Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. miRNAs, Plasmids and Target Prediction
2.3. Triglyceride (TG) Level Determination
2.4. Transcriptomic Analysis
2.5. Quantitative Real-Time PCR
2.6. Western Blot
2.7. Luciferase Reporter Assay
2.8. Statistical Analysis
2.9. Data Available
3. Results
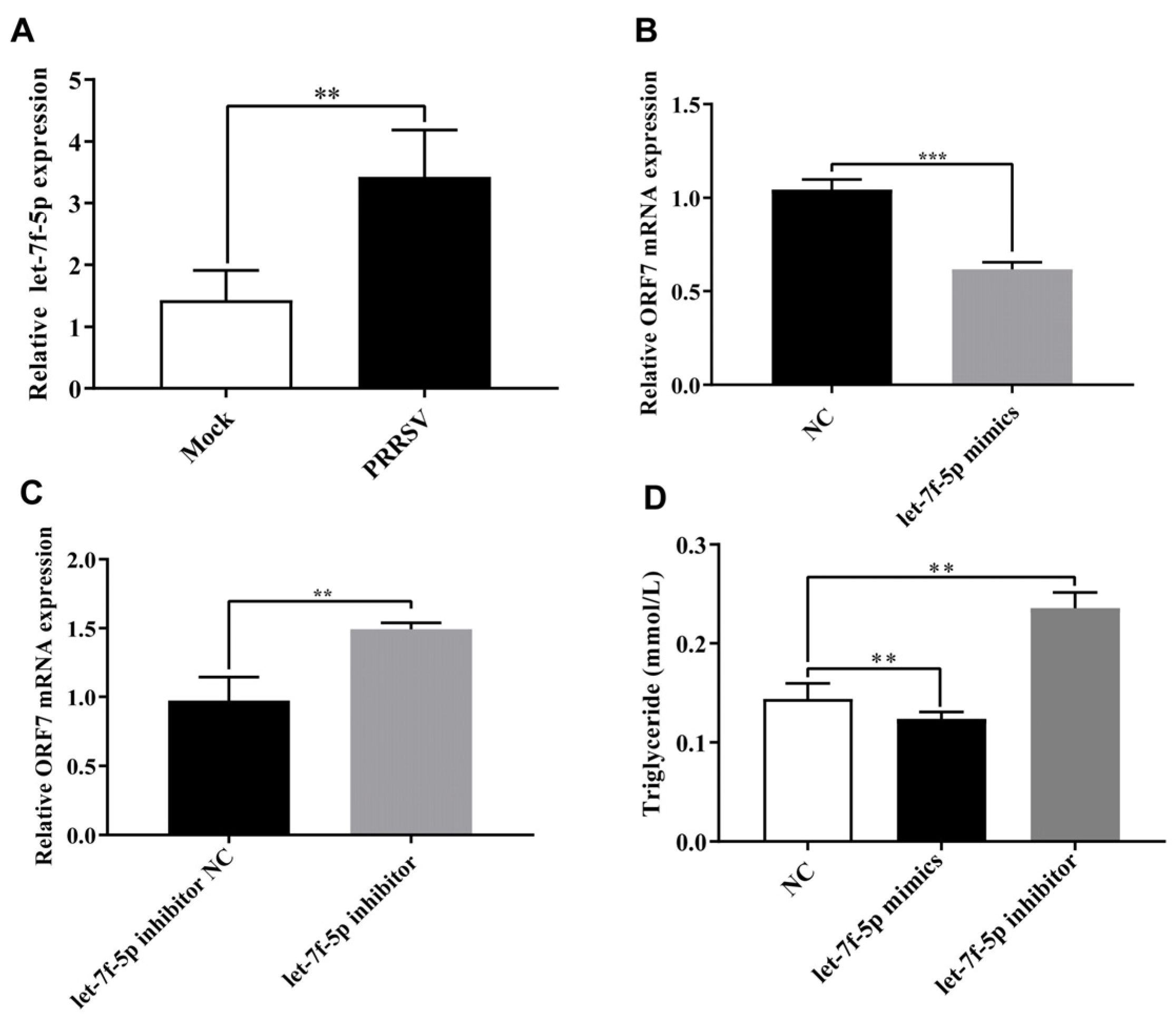
3.1. Let-7f-5p Restricts PRRSV Replication and Regulates Lipid Metabolism
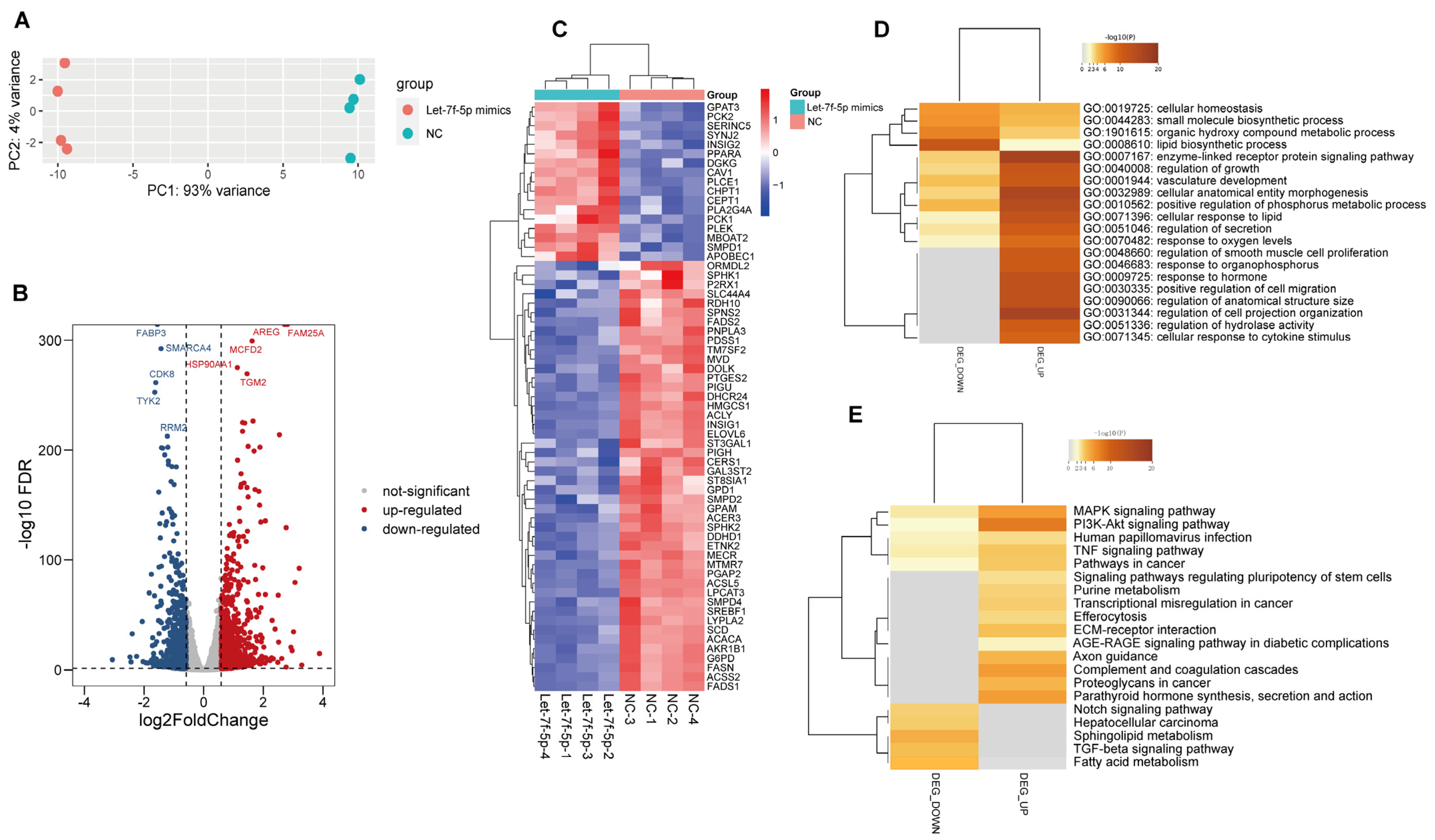
3.2. Let-7f-5p Altered Lipogenesis Pathway in PRRSV-Infected PK-15CD163 Cells
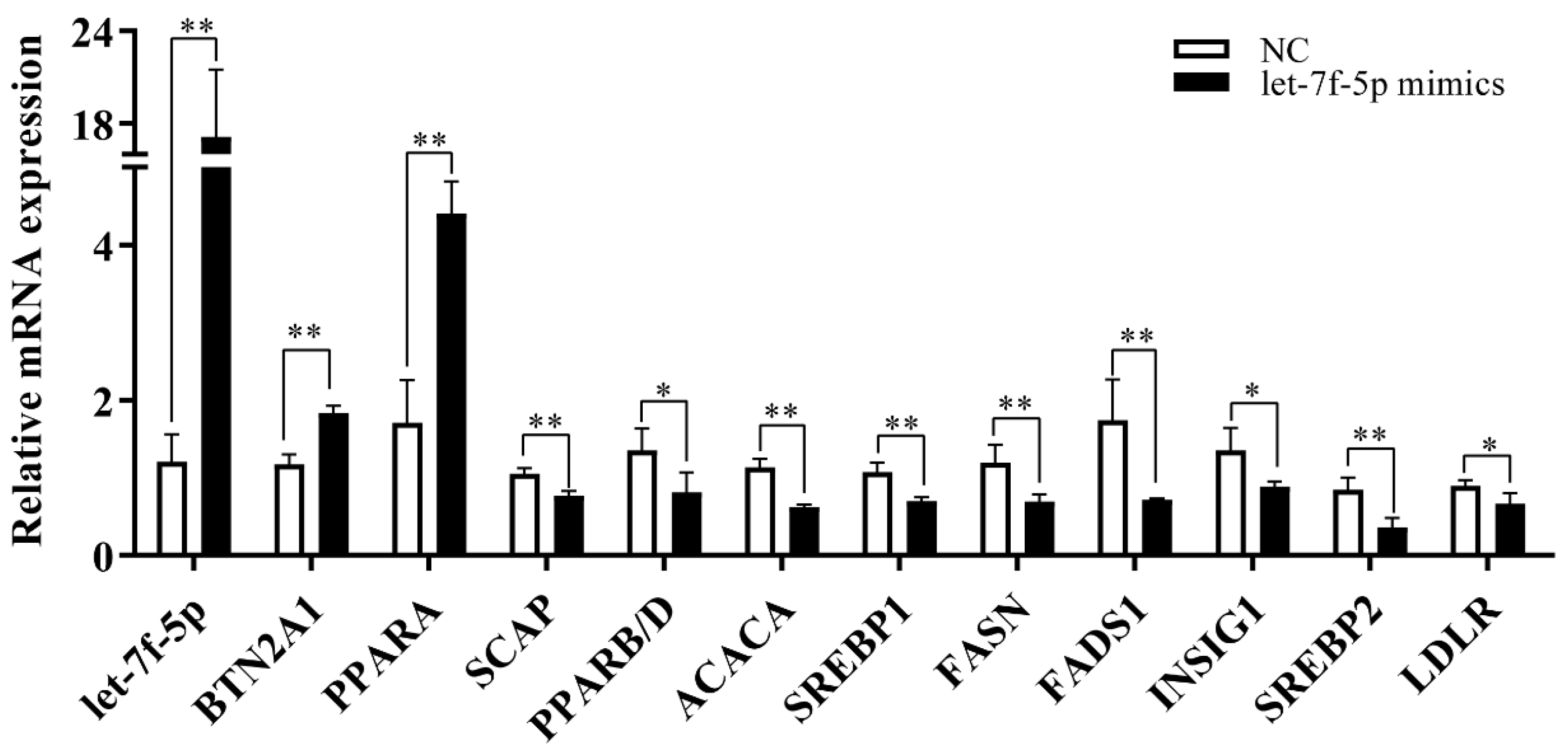
3.3. qRT-PCR Verified That Let-7f-5p Targeted Lipid Metabolism Pathway
3.4. Let-7f-5p Directly Targets SREBP2 to Modulate Lipid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renken, C.; Nathues, C.; Swam, H.; Fiebig, K.; Weiss, C.; Eddicks, M.; Ritzmann, M.; Nathues, H. Application of an economic calculator to determine the cost of porcine reproductive and respiratory syndrome at farm-level in 21 pig herds in Germany. Porc. Health Manag. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Dokland, T. The structural biology of PRRSV. Virus Res. 2010, 154, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lobo, F.; Díez-Fuertes, F.; Segalés, J.; García-Artiga, C.; Simarro, I.; Castro, J.; Prieto, C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet. Microbiol. 2011, 154, 58–68. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Y.; Ding, Y.; Zhang, Y.; Zhang, J. PRRSV receptors and their roles in virus infection. Arch. Microbiol. 2015, 197, 503–512. [Google Scholar] [CrossRef]
- Arruda, A.G.; Tousignant, S.; Sanhueza, J.; Vilalta, C.; Poljak, Z.; Torremorell, M.; Alonso, C.; Corzo, C.A. Aerosol detection and transmission of porcine reproductive and respiratory syndrome virus (PRRSV): What is the evidence, and what are the knowledge gaps? Viruses 2019, 11, 712. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Cai, X.; Zhou, E.-M.; Zimmerman, J.J. Effects of PRRSV infection on the porcine thymus. Trends Microbiol. 2020, 28, 212–223. [Google Scholar] [CrossRef]
- He, Y.; Wang, G.; Liu, Y.; Shi, W.; Han, Z.; Wu, J.; Jiang, C.; Wang, S.; Hu, S.; Wen, H. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2012, 160, 455–462. [Google Scholar] [CrossRef]
- Guan, K.; Su, Q.; Kuang, K.; Meng, X.; Zhou, X.; Liu, B. MiR-142-5p/FAM134B Axis Manipulates ER-Phagy to Control PRRSV Replication. Front. Immunol. 2022, 13, 842077. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, J.; Li, X.; Ren, W.; Li, F.; Wang, T.; Li, C.; Dong, Z.; Tian, X.; Zhang, L.; et al. Identification and integrated analysis of lncRNAs and miRNAs in IPEC-J2 cells provide novel insight into the regulation of the innate immune response by PDCoV infection. BMC Genom. 2022, 23, 486. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xue, M.; Wu, P.; Wang, H.; Liu, Z.; Wu, G.; Liu, P.; Wang, K.; Xu, W.; Feng, L. Coronavirus transmissible gastroenteritis virus antagonizes the antiviral effect of the microRNA miR-27b via the IRE1 pathway. Sci. China Life Sci. 2022, 65, 1413–1429. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, L.; Liu, Y.; Li, C.; Zhang, L.; Wang, T.; Zhao, D.; Xu, X.; Zhang, Y. MicroRNA-221-5p Inhibits Porcine Epidemic Diarrhea Virus Replication by Targeting Genomic Viral RNA and Activating the NF-kappaB Pathway. Int. J. Mol. Sci. 2018, 19, 3381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Sun, P.; Wang, J.; Li, K.; Zhao, Z.; Bai, X.; Cao, Y.; Bao, H.; Li, D. Downregulation of miR-122 by porcine reproductive and respiratory syndrome virus promotes viral replication by targeting SOCS3. Vet. Microbiol. 2022, 275, 109595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, X.-K.; Gao, L.; Huang, C.; Li, N.; Jia, X.; Liu, W.; Feng, W.-H. MicroRNA-23 inhibits PRRSV replication by directly targeting PRRSV RNA and possibly by upregulating type I interferons. Virology 2014, 450, 182–195. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Yao, Y.; Zhu, Y.; Zheng, X.; Liu, F.; Feng, W. Inducible miR-150 inhibits porcine reproductive and respiratory syndrome virus replication by targeting viral genome and suppressor of cytokine signaling 1. Viruses 2022, 14, 1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Pan, Y.; Gao, J.; Xu, Y.; Li, X.; Tian, Z.; Chen, H.; Wang, Y. Downregulation of miR-218 by porcine reproductive and respiratory syndrome virus facilitates viral replication via inhibition of type I interferon responses. J. Biol. Chem. 2021, 296, 100683. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Xu, Z.; Fang, L.; Zhong, Y.; Chen, Q.; Luo, R.; Chen, H.; Li, K.; Xiao, S. MiR-125b reduces porcine reproductive and respiratory syndrome virus replication by negatively regulating the NF-κB pathway. PLoS ONE 2013, 8, e55838. [Google Scholar] [CrossRef]
- Abbott, A.; Ambros, V. Functional analysis of the microRNA genes of: Roles of the -4 and -7 families of microRNAs in nematode development. Birth Defects Res. A 2006, 76, 366. [Google Scholar]
- Ma, Y.; Shen, N.; Wicha, M.; Luo, M. The Roles of the Let-7 Family of MicroRNAs in the Regulation of Cancer Stemness. Cells 2021, 10, 2415. [Google Scholar] [CrossRef]
- Dou, R.; Nishihara, R.; Cao, Y.; Mima, T.; Masuda, A.; Masugi, Y.; Shi, Y.; Gu, M.C.; Li, W.W.; da Silva, A.; et al. MicroRNA-, T Cells, and Patient Survival in Colorectal Cancer. Cancer Immunol. Res. 2016, 4, 927–935. [Google Scholar] [CrossRef]
- You, X.; Liu, M.; Liu, Q.; Li, H.; Qu, Y.; Gao, X.; Huang, C.; Luo, G.; Cao, G.; Xu, D. miRNA let-7 family regulated by NEAT1 and ARID3A/NF-κB inhibits PRRSV-2 replication in vitro and in vivo. PLoS Pathog. 2022, 18, e1010820. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, S.; Xiao, Y.; Wang, X.; Zhang, C.; Zhao, Q.; Nan, Y.; Huang, B.; Liu, H.; Liu, N. MYH9 is an essential factor for porcine reproductive and respiratory syndrome virus infection. Sci. Rep. 2016, 6, 25120. [Google Scholar] [CrossRef]
- Li, N.; Du, T.; Yan, Y.; Zhang, A.; Gao, J.; Hou, G.; Xiao, S.; Zhou, E.-M. MicroRNA let-7f-5p inhibits porcine reproductive and respiratory syndrome virus by targeting MYH9. Sci. Rep. 2016, 6, 34332. [Google Scholar] [CrossRef] [PubMed]
- Simino, L.A.P.; Panzarin, C.; Fontana, M.F.; de Fante, T.; Geraldo, M.V.; Ignacio-Souza, L.M.; Milanski, M.; Torsoni, M.A.; Ross, M.G.; Desai, M.; et al. MicroRNA Let-7 targets AMPK and impairs hepatic lipid metabolism in offspring of maternal obese pregnancies. Sci. Rep. 2021, 11, 8980. [Google Scholar] [CrossRef] [PubMed]
- Panzarin, C.; Simino, L.A.P.; Mancini, M.C.S.; Ignacio-Souza, L.M.; Milanski, M.; Torsoni, M.A.; Torsoni, A.S. Hepatic microRNA modulation might be an early event to non-alcoholic fatty liver disease development driven by high-fat diet in male mice. Mol. Biol. Rep. 2022, 49, 2655–2666. [Google Scholar] [CrossRef]
- Altan-Bonnet, N. Lipid tales of viral replication and transmission. Trends Cell Biol. 2017, 27, 201–213. [Google Scholar] [CrossRef]
- Roingeard, P.; Melo, R.C. Lipid droplet hijacking by intracellular pathogens. Cell. Microbiol. 2017, 19, e12688. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Yi, S.; Gao, Q.; Gong, T.; Feng, Y.; Wu, D.; Zheng, X.; Wang, H.; Zhang, G. Porcine reproductive and respiratory syndrome virus regulates lipid droplet accumulation in lipid metabolic pathways to promote viral replication. Virus Res. 2023, 333, 199139. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, Q.; Wen, Y.; Wu, R.; Du, S.; Huang, X.; Wen, X.; Cao, S.; Zeng, L.; Yan, Q. Porcine reproductive and respiratory syndrome virus upregulates SMPDL3B to promote viral replication by modulating lipid metabolism. Iscience 2023, 26, 107450. [Google Scholar] [CrossRef]
- Bommer, G.T.; MacDougald, O.A. Regulation of lipid homeostasis by the bifunctional SREBF2-miR33a locus. Cell Metab. 2011, 13, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Wanninger, J.; Schmidhofer, S.; Weigert, J.; Neumeier, M.; Dorn, C.; Hellerbrand, C.; Zimara, N.; Schäffler, A.; Aslanidis, C. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology 2011, 152, 26–35. [Google Scholar] [CrossRef]
- Shao, J.; Jiang, G.; Li, Y.; Wang, M.; Tang, T.; Wang, J.; Jia, X.; Lai, S. Let-7a-5p Regulates Animal Lipid Accumulation by Targeting Srebf2 and Thbs1 Signaling. Int. J. Mol. Sci. 2024, 25, 894. [Google Scholar] [CrossRef]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Mazzon, M.; Mercer, J. Lipid interactions during virus entry and infection. Cell Microbiol. 2014, 16, 1493–1502. [Google Scholar] [CrossRef]
- de Castro, I.F.; Tenorio, R.; Risco, C. Virus assembly factories in a lipid world. Curr. Opin. Virol. 2016, 18, 20–26. [Google Scholar] [CrossRef]
- Dutta, A.; Sharma-Walia, N. Curbing lipids: Impacts on cancer and viral infection. Int. J. Mol. Sci. 2019, 20, 644. [Google Scholar] [CrossRef]
- Villareal, V.A.; Rodgers, M.A.; Costello, D.A.; Yang, P.L. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antivir. Res. 2015, 124, 110–121. [Google Scholar] [CrossRef]
- Zheng, Z.; Ling, X.; Li, Y.; Qiao, S.; Zhang, S.; Wu, J.; Ma, Z.; Li, M.; Guo, X.; Li, Z.; et al. Host cells reprogram lipid droplet synthesis through YY1 to resist PRRSV infection. mBio 2024, 15, e0154924. [Google Scholar] [CrossRef]
- Yu, P.-W.; Fu, P.-F.; Zeng, L.; Qi, Y.-L.; Li, X.-Q.; Wang, Q.; Yang, G.-Y.; Li, H.-W.; Wang, J.; Chu, B.-B. EGCG restricts PRRSV proliferation by disturbing lipid metabolism. Microbiol. Spectr. 2022, 10, e0227621. [Google Scholar] [CrossRef]
- Wang, X.P.; Wei, R.F.; Li, Q.Y.; Liu, H.L.; Huang, B.C.; Gao, J.M.; Mu, Y.; Wang, C.B.; Hsu, W.H.; Hiscox, J.A.; et al. PK-15 cells transfected with porcine CD163 by PiggyBac transposon system are susceptible to porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2013, 193, 383–390. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Andersen, C.B.F.; Moestrup, S.K. CD163 Binding to Haptoglobin-Hemoglobin Complexes Involves a Dual-point Electrostatic Receptor-Ligand Pairing. J. Biol. Chem. 2013, 288, 18834–18841. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouche, E.; Lasserre, F.; Naylies, C.; Betoulieres, C.; et al. Hepatocyte-specific deletion of Pparalpha promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 6489. [Google Scholar] [CrossRef] [PubMed]
- Branche, E.; Wang, Y.-T.; Viramontes, K.M.; Valls Cuevas, J.M.; Xie, J.; Ana-Sosa-Batiz, F.; Shafee, N.; Duttke, S.H.; McMillan, R.E.; Clark, A.E. SREBP2-dependent lipid gene transcription enhances the infection of human dendritic cells by Zika virus. Nat. Commun. 2022, 13, 5341. [Google Scholar] [CrossRef]
- Yuan, S.; Chu, H.; Chan, J.F.-W.; Ye, Z.-W.; Wen, L.; Yan, B.; Lai, P.-M.; Tee, K.-M.; Huang, J.; Chen, D. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Yang, L.; Meng, X.; Xu, Q.; Zhou, X.; Liu, B. Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection. Vet. Sci. 2024, 11, 392. https://doi.org/10.3390/vetsci11090392
Jiang D, Yang L, Meng X, Xu Q, Zhou X, Liu B. Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection. Veterinary Sciences. 2024; 11(9):392. https://doi.org/10.3390/vetsci11090392
Chicago/Turabian StyleJiang, Dongfeng, Liyu Yang, Xiangge Meng, Qiuliang Xu, Xiang Zhou, and Bang Liu. 2024. "Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection" Veterinary Sciences 11, no. 9: 392. https://doi.org/10.3390/vetsci11090392
APA StyleJiang, D., Yang, L., Meng, X., Xu, Q., Zhou, X., & Liu, B. (2024). Let-7f-5p Modulates Lipid Metabolism by Targeting Sterol Regulatory Element-Binding Protein 2 in Response to PRRSV Infection. Veterinary Sciences, 11(9), 392. https://doi.org/10.3390/vetsci11090392






