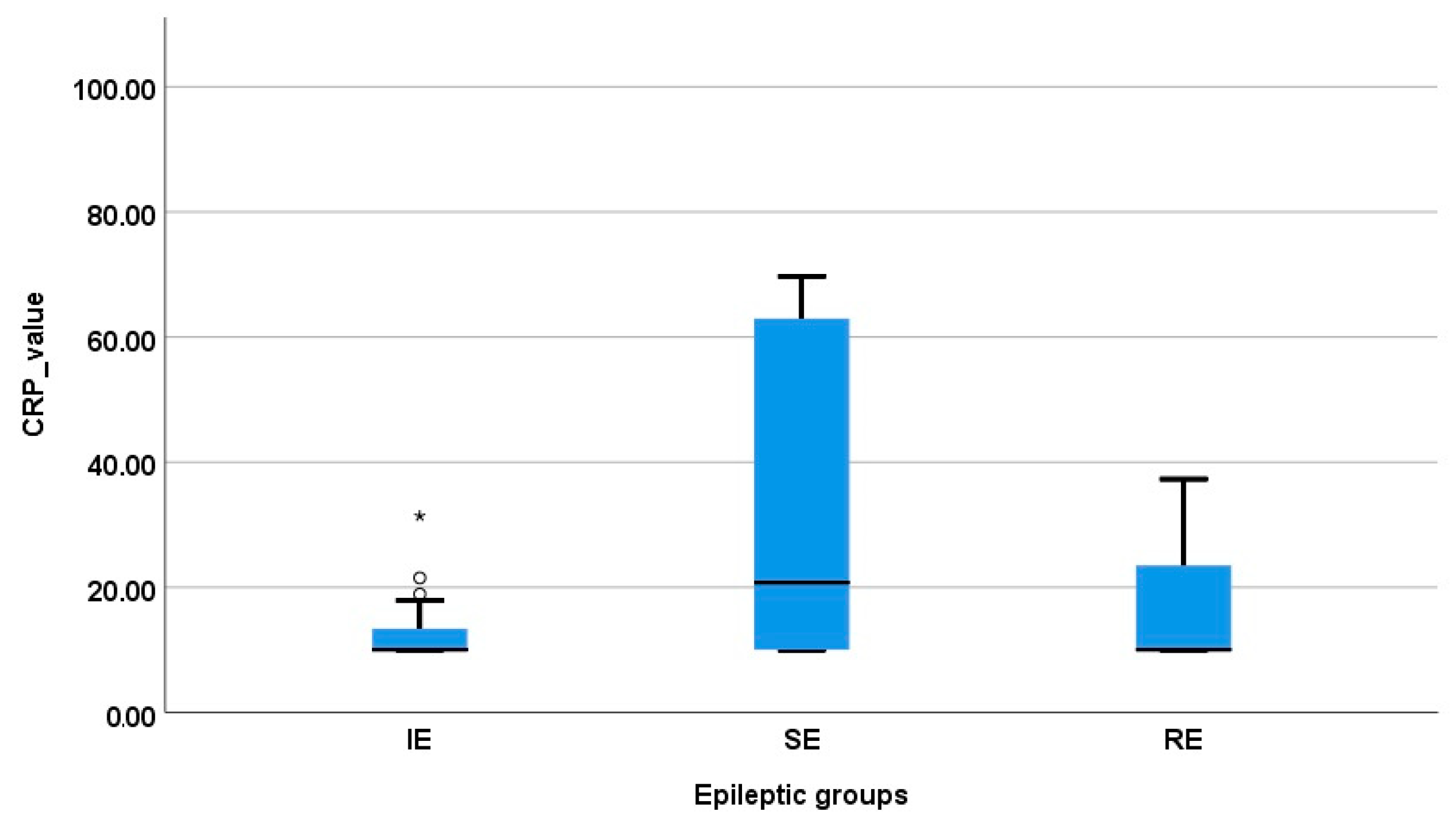
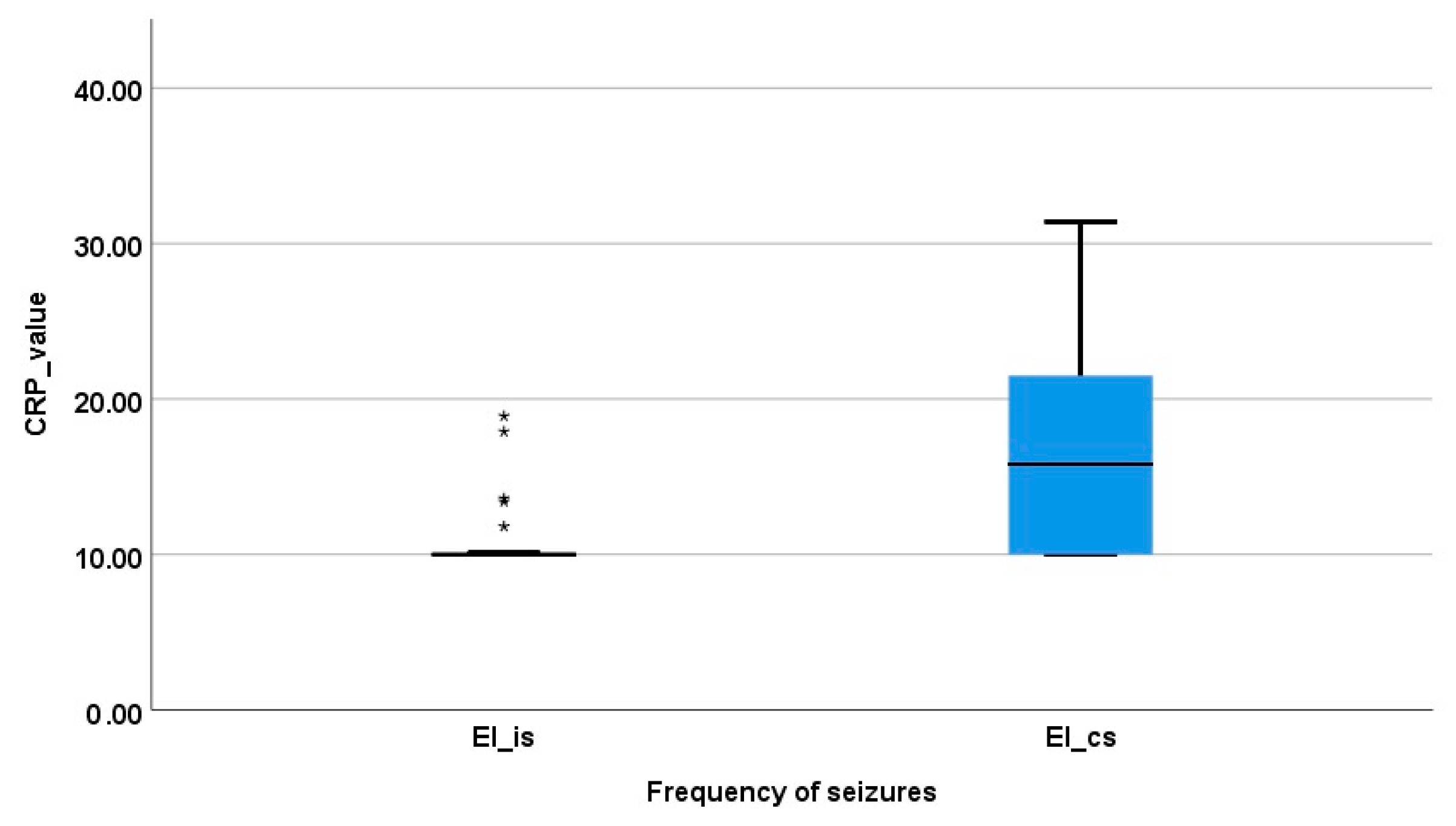
3.2. Serum CRP Concentration
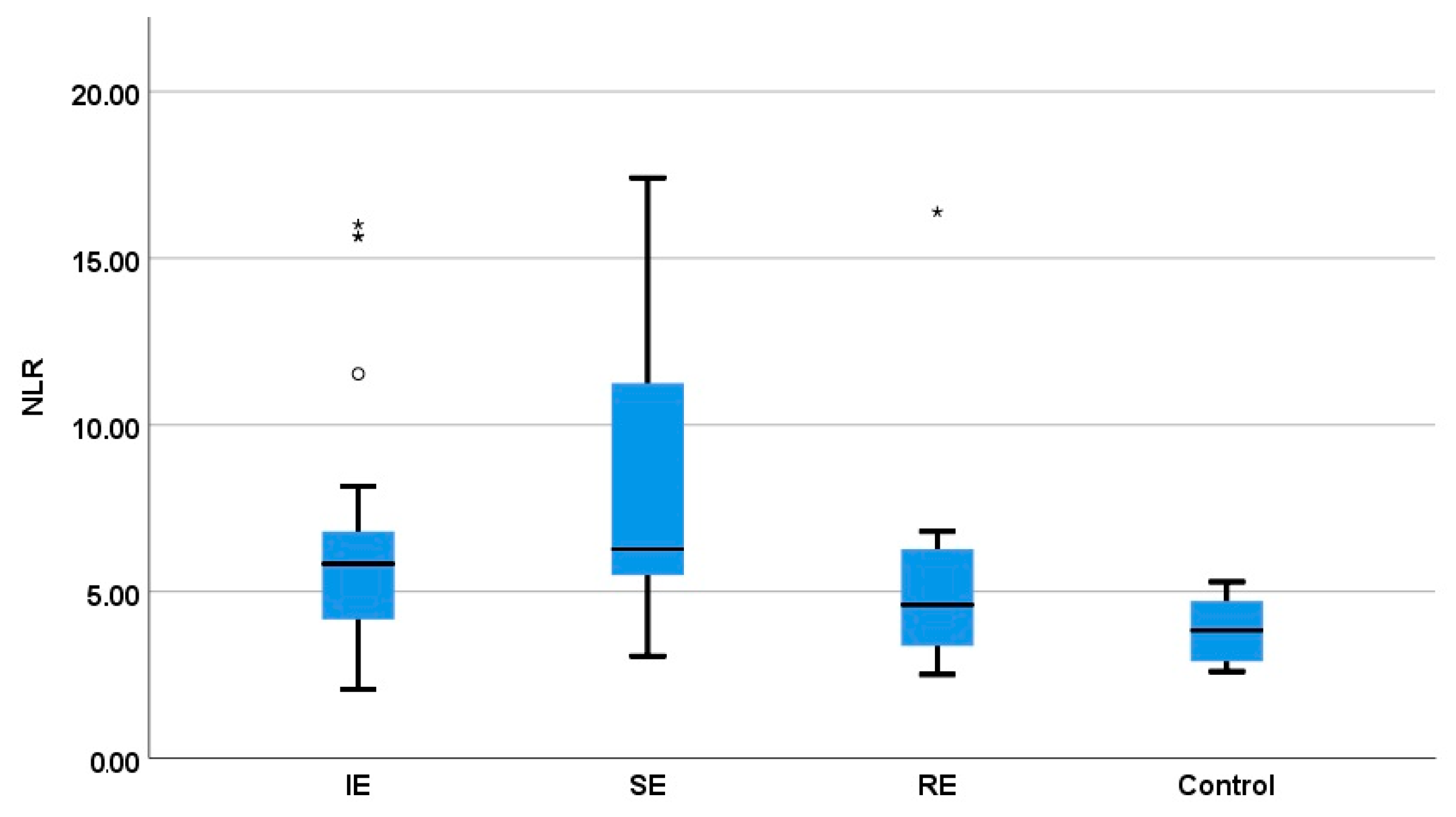
3.3. Comparison of the NLR between Healthy Dogs and Epileptic Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berendt, M.; Farquhar, R.G.; Mandigers, P.J.J.; Pakozdy, A.; Bhatti, S.F.M.; De Risio, L.; Fischer, A.; Long, S.; Matiasek, K.; Muñana, K.; et al. International Veterinary Epilepsy Task Force Consensus Report on Epilepsy Definition, Classification and Terminology in Companion Animals. BMC Vet. Res. 2015, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, A.; Maestro Saiz, I. Canine versus Human Epilepsy: Are We up to Date? J. Small Anim. Pract. 2016, 57, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Mofrad, A.M.E.; Mokarian, P.; Nourigheimasi, S.; Azarhomayoun, A.; Khanzadeh, S.; Habibzadeh, S.; Ghaedi, A. Neutrophil to Lymphocyte Ratio in Epilepsy: A Systematic Review. Mediat. Inflamm. 2022, 2022, 4973996. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The Role of Inflammation in the Development of Epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef]
- Hanael, E.; Veksler, R.; Friedman, A.; Bar-Klein, G.; Senatorov, V.V., Jr.; Kaufer, D.; Konstantin, L.; Elkin, M.; Chai, O.; Peery, D.; et al. Blood-Brain Barrier Dysfunction in Canine Epileptic Seizures Detected by Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Epilepsia 2019, 60, 1005–1016. [Google Scholar] [CrossRef]
- Hindenberg, S.; Bauer, N.; Moritz, A. Extremely High Canine C-Reactive Protein Concentrations > 100 Mg/l–Prevalence, Etiology and Prognostic Significance. BMC Vet. Res. 2020, 16, 147. [Google Scholar] [CrossRef]
- Olson, M.E.; Hornick, M.G.; Stefanski, A.; Albanna, H.R.; Gjoni, A.; Hall, G.D.; Hart, P.C.; Rajab, I.M.; Potempa, L.A. A Biofunctional Review of C-Reactive Protein (CRP) as a Mediator of Inflammatory and Immune Responses: Differentiating Pentameric and Modified CRP Isoform Effects. Front. Immunol. 2023, 14, 1264383. [Google Scholar] [CrossRef]
- de la Fuente, C.; Monreal, L.; Cerón, J.; Pastor, J.; Viu, J.; Añor, S. Fibrinolytic Activity in Cerebrospinal Fluid of Dogs with Different Neurological Disorders. J. Vet. Intern. Med. 2012, 26, 1365–1373. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Kim, S.; Kang, J.-H.; Park, J.; Yu, D. Alterations in Serum Protein Electrophoresis Profiles during the Acute Phase Response in Dogs with Acute Pancreatitis. Can. J. Vet. Res. 2020, 84, 74–78. [Google Scholar]
- Dik, I.; Hatipoglu, D.; Gulersoy, E. Comparison of Some Cytokines, Acute Phase Proteins and Citrulline Levels in Healthy and Canine Distemper Infected Dogs. J. Vet. Med. Sci. 2023, 85, 76–82. [Google Scholar] [CrossRef]
- Andersen-Ranberg, E.; Berendt, M.; Gredal, H. Biomarkers of Non-Infectious Inflammatory CNS Diseases in Dogs-Where Are We Now? Part I: Meningoencephalitis of Unknown Origin. Vet. J. 2021, 273, 105678. [Google Scholar] [CrossRef]
- Cray, C. Acute Phase Proteins in Animals. Prog. Mol. Biol. Transl. Sci. 2012, 105, 113–150. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.M.; Kidney, B.A.; Snead, E.C.R.; Myers, S.L.; Jackson, M.L. Serum C-Reactive Protein Concentrations in Healthy Miniature Schnauzer Dogs. Vet. Clin. Pathol. 2011, 40, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Segers, E.; Martlé, V.; Piepers, S.; Ham, L.V.; Bhatti, S.F.M. Serum C-Reactive Protein Concentrations in Dogs with Idiopathic Epilepsy. Vlaams Diergeneeskd. Tijdschr. 2017, 86, 79–83. [Google Scholar] [CrossRef]
- Mahon, E.K.; Williams, T.L.; Alves, L. Serum C-reactive Protein Concentrations in Dogs with Structural and Idiopathic Epilepsy. Vet. Rec. 2023, 193, e3211. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, J.; Miao, J.; Wen, H. Changes in Routine Blood Parameters of Patients with Generalized Tonic Clonic Seizure: A Retrospective Study. Neurosci. J. 2023, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mutz, M.; Boudreaux, B.; Kearney, M.; Stroda, K.; Gaunt, S.; Shiomitsu, K. Prognostic Value of Baseline Absolute Lymphocyte Concentration and Neutrophil/Lymphocyte Ratio in Dogs with Newly Diagnosed Multi-Centric Lymphoma. Vet. Comp. Oncol. 2015, 13, 337–347. [Google Scholar] [CrossRef]
- Macfarlane, L.; Morris, J.; Pratschke, K.; Mellor, D.; Scase, T.; Macfarlane, M.; Mclauchlan, G. Diagnostic Value of Neutrophil–Lymphocyte and Albumin–Globulin Ratios in Canine Soft Tissue Sarcoma. J. Small Anim. Pract. 2016, 57, 135–141. [Google Scholar] [CrossRef]
- Naito, E.; Yuki, M.; Hirano, T.; Kainuma, D.; Aoyama, R. Prognostic Utility of Preoperative Neutrophil–Lymphocyte Ratio in Cats with Malignant Mammary Tumors. Res. Vet. Sci. 2021, 135, 349–354. [Google Scholar] [CrossRef]
- Rejec, A.; Butinar, J.; Gawor, J.; Petelin, M. Evaluation of Complete Blood Count Indices (NLR, PLR, MPV/PLT, and PLCRi) in Healthy Dogs, Dogs With Periodontitis, and Dogs With Oropharyngeal Tumors as Potential Biomarkers of Systemic Inflammatory Response. J. Vet. Dent. 2017, 34, 231–240. [Google Scholar] [CrossRef]
- Chiti, L.E.; Ferrari, R.; Boracchi, P.; Morello, E.; Marconato, L.; Roccabianca, P.; Avallone, G.; Iussich, S.; Giordano, A.; Ferraris, E.I.; et al. Prognostic Impact of Clinical, Haematological, and Histopathological Variables in 102 Canine Cutaneous Perivascular Wall Tumours. Vet. Comp. Oncol. 2021, 19, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Romero-Romero, L.; Govezensky, T.; Rosales, C. Neutrophil to Lymphocyte Ratio and Principal Component Analysis Offer Prognostic Advantage for Dogs with Mammary Tumors. Front. Vet. Sci. 2023, 10, 1187271. [Google Scholar] [CrossRef]
- Jung, M.-J.; Kim, J.-H. Prognostic Efficacy of Complete Blood Count Indices for Assessing the Presence and the Progression of Myxomatous Mitral Valve Disease in Dogs. Animals 2023, 13, 2821. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.A.; Pizarro Del Valle, C.; Waugh, E.M.; French, A.; Ridyard, A.E. Retrospective Investigation of the Neutrophil-to-Lymphocyte Ratio in Dogs with Pneumonia: 49 Cases (2011–2016). J. Vet. Emerg. Crit. Care 2021, 31, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Blood Neutrophil-to-Lymphocyte Ratio (NLR) as a Diagnostic Marker in Dogs with Chronic Enteropathy. J. Vet. Diagn. Investig. 2021, 33, 516–527. [Google Scholar] [CrossRef]
- Benvenuti, E.; Pierini, A.; Gori, E.; Lucarelli, C.; Lubas, G.; Marchetti, V. Neutrophil-to-Lymphocyte Ratio (NLR) in Canine Inflammatory Bowel Disease (IBD). Vet. Sci. 2020, 7, 141. [Google Scholar] [CrossRef]
- Becher, A.; Acke, E.; Serrano, G.; Kiefer, I.; Alef, M.; von Bomhard, W.; Heilmann, R.M. Evaluation of the Blood Neutrophil-to-Lymphocyte Ratio (NLR) as a Diagnostic and Prognostic Biomarker in Dogs with Portosystemic Shunt. Vet. Sci. 2024, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, N.; Llewellyn, E.A.; Schaeffer, D.J. Utility and Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Dogs with Septic Peritonitis. J. Am. Anim. Hosp. Assoc. 2018, 54, 351–359. [Google Scholar] [CrossRef]
- Pierini, A.; Gori, E.; Lippi, I.; Ceccherini, G.; Lubas, G.; Marchetti, V. Neutrophil-to-Lymphocyte Ratio, Nucleated Red Blood Cells and Erythrocyte Abnormalities in Canine Systemic Inflammatory Response Syndrome. Res. Vet. Sci. 2019, 126, 150–154. [Google Scholar] [CrossRef]
- Durán-Galea, A.; Cristóbal-Verdejo, J.I.; Barrera-Chacón, R.; Macías-García, B.; González-Solís, M.A.; Nicolás-Barceló, P.; García-Ibáñez, A.B.; Ruíz-Tapia, P.; Duque-Carrasco, F.J. Clinical Importance of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Systemic Immune-Inflammation Index in Dogs with Leishmaniasis. Comp. Immunol. Microbiol. Infect. Dis. 2024, 107, 102148. [Google Scholar] [CrossRef]
- Park, J.; Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Kim, H.; Yang, M.-P.; Kang, B.-T. Evaluation of the Blood Neutrophil-to-Lymphocyte Ratio as a Biomarker for Meningoencephalitis of Unknown Etiology in Dogs. J. Vet. Intern. Med. 2022, 36, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- De Risio, L.; Bhatti, S.; Muñana, K.; Penderis, J.; Stein, V.; Tipold, A.; Berendt, M.; Farqhuar, R.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force Consensus Proposal: Diagnostic Approach to Epilepsy in Dogs. BMC Vet. Res. 2015, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Armaşu, M.; Packer, R.M.A.; Cook, S.; Solcan, G.; Volk, H.A. An Exploratory Study Using a Statistical Approach as a Platform for Clinical Reasoning in Canine Epilepsy. Vet. J. 2014, 202, 292–296. [Google Scholar] [CrossRef]
- Charalambous, M.; Muñana, K.; Patterson, E.E.; Platt, S.R.; Volk, H.A. ACVIM Consensus Statement on the Management of Status Epilepticus and Cluster Seizures in Dogs and Cats. J. Vet. Intern. Med. 2024, 38, 19–40. [Google Scholar] [CrossRef]
- Malin, K.; Witkowska-Piłaszewicz, O. C-Reactive Protein as a Diagnostic Marker in Dogs: A Review. Animals 2022, 12, 2888. [Google Scholar] [CrossRef] [PubMed]
- Galezowski, A.M.; Snead, E.C.R.; Kidney, B.A.; Jackson, M.L. C-Reactive Protein as a Prognostic Indicator in Dogs with Acute Abdomen Syndrome. J. Vet. Diagn. Investig. 2010, 22, 395–401. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Bell, R. Acute Phase Proteins: Biomarkers of Infection and Inflammation in Veterinary Medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Rozanski, E.; Wells, J. Validation of a Point-of-Care Assay for Serum Canine Pancreatic Lipase and C-Reactive Protein in the Clinical Setting. Available online: https://www.bionote.com/_files/ugd/934ee6_8a911d84d7794154a9b8a0048d115df5.pdf (accessed on 21 August 2024).
- Cavalerie, R.; Santos, A.; Leonardi, H.; Blond, L.; Beurlet, S.; Dumont, R.; Piazza, S. C-Reactive Protein Concentration Has Limited Value in the Diagnosis of Meningoencephalitis of Unknown Origin in Dogs. J. Am. Vet. Med. Assoc. 2024, 262, 1–8. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, M.; Ohno, K.; Koshino, A.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-Reactive Protein Concentration in Dogs with Various Diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef]
- Konstantinidis, A.O.; Patsikas, M.N.; Papazoglou, L.G.; Adamama-Moraitou, K.K. Congenital Portosystemic Shunts in Dogs and Cats: Classification, Pathophysiology, Clinical Presentation and Diagnosis. Vet. Sci. 2023, 10, 160. [Google Scholar] [CrossRef]
- Löscher, W. Dogs as a Natural Animal Model of Epilepsy. Front. Vet. Sci. 2022, 9, 928009. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.; Fischer, A.; Potschka, H.; Walker, M.C.; Raedt, R.; Vonck, K.; Boon, P.; Lohi, H.; Löscher, W.; Worrell, G.; et al. Translational Veterinary Epilepsy: A Win-Win Situation for Human and Veterinary Neurology. Vet. J. 2023, 293, 105956. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The Role of Inflammation in Epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Vezzani, A. Brain Inflammation and Seizures: Evolving Concepts and New Findings in the Last 2 Decades. Epilepsy Curr. 2020, 20, 40S–43S. [Google Scholar] [CrossRef]
- Ezer, R.; Toydemir, H.E.; Gökyiğit, F.M. The Relationship between Interleukin-6 and Epileptic Seizure. Ortadogu Tıp Derg. 2020, 12, 225–232. [Google Scholar] [CrossRef]
- Zattoni, M.; Mura, M.L.; Deprez, F.; Schwendener, R.A.; Engelhardt, B.; Frei, K.; Fritschy, J.-M. Brain Infiltration of Leukocytes Contributes to the Pathophysiology of Temporal Lobe Epilepsy. J. Neurosci. 2011, 31, 4037–4050. [Google Scholar] [CrossRef]
- Vega, J.L.; Komisaruk, B.R.; Stewart, M. Hiding in Plain Sight? A Review of Post-Convulsive Leukocyte Elevations. Front. Neurol. 2022, 13, 1021042. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.; Bhatti, S.F.M.; Volk, H.A.; Platt, S. Defining and Overcoming the Therapeutic Obstacles in Canine Refractory Status Epilepticus. Vet. J. 2022, 283–284, 105828. [Google Scholar] [CrossRef]
- Fischer, A.; Hülsmeyer, V.-I.; Munoz Schmieder, V.P.; Tipold, A.; Kornberg, M.; König, F.; Gesell, F.K.; Ahrend, L.K.; Volk, H.A.; Potschka, H. Cyclooxygenase-2 Inhibition as an Add-On Strategy in Drug Resistant Epilepsy-A Canine Translational Study. Front. Vet. Sci. 2022, 9, 864293. [Google Scholar] [CrossRef]
- von Rüden, E.-L.; Potschka, H.; Tipold, A.; Stein, V.M. The Role of Neuroinflammation in Canine Epilepsy. Vet. J. 2023, 298–299, 106014. [Google Scholar] [CrossRef]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and Immunoregulation in Glioblastoma and Brain Metastases: Recent Developments in Imaging Approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef]
- Sloma, E.A.; Creneti, C.T.; Erb, H.N.; Miller, A.D. Characterization of Inflammatory Changes Associated with Canine Oligodendroglioma. J. Comp. Pathol. 2015, 153, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wanamaker, M.W.; Vernau, K.M.; Taylor, S.L.; Cissell, D.D.; Abdelhafez, Y.G.; Zwingenberger, A.L. Classification of Neoplastic and Inflammatory Brain Disease Using Magnetic Resonance Imaging Texture Analysis in 119 Dogs. Vet. Radiol. Ultrasound 2021, 62, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, K.; Piedavent, M.; Bauer, S.; Neumann, J.T.; Friese, M.A. Neutrophils Amplify Autoimmune Central Nervous System Infiltrates by Maturing Local APCs. J. Immunol. 2013, 191, 4531–4539. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.R.; Wagner, C.A.; Goverman, J.M. The Contribution of Neutrophils to CNS Autoimmunity. Clin. Immunol. 2018, 189, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, A.; Hiroki, C.H.; da Fonseca, D.M.; Birbrair, A.; Ferreira, R.G.; Bassi, G.S.; Fonseca, M.D.; Kusuda, R.; Cebinelli, G.C.M.; da Silva, K.P.; et al. The Role of Neutrophils in Neuro-Immune Modulation. Pharmacol. Res. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Nowakowska, M.; Üçal, M.; Charalambous, M.; Bhatti, S.F.M.; Denison, T.; Meller, S.; Worrell, G.A.; Potschka, H.; Volk, H.A. Neurostimulation as a Method of Treatment and a Preventive Measure in Canine Drug-Resistant Epilepsy: Current State and Future Prospects. Front. Vet. Sci. 2022, 9, 889561. [Google Scholar] [CrossRef]
- Vega, J.L.; Emmady, P.; Roels, C.; Conforti, J.; Ramirez, C.; Dorak, M.T. The Magnitude of Postconvulsive Leukocytosis Mirrors the Severity of Periconvulsive Respiratory Compromise: A Single Center Retrospective Study. Front. Neurol. 2019, 10, 1291. [Google Scholar] [CrossRef]
- Lim, H.-K.; Bae, S.; Han, K.; Kang, B.-M.; Jeong, Y.; Kim, S.-G.; Suh, M. Seizure-Induced Neutrophil Adhesion in Brain Capillaries Leads to a Decrease in Postictal Cerebral Blood Flow. iScience 2023, 26, 106655. [Google Scholar] [CrossRef]
| Percentage of Dogs with: | ||||
|---|---|---|---|---|
| CRP Value | NLR | |||
| Normal | Equivocal/Abnormal | Normal | Abnormal | |
| IEis | 95.70% | 4.30% | 30% | 70% |
| IEcs | 66.66% | 33.33% | - | 100% |
| SE | 50% | 50% | 5% | 95% |
| RE | 60% | 40% | 30% | 70% |
| IE Isolated Seizures (n = 23) | IE Cluster Epilepticus (n = 6) | p Value | |
|---|---|---|---|
| Neutrophil count (× 109/L), Median ± standard error (Range) | 8.76 ± 0.66 (3.7–17.54) | 16.02 ± 0.94 (13.18–19.36) | 0.001 |
| Lymphocyte count (× 109/L), Median ± standard error (Range) | 1.71± 0.14 (0.75–3.04) | 2.10 ± 0.29 (1.21–3.04) | 0.196 |
| NLR, Median ± standard error (Range) | 5.92 ± 0.71 (2.06–15.64) | 8.69 ± 1.74 (5.71–16.01) | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despa, A.; Musteata, M.; Solcan, G. Evaluation of Blood C Reactive Protein (CRP) and Neutrophil-to-Lymphocyte Ratio (NLR) Utility in Canine Epilepsy. Vet. Sci. 2024, 11, 408. https://doi.org/10.3390/vetsci11090408
Despa A, Musteata M, Solcan G. Evaluation of Blood C Reactive Protein (CRP) and Neutrophil-to-Lymphocyte Ratio (NLR) Utility in Canine Epilepsy. Veterinary Sciences. 2024; 11(9):408. https://doi.org/10.3390/vetsci11090408
Chicago/Turabian StyleDespa, Andreea, Mihai Musteata, and Gheorghe Solcan. 2024. "Evaluation of Blood C Reactive Protein (CRP) and Neutrophil-to-Lymphocyte Ratio (NLR) Utility in Canine Epilepsy" Veterinary Sciences 11, no. 9: 408. https://doi.org/10.3390/vetsci11090408
APA StyleDespa, A., Musteata, M., & Solcan, G. (2024). Evaluation of Blood C Reactive Protein (CRP) and Neutrophil-to-Lymphocyte Ratio (NLR) Utility in Canine Epilepsy. Veterinary Sciences, 11(9), 408. https://doi.org/10.3390/vetsci11090408






