Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
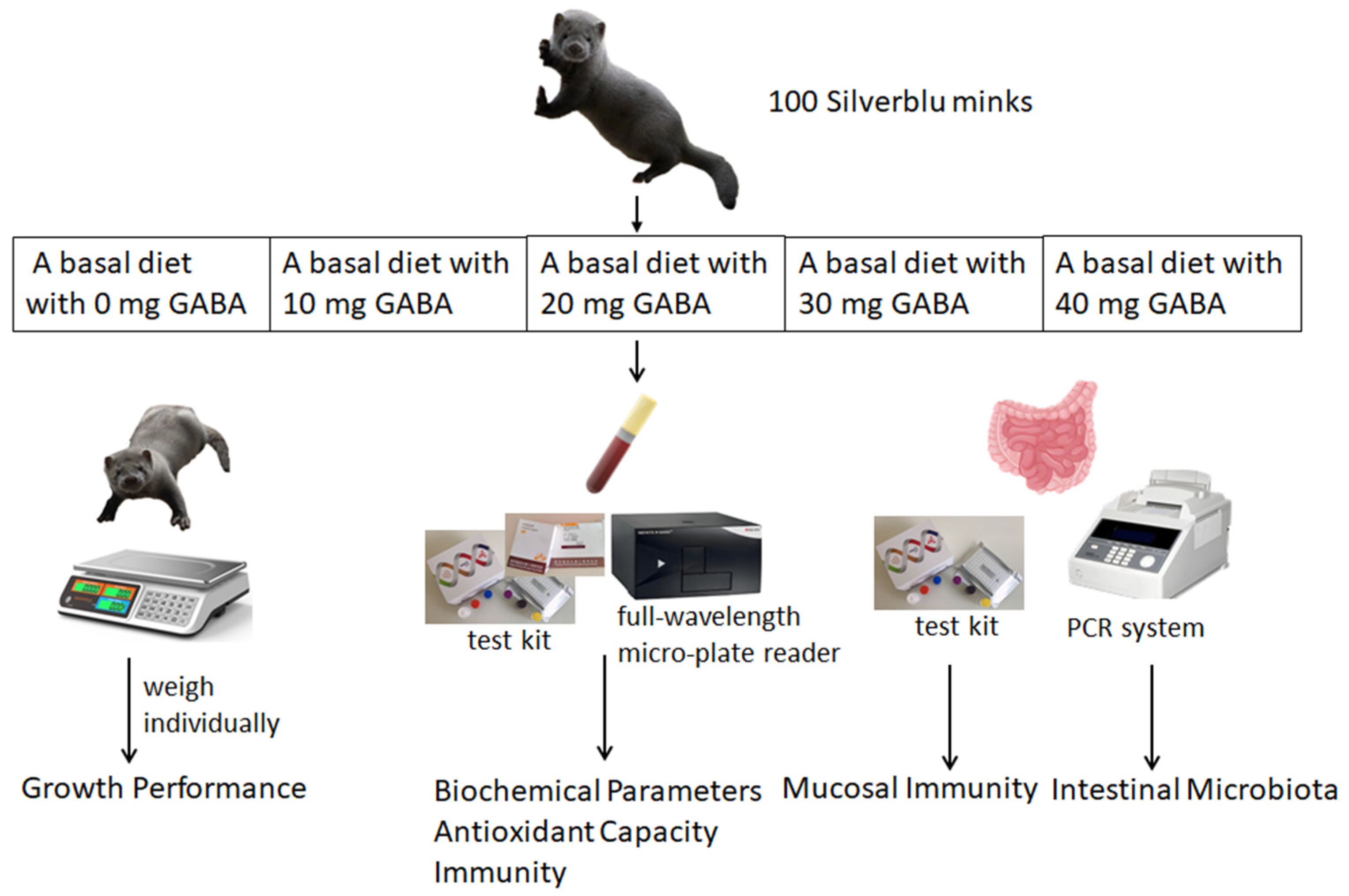
2.1. Experimental Animals, Design and Management
2.2. Sampling and Measurements
2.3. Biochemical Parameters in Serum
2.4. Immunity Analysis
2.5. Antioxidant Capacity Analysis
2.6. Intestinal Flora Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Biochemical Parameters in Serum
3.3. Immune Function
3.3.1. Serum Immune Indexes
3.3.2. Mucosal Immune Indexes
3.4. Antioxidant Capacity
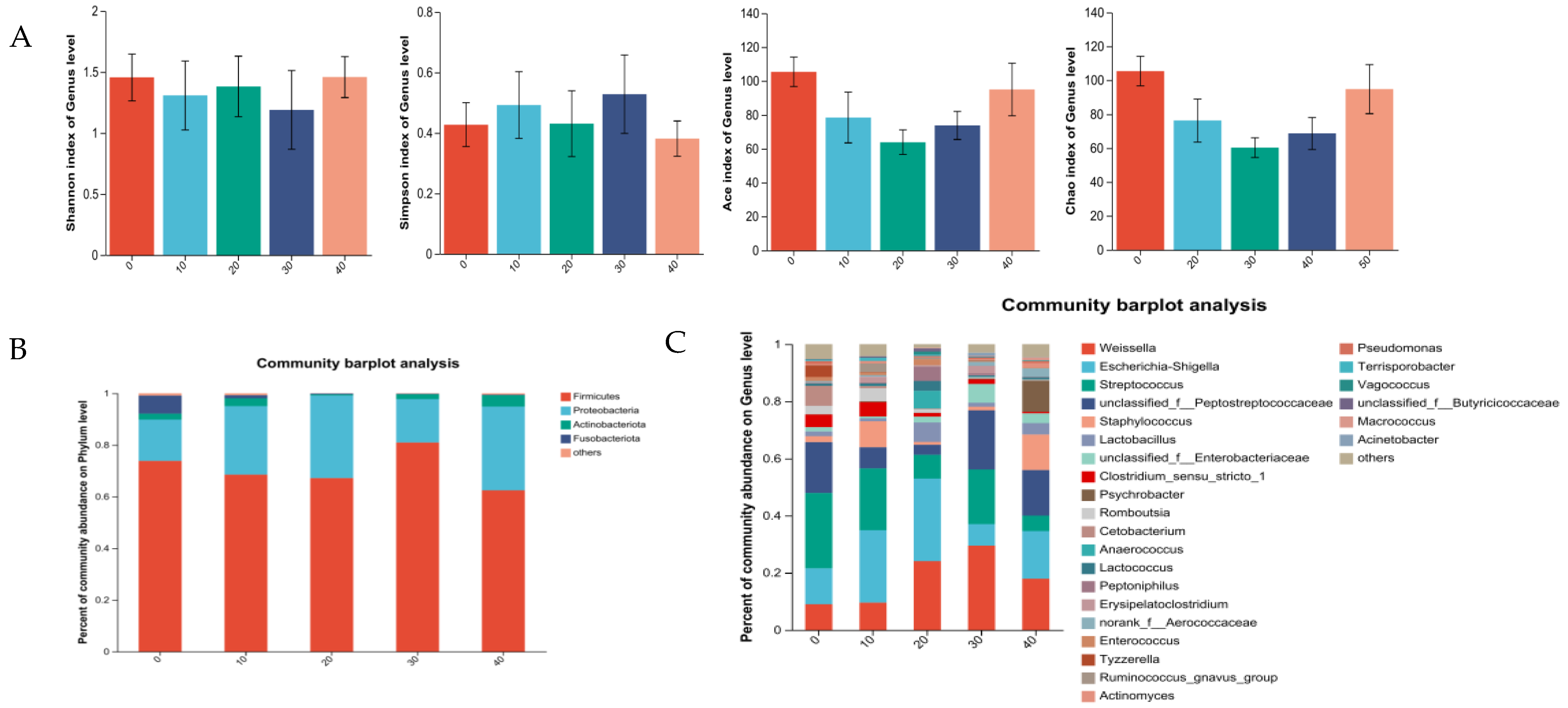
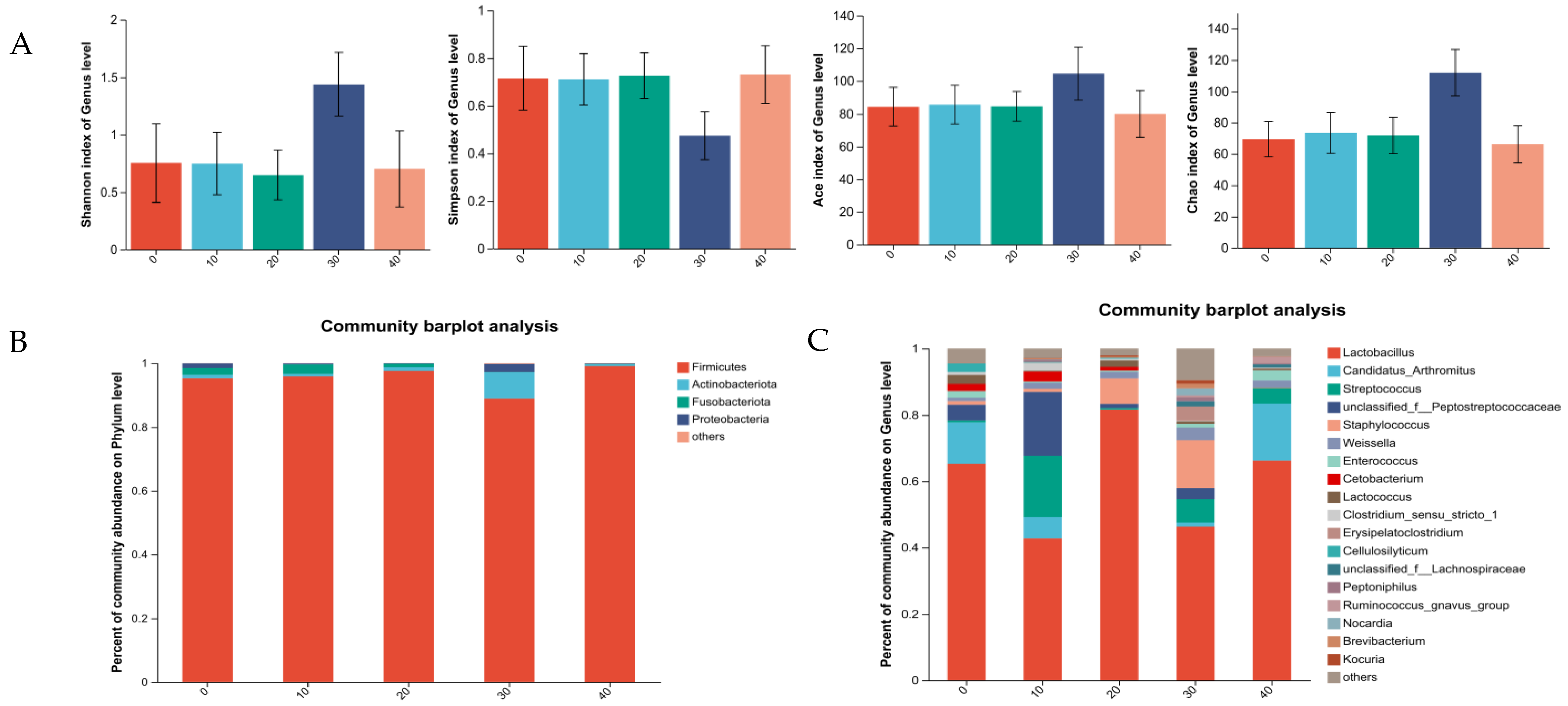
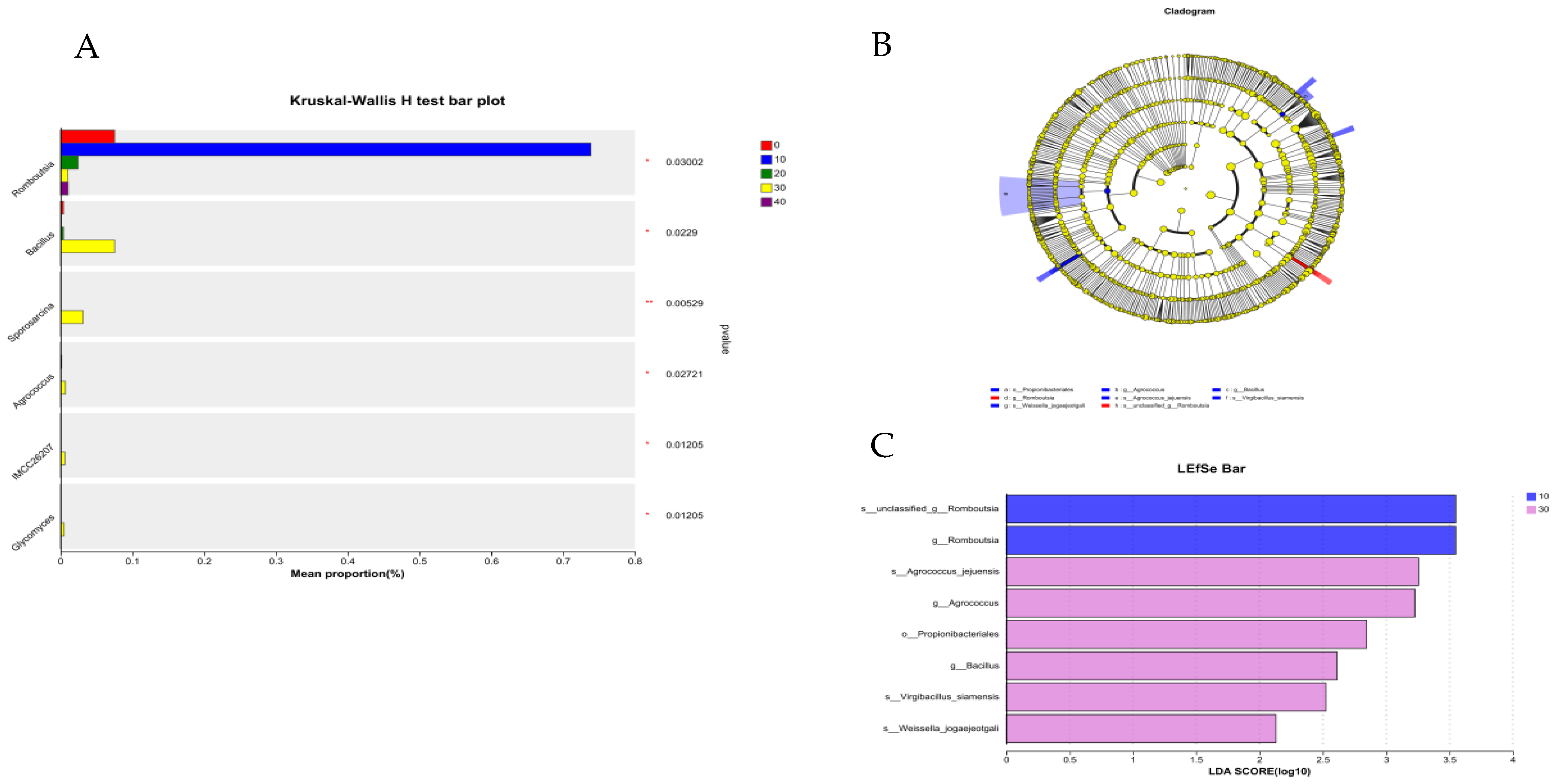
3.5. Intestinal Microbiota
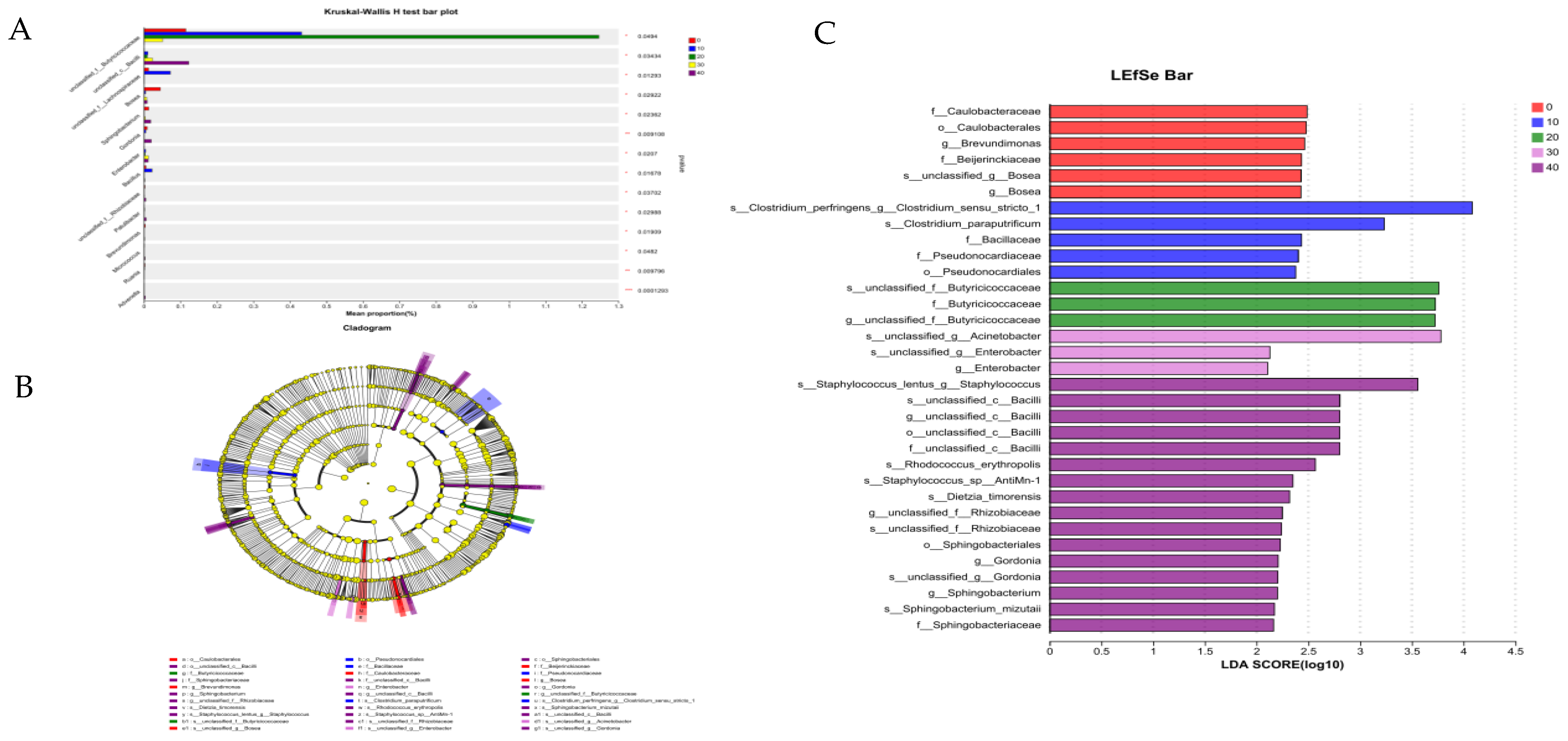
3.5.1. Intestine Microbiota of Male Minks
3.5.2. Intestine Microbiota of Female Minks
3.5.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Zou, X.T.; Li, H.; Dong, X.Y.; Zhao, W. Effect of dietary γ-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim. Sci. J. 2012, 83, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.; Vo, S.T.; Torres, D.M.; Falqué López, E. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.M.; Shen, L.M.; Wu, X.R. Advances in enzymology of gamma-aminobutyric acid metabolism in higher plants (summarize). J. China Agric. Univ. 1996, 1, 29–33. [Google Scholar]
- Narayan, V.S.; Nair, P.M. The 4-aminobutyrate shunt in solanum tuberosum. Phytochemistry 1986, 25, 997–1001. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Wang, C.; Liu, J.; Ferguson, J. Effects of rumen-protected gamma-aminobutyric acid on feed intake, performance and antioxidative status in transition cows. Livest. Sci. 2013, 153, 66–72. [Google Scholar] [CrossRef]
- Guang, Z.; Dan, S.; Qiang, W.; Haibing, T.; Shourong, S. Effects of dietary supplemented of γ-amino butyric acid on growth performance, blood biochemical indices and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment. Ital. J. Anim. Sci. 2020, 19, 1431–1438. [Google Scholar]
- Mokhtar, F.; Shahryar, S.; Majid, K. Effect of melatonin on oxidative stress, inflammation cytokines, biochemical parameters and growth performance in broiler chicken under induced stress by dexamethasone. Acta Agric. Scand. Sect. A—Anim. Sci. 2023, 72, 149–157. [Google Scholar]
- Dong, S.; Deng, W.; Lei, X.; Wang, Z.; Wen, F.; Ma, B. Effects of Heat Stress on the Physicochemical Properties and Production Performance of Animals. J. Henan Univ. Sci. Technol. (Agric. Ed.) 2003, 1, 59–62+66. (In Chinese) [Google Scholar]
- Ji, J.; Ren, H.; Zhang, T.; Zhao, W.; Li, W. Effects of Glutamine on Growth Performance, Serum Biochemical Indexes and Antioxidant Capacity of Minks in Summer. Chin. J. Anim. Nutr. 2018, 30, 1415–1422. (In Chinese) [Google Scholar]
- Wang, Z.; Niu, J.; Wang, B. How to Prevent and Treat Heatstroke in Mink. Spec. Econ. Anim. Plants 2017, 20, 10. (In Chinese) [Google Scholar]
- Xiang, Q. The Impact of Sudden Temperature Changes on Mink Appetite. Fur. Anim. Husb. 1983, 4, 18. (In Chinese) [Google Scholar]
- Li, Y.; Zhen, S.; Cao, L.; Sun, F.; Wang, L. Effects ofLactobacillus plantarumPostbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals 2023, 13, 2958. [Google Scholar] [CrossRef] [PubMed]
- LI, B.; Zhang, K.; Li, C.; Wang, X.; Chen, Y.; Yang, Y. Characterization and Comparison of Microbiota in the Gastrointestinal Tracts of the Goat (Capra hircus) During Preweaning Development. Front. Microbiol. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, B.; Wang, L.; Sun, Q.; Xi, Y.; Yuan, Y.; Wang, L.; Fu, C.; LI, S. Effects of enzymatic hydrolysis of artemisia annua combined with Bacillus licheniformis on growth performance and cecal flora of broilers. Chin. J. Org. Chem. 2021, 33, 3810–3820. [Google Scholar]
- Zhang, S.; Zhao, J.; Hu, J.; He, H.; Wei, Y.; Ji, L.; Ma, X. Gama-aminobutyric acid (GABA) alleviates hepatic inflammation via GABA receptors/TLR4/NF-κB pathways in growing-finishing pigs generated by super-multiparous sows. Anim. Nutr. 2022, 9, 280–290. [Google Scholar] [CrossRef]
- Jonaidi, H.; Noori, Z. Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012, 198, 827–832. [Google Scholar] [CrossRef]
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: A meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 45019–45028. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Li, J.; Wang, Y. The physiological function of γ-aminobutyric acid and the general research about γ-aminobutyric acid. J. Guangxi Univ. 2007, 32, 464–466. [Google Scholar]
- Zhang, Q.; Zhang, S.; Cong, G.; Zhang, Y.; Madsen, M.H.; Tan, B.; Shi, S. Effects of Soy Protein Concentrate in Starter Phase Diet on Growth Performance, Blood Biochemical Indices, Carcass Traits, Immune Organ Indices and Meat Quality of Broilers. Animals 2021, 11, 281. [Google Scholar] [CrossRef]
- Liu, X. Identification of serum m-AST in alcoholic liver disease and non-alcoholic liver disease. Chin. J. Lab. Diagn. 2008, 12, 254. [Google Scholar]
- Cheng, J.; Zheng, N.; Sun, X.; Li, S.; Wang, J.; Zhang, Y. Feeding rumen-protected gamma-aminobutyric acid enhances the immune response and antioxidant status of heat-stressed lactating dairy cows. J. Therm. Biol. 2016, 60, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tand, J.; Chen, Z. The protective effect of γ-aminobutyric acid on the development of immune function in chickens under heat stress. J. Anim. Physiol. Anim. Nutr. 2016, 4, 768–777. [Google Scholar]
- Schroeder, W.H.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immun. 2010, 2, 41–52. [Google Scholar] [CrossRef]
- Weigert, N.; Schepp, W.; Haller, A.; Schusdziarra, V. Regulation of Gastrin, Somatostatin and Bombesin Release from the Isolated Rat Stomach by Exogenous and Endogenous Gamma-Aminobutyric Acid. Digestion 1998, 59, 16–25. [Google Scholar] [CrossRef]
- Herman, J.P.; Mueller, N.K.; Figueiredo, H. Role of GABA and Glutamate Circuitry in Hypothalamo-Pituitary-Adrenocortical Stress Integration. Ann. N. Y. Acad. Sci. 2004, 1018, 35–45. [Google Scholar] [CrossRef]
- Abdou, M.A.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-Aminobutyric acid (GABA) administration in humans. BioFactors 2006, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, X.; Xia, Y.; Wang, M.; Liao, S.; Li, F.; Yin, J.; Ren, W.; Tan, B.; Yin, Y. Effects of dietary gamma-aminobutyric acid supplementation on amino acid profile, intestinal immunity, and microbiota in ETEC-challenged piglets. Food Funct. 2020, 11, 9067–9074. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, B.; Xia, Y.; Liao, S.; Wang, M.; Yin, J.; Wang, J.; Xiao, H.; Qi, M.; Bin, P.; et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct. 2019, 10, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, A.F.; Pierre, J.F.; Kudsk, K.A. JAK-STAT and intestinal mucosal immunology. Gut Microbes 2014, 5, 1. [Google Scholar] [CrossRef]
- Ren, W.; Wang, K.; Yin, J.; Chen, S.; Liu, G.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Glutamine-Induced Secretion of Intestinal Secretory Immunoglobulin A: A Mechanistic Perspective. Front. Immunol. 2016, 11, 503. [Google Scholar] [CrossRef]
- Cerutti, A.; Rescigno, M. The Biology of Intestinal Immunoglobulin A Responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Tanga, F.; Nutile-Mcmenemy, N.; Deleo, J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA 2005, 16, 5856–5861. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Ehrentraut, S.F.; Wilson, K.E.; Kelly, C.J.; Glover, L.E.; Collins, C.B.; Bayless, A.J.; Saeedi, B.; Dobrinskikh, E.; et al. IFN-γ-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 2014, 3, 1267–1276. [Google Scholar] [CrossRef]
- Lorén, V.; Cabré, E.; Ojanguren, I.; Domènech, E.; Pedrosa, E.; Garcia-Jaraquemada, A.; Mañosa, M.; Manyé, J. Interleukin-10 enhances the intestinal epithelial barrier in the presence of corticosteroids through p38 MAPK activity in Caco-2 monolayers: A possible mechanism for steroid responsiveness in ulcerative colitis. PLoS ONE 2015, 10, 0130921. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.; Kominsky, D.; Colgan, S.P. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Bjurstöm, H.; Wang, J.; Ericsson, I.; Bengtsson, M.; Liu, Y.; Kumar-Mendu, S.; Issazadeh-Navikas, S.; Birnir, B. GABA, a natural immunomodulator of T lymphocytes. J. Neuroimmunol. 2008, 205, 44–50. [Google Scholar] [CrossRef]
- Jin, Z.; Mendu, S.K.; Birnir, B. GABA is an effective immunomodulatory molecule. Amino Acids 2013, 45, 87–94. [Google Scholar] [CrossRef]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef]
- Bi, C.; Yin, J.; Yang, W.; Shi, B.; Shan, A. Effects of dietary γ-aminobutyric acid supplementation on antioxidant status, blood hormones and meat quality in growing-finishing pigs undergoing transport stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 590–596. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Sun, Y.; Xie, Q. Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of wenchang chicken induced by heat stress. J. Anim. Plant Sci. 2013, 23, 1634–1641. [Google Scholar]
- Üner, N.; Oruc, E.; Sevgiler, Y.; Sahin, N.; Durmaz, H.; Usta, D. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2006, 21, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Bahl, M.I.; Hammer, A.S.; Clausen, T.; Jakobsen, A.; Skov, S.; Andresen, L. The gastrointestinal tract of farmed mink (Neovison vison) maintains a diverse mucosa-associated microbiota following a 3-day fasting period. MicrobiologyOpen 2017, 6, e00434. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhan, Q.; Shi, H.; Li, Y.; Li, D.; Li, Y.; Yang, X. Dietary oregano aqueous extract improves growth performance and intestinal health of broilers through modulating gut microbial compositions. J. Anim. Sci. Biotechnol. 2023, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Francesca, F.; Marco, M.; Michela, V.; Antonella, G.; Rita, B. Probiotic Potential and Safety Assessment of Type Strains of Weissella and Periweissella Species. Microbiol. Spectr. 2023, 11, e03047-22. [Google Scholar]
- Willems, A.; Collins, M.D. Phylogenetic relationships of the genera Acetobacterium and Eubacterium sensu stricto and reclassification of Eubacterium alactolyticum as Pseudoramibacter alactolyticus gen. nov. comb. nov. Int. J. Syst. Bacteriol. 1996, 46, 1083–1087. [Google Scholar] [CrossRef]
- Mu, X.; Song, T.; Zhou, J.; Sun, J. Identification of infection caused by prevotella buccae and at opobium parvulum. Chin. J. Nosocomiol. 2018, 24, 3713–3717. [Google Scholar]
- Tan, F.; Liu, G.; Lau, S.A.; Jaafar, M.H.; Park, Y.; Azzam, G.; Li, Y.; Liong, M. Lactobacillus probiotics improved the gut microbiota profile of a Drosophila melanogaster Alzheimer’s disease model and alleviated neurodegeneration in the eye. Benef. Microbes 2020, 11, 79–89. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, D.; Wu, X.; Feng, Y.; Ni, Y. Dietary γ-Aminobutyric Acid Supplementation Inhibits High-Fat Diet-Induced Hepatic Steatosis via Modulating Gut Microbiota in Broilers. Microorganisms 2022, 10, 1281. [Google Scholar] [CrossRef]
- Myriam, F.; Daniel, S.; Helene, R.; Thierry, V.; Daphne, S.; Didier, G.; Jerome, G.; Jacques, J.; Norbert, R. Integral membrane proteins of the chloroplast envelope: Identification and subcellular localization of new transporters. Proc. Natl. Acad. Sci. USA 2002, 17, 11487–11492. [Google Scholar]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 2017, 5, 3019. [Google Scholar] [CrossRef] [PubMed]
- Esakkiraj, P.; Bharathi, C.; Ayyanna, R.; Jha, N.; Panigrahi, A. Functional and molecular characterization of a cold-active lipase from Psychrobacter celer PU3 with potential a*ntibiofilm property. Int. J. Biol. Macromol. 2022, 211, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Chen, J.; Liang, S.; Chen, H.; Liu, S. Effects of the Probiotic Psychrobacter sp. B6 on the Growth, Digestive Enzymes, Antioxidant Capacity, Immunity, and Resistance of Exopalaemon carinicauda to Aeromonas hydrophila. Probiotics Antimicrob. Proteins 2022, 15, 813–820. [Google Scholar] [CrossRef]

| Items | Content (%) |
|---|---|
| Sea fishes | 30.00 |
| Unhatched fertilized egg | 24.00 |
| Chicken ribs | 10.00 |
| Chicken head | 15.00 |
| Extruded corn | 7.00 |
| Chicken livers | 5.00 |
| Wheat bran | 3.00 |
| Porcine spray-dried blood cells | 2.00 |
| Soybean meal | 3.00 |
| Premix 1 | 1.00 |
| Total | 100.00 |
| Nutrient levels | |
| ME (MJ/kg) 2 | 16.59 |
| Ether extract | 20.30 |
| Crude protein | 33.55 |
| Calcium | 0.90 |
| Phosphorus | 0.97 |
| GABA Supplemental Levels/(mg/kg) | SEM | Gender | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | Male | Female | PGABA | PGender | PGABA×Gender | |||
| Wt/g | ||||||||||||
| Initial (wk 0) | 1130.50 | 1100.15 | 1132.12 | 1148.42 | 1124.14 | 32.59 | 1308.53 | 945.60 | 19.86 | 0.880 | <0.001 | 0.967 |
| Final (wk 8) | 1835.25 | 1837.25 | 1908.74 | 1969.01 | 1936.89 | 55.55 | 2383.21 | 1411.64 | 33.85 | 0.327 | <0.001 | 0.225 |
| ADG, g | 12.59 | 13.16 | 13.87 | 14.65 | 14.51 | 0.75 | 19.19 | 8.32 | 0.49 | 0.288 | <0.001 | 0.100 |
| ADFI, g | 271.36 b | 283.55 a | 294.58 a | 288.64 a | 294.14 a | 5.80 | 349.50 | 223.41 | 3.75 | 0.044 | <0.001 | 0.033 |
| F:G | 23.92 | 24.94 | 23.88 | 21.96 | 24.01 | 1.26 | 19.07 | 28.42 | 0.82 | 0.640 | <0.001 | 0.407 |
| GABA Supplemental Levels/(mg/kg) | SEM | Gender | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | Male | Female | PGABA | PGender | PGABA×Gender | |||
| ALB, g/L | 46.35 | 46.23 | 51.48 | 51.00 | 49.69 | 1.82 | 47.36 | 50.54 | 1.15 | 0.131 | 0.058 | 0.780 |
| BUN, mmol/L | 10.44 a | 6.48 b | 6.05 b | 6.62 b | 7.58 b | 0.84 | 7.51 | 7.35 | 0.54 | 0.006 | 0.833 | 0.474 |
| TP, g/L | 33.07 c | 35.46 bc | 41.88 ab | 47.93 a | 42.60 ab | 2.65 | 42.27 | 38.11 | 1.68 | 0.002 | 0.089 | 0.087 |
| AST, U/L | 91.55 | 74.90 | 80.73 | 55.57 | 51.33 | 10.92 | 71.14 | 70.49 | 6.91 | 0.062 | 0.948 | 0.282 |
| ALT, U/L | 63.55 | 56.39 | 68.78 | 48.61 | 74.21 | 9.91 | 52.92 | 71.70 | 6.27 | 0.401 | 0.041 | 0.455 |
| GABA Supplemental Levels/(mg/kg) | SEM | Gender | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | Male | Female | PGABA | PGender | PGABA×Gender | |||
| IgA, g/L | 0.34 c | 0.31 c | 0.38 b | 0.54 a | 0.40 b | 0.01 | 0.38 | 0.42 | 0.01 | <0.001 | <0.001 | 0.195 |
| IgM, g/L | 2.87 c | 3.31 bc | 3.67 ab | 4.05 a | 3.44 b | 0.17 | 2.93 | 4.01 | 0.11 | <0.001 | <0.001 | 0.980 |
| IgG, g/L | 18.50 bc | 11.33 d | 15.51 cd | 25.46 a | 23.60 ab | 2.00 | 20.20 | 17.56 | 1.26 | <0.001 | 0.147 | 0.192 |
| GABA Supplemental Levels/(mg/kg) | SEM | Gender | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | Male | Female | PGABA | PGender | PGABA×Gender | |||
| IL-2, pg/mL | 212.31 a | 194.50 ab | 190.90 ab | 173.07 b | 196.29 ab | 8.64 | 203.59 | 183.24 | 5.47 | 0.045 | 0.011 | 0.278 |
| IL-4, pg/mL | 98.75 c | 110.42 ab | 118.80 a | 116.88 ab | 108.97 b | 2.99 | 119.93 | 101.60 | 1.89 | <0.001 | <0.001 | 0.428 |
| IL-10, pg/mL | 56.83 b | 66.65 a | 69.07 a | 67.43 a | 63.58 ab | 2.71 | 66.55 | 62.88 | 1.71 | 0.021 | 0.136 | 0.364 |
| IL-12, pg/mL | 15.74 a | 15.28 a | 13.85 ab | 11.62 b | 15.29 a | 0.88 | 15.58 | 13.14 | 0.56 | 0.015 | 0.004 | 0.135 |
| IFN-γ, pg/mL | 605.40 a | 511.46 ab | 527.87 a | 405.02 b | 565.79 a | 38.64 | 592.09 | 454.13 | 24.44 | 0.009 | <0.001 | 0.592 |
| TNF-α, pg/mL | 382.72 | 367.05 | 324.52 | 343.07 | 365.12 | 25.16 | 269.96 | 443.03 | 15.91 | 0.519 | <0.001 | 0.872 |
| SIgA, ng/mL | 1672.03 b | 1861.26 b | 2310.15 a | 2363.17 a | 1805.48 b | 123.99 | 2429.79 | 1575.05 | 78.42 | <0.001 | <0.001 | 0.104 |
| GABA Supplemental Levels/(mg/kg) | SEM | Gender | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | Male | Female | PGABA | PGender | PGABA×Gender | |||
| SOD, U/mL | 60.61 | 52.94 | 56.59 | 71.40 | 57.36 | 5.74 | 73.21 | 46.35 | 3.63 | 0.214 | <0.001 | 0.170 |
| T-AOC, U/mL | 12.46 b | 10.55 b | 20.11 a | 19.67 a | 18.41 a | 2.05 | 13.29 | 19.18 | 1.30 | 0.003 | 0.003 | 0.069 |
| MDA, nmol/mL | 8.23 | 7.73 | 7.94 | 14.42 | 8.90 | 2.25 | 8.88 | 10.01 | 1.42 | 0.198 | 0.578 | 0.687 |
| GSH-Px, U/mL | 828.89 c | 889.07 bc | 948.11 ab | 1017.20 a | 1056.99 a | 39.77 | 1048.56 | 847.55 | 25.15 | 0.001 | <0.001 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhen, S.; Sun, F.; Cao, L.; Wang, L. Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks. Vet. Sci. 2024, 11, 398. https://doi.org/10.3390/vetsci11090398
Li Y, Zhen S, Sun F, Cao L, Wang L. Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks. Veterinary Sciences. 2024; 11(9):398. https://doi.org/10.3390/vetsci11090398
Chicago/Turabian StyleLi, Yalin, Shibo Zhen, Fengxue Sun, Lin Cao, and Lihua Wang. 2024. "Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks" Veterinary Sciences 11, no. 9: 398. https://doi.org/10.3390/vetsci11090398
APA StyleLi, Y., Zhen, S., Sun, F., Cao, L., & Wang, L. (2024). Effects of γ-Aminobutyric Acid on Growth Performance, Immunity, Antioxidant Capacity, and Intestinal Microbiota of Growing Minks. Veterinary Sciences, 11(9), 398. https://doi.org/10.3390/vetsci11090398






