Sero-Prevalence of Hemorrhagic Septicaemia in Cattle and Buffalo Population of Indian States Karnataka and Gujarat
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. General Description of the Study Sites
2.2.1. Sample Size Estimation
2.2.2. Data Collection
2.2.3. Collection of Serum Samples
2.2.4. Laboratory Procedures
2.2.5. Statistical Analysis
3. Results
3.1. Sero-Prevalence of Hemorrhagic Septicemia (HS) at Different Strata
3.2. Assessment of Risk Factor for Hemorrhagic Septicemia (HS) Sero-Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almoheer, R.; Abd, W.M.E.; Zakaria, H.A.; Jonet, M.A.B.; Al-Shaibani, M.M.; Al-Gheethi, A.; Addis, S.N.K. Spatial, temporal, and demographic patterns in the prevalence of hemorrhagic septicemia in 41 countries in 2005–2019: A systematic analysis with special focus on the potential development of a new-generation vaccine. Vaccines 2022, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Foggin, C.M.; Rosen, L.E.; Henton, M.M.; Buys, A.; Floyd, T.; Turner, A.D.; Tarbin, J.; Lloyd, A.S.; Chaitezvi, C.; Ellis, R.J.; et al. Pasteurella sp. associated with fatal septicaemia in six African elephants. Nat. Commun. 2023, 14, 6398. [Google Scholar] [CrossRef] [PubMed]
- OIE. Haemorrhagic Septicaemia. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021; World Organisation for Animal Health (WOAH): Paris, France, 2021; Chapter 3.4.10; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.04.10_HAEMORRHAGIC_SEPTICAEMIA.pdf (accessed on 4 January 2024).
- Peng, Z.; Wang, X.; Zhou, R.; Chen, H.; Wilson, B.A.; Wu, B. Pasteurella multocida: Genotypes and genomics. Microbiol. Mol. Biol. 2019, 83, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Ullah, N.; Farhan Ul Haque, M.; Rauf, W.; Iqbal, M.; Ali, A.; Rahman, M. Genomic characterization and comparative genomic analysis of HS-associated Pasteurella multocida serotype B: 2 strains from Pakistan. BMC Genom. 2023, 24, 546. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.; Yogisharadhya, R.; Mohanty, N.N.; Mendem, S.K.; Nizamuddin, A.; Chanda, M.M.; Shivachandra, S.B. Comparative genome analysis of Pasteurella multocida serogroup B: 2 strains causing haemorrhagic septicaemia (HS) in bovines. Gene 2022, 826, 146452. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liang, W.; Wang, F.; Xu, Z.; Xie, Z.; Lian, Z.; Hua, L.; Zhou, R.; Chen, H.; Wu, B. Genetic and phylogenetic characteristics of Pasteurella multocida isolates from different host species. Front. Microbiol. 2018, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Narcana, I.K.; Suardana, I.W.; Besung, I.N.K. Molecular characteristic of Pasteurella multocida isolates from Sumba Island at East Nusa Tenggara Province, Indonesia. Vet. World 2020, 13, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, L.N.; Priyadarshini, A.; Kumar, S.; Thomas, P.; Gupta, S.K.; Nagaleekar, V.K.; Singh, V.P. Virulence genotyping of Pasteurella multocida isolated from multiple hosts from India. Sci. World J. 2014, 2014, 814109. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.; Chanda, M.M.; Yogisharadhya, R.; Parveen, A.; Ummer, J.; Dhayalan, A.; Mohanty, N.N.; Shivachandra, S.B. Comparative genetic diversity analysis based on virulence and repetitive genes profiling of circulating Pasteurella multocida isolates from animal hosts. Infect. Genet. Evol. 2020, 85, 104564. [Google Scholar] [CrossRef]
- Moustafa, A.M.; Ali, S.N.; Bennett, M.D.; Hyndman, T.H.; Robertson, I.D.; Edwards, J. A case–control study of haemorrhagic septicaemia in buffaloes and cattle in Karachi, Pakistan, in 2012. Transbound. Emerg. Dis. 2017, 64, 520–527. [Google Scholar] [CrossRef]
- Singh, B.; Prasad, S.; Verma, M.R.; Sinha, D. Estimation of economic losses due to haemorrhagic septicaemia in cattle and buffaloes in India. Agric. Econ. Res. Rev. 2014, 27, 271–279. [Google Scholar] [CrossRef]
- Govindaraj, G.; Krishnamoorthy, P.; Nethrayini, K.R.; Shalini, R.; Rahman, H. Epidemiological features and financial loss due to clinically diagnosed Haemorrhagic Septicemia in bovines in Karnataka, India. Prev. Vet. Med. 2017, 144, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.M.; Purse, B.V.; Hemadri, D.; Patil, S.S.; Yogisharadhya, R.; Prajapati, A.; Shivachandra, S.B. Spatial and temporal analysis of haemorrhagic septicaemia outbreaks in India over three decades (1987–2016). Sci. Rep. 2024, 14, 6773. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, A.; De Alwis, M. Haemorrhagic septicaemia, its significance, prevention and control in Asia. Vet. Med. 2002, 47, 234–240. [Google Scholar] [CrossRef]
- Jindal, N.; Kumar, S.; Narang, G.; Chaturvedi, G.C.; Tomer, P.; Garg, D.N. Some epidemiological observations on haemorrhagic septicaemia in buffaloes and cattle in Haryana state of India. Buffalo J. 2002, 2, 273–280. [Google Scholar]
- Shivachandra, S.B.; Viswas, K.N.; Kumar, A.A. A review of hemorrhagic septicemia in cattle and buffalo. Anim. Health Res. Rev. 2011, 12, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Köndgen, S.; Leider, M.; Lankester, F.; Bethe, A.; Lübke-Becker, A.; Leendertz, F.H.; Ewers, C. Pasteurella multocida involved in respiratory disease of wild chimpanzees. PLoS ONE 2011, 6, e24236. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Singh, S.; Gazal, S.; Jadhao, A.; Kantale, R.; Basak, G.; Singh, P. Haemorrhagic septicemia: A persistent nuisance in Indian livestock review. J. Pharm. Innov. 2022, 11, 4382–4394. [Google Scholar]
- Wijewardana, T.G.; Alwis, M.D.; Bastianz, H.L.G. Cultural, biochemical, serological and pathogenicity studies of strains of Pasteurella multocida isolated from carrier animals and outbreaks of haemorrhagic septicaemia. Sri Lanka Vet. J. 1986, 34, 43–57. [Google Scholar]
- Sanchez, S.; Mizan, S.; Quist, C.; Schroder, P.; Juneau, M.; Dawe, D.; Ritchie, B.; Lee, M.D. Serological response to Pasteurella multocida NanH sialidase in persistently colonized rabbits. Clin. Vaccine Immunol. 2004, 11, 825–834. [Google Scholar] [CrossRef]
- Calderón, B.J.M.; Fernández, A.; Arnal, J.L.; Sanz, T.C.; Fernández, G.J.F.; Vela, A.I.; Cid, D. Molecular epidemiology of Pasteurella multocida associated with bovine respiratory disease outbreaks. Animals 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Khamesipour, F.; Momtaz, H.; Azhdary, M.M. Occurrence of virulence factors and antimicrobial resistance in Pasteurella multocida strains isolated from slaughter cattle in Iran. Front. Microbiol. 2014, 5, 536. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Natesan, K.; Prajapati, A.; Kalleshmurthy, T.; Shome, B.R.; Rahman, H.; Shome, R. Prevalence and antibiotic susceptibility of Mannheimia haemolytica and Pasteurella multocida isolated from ovine respiratory infection: A study from Karnataka, southern India. Vet. World 2020, 13, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Shome, R.; Deka, R.P.; Sahay, S.; Grace, D.; Lindahl, J.F. Seroprevalence of hemorrhagic septicemia in dairy cows in Assam, India. Infect. Ecol. Epidemiol. 2019, 9, 1604064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Aziz, M.; Tunio, M.T.; Rehman, R.; Rehman, A.; Rehman, K.; Hameed, A.; Naveed, T.; Tanveer, M.; Ali, M. Seroprevalence of hemorrhagic septicemia in buffalo and cattle in flood, irrigated and sandy areas of Punjab, Pakistan. Pure Appl. Biol. 2018, 7, 1234–1243. [Google Scholar] [CrossRef]
- Ahuja, V.; Rajasekhar, M.; Raju, R. Animal health for poverty alleviation: A review of key issues for India. In Background Paper Prepared for “Livestock Sector Review” of the World Bank; World Bank: Washington, DC, USA, 2008; p. 60. [Google Scholar]
- De Alwis, M.C.; Wijewardana, T.G.; Gomis, A.I.; Vipulasiri, A.A. Persistence of the carrier status in haemorrhagic septicaemia (Pasteurella multocida serotype 6: B infection) in buffaloes. Trop. Anim. Health Prod. 1990, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Saharee, A.A.; Salim, N.B. The epidemiology of haemorrahgic septicemia in cattle and buffaloes in Malaysia. In Proceedings of the Fourth International Workshop on Haemorrhagic Septicaemia, Kandy, Sri Lanka, 11–15 February 1991; pp. 109–112. [Google Scholar]
- Horadagoda, N.U.; De Alwis, M.C.L.; Wijewardana, T.G.; Belak, K.; Gomis, A.I.U.; Vipulasiri, A.A. Experimental Haemorrhagic Septicaemia in Buffalo Calves; FAO: Rome, Italy, 1991; pp. 73–80. [Google Scholar]
- Tankaew, P.; Srisawat, W.; Singhla, T.; Tragoolpua, K.; Kataoka, Y.; Sawada, T.; Sthitmatee, N. Comparison of two indirect ELISA coating antigens for the detection of dairy cow antibodies against Pasteurella multocida. J. Microbiol. Methods. 2018, 145, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, Q.; Puspitasari, Y.; Othman, S.; Zamri-Saad, M.; Salleh, A. Phagocytosis and intracellular killing of Pasteurella multocida B: 2 by macrophages: A comparative study between buffalo and cattle. Vet. World 2022, 15, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.H.; Jan, M.H.; Verma, H.K.; Sharma, R.K. Epidemiology, economic losses due to Haemorrhagic Septicemia and cost-benefit analysis of interventions in dairy animals of Punjab. Indian J. Anim. Sci. 2020, 90, 1447–1452. [Google Scholar] [CrossRef]
- AICRP-ADMAS Annual Report 2017–2018. Available online: https://nivedi.res.in/pdf/Reports/AICRP%2017-18.pdf (accessed on 6 January 2024).
- Saxena, M.K.; Singh, V.P.; Kumar, A.; Choudhuri, P.; Singh, V.P.; Shivachandra, S.B.; Biswas, A.; Sharma, B. REP-PCR analysis of P. multocida isolates from wild and domestic animals in India. Vet. Res. Commun. 2006, 30, 851–861. [Google Scholar] [CrossRef]
- Aiswarya, V.; Mathakiya, R.A.; Bhanderi, B.B.; Roy, A. Characterization of Pasteurella multocida isolates of buffalo origin from Gujarat state of India by outer membrane protein profile analysis. Buffalo Bull. 2017, 36, 313–322. [Google Scholar]

| States Name | Total Positives/Samples, CI 95% | p Value between States | Districts Name | Total Positives/Samples, CI 95% | p Value between Districts | Clusters Name | Total Positives/Samples, CI 95% | p Value between Clusters | Epiunit Positivity, CI 95% | HH Positivity, CI 95% |
|---|---|---|---|---|---|---|---|---|---|---|
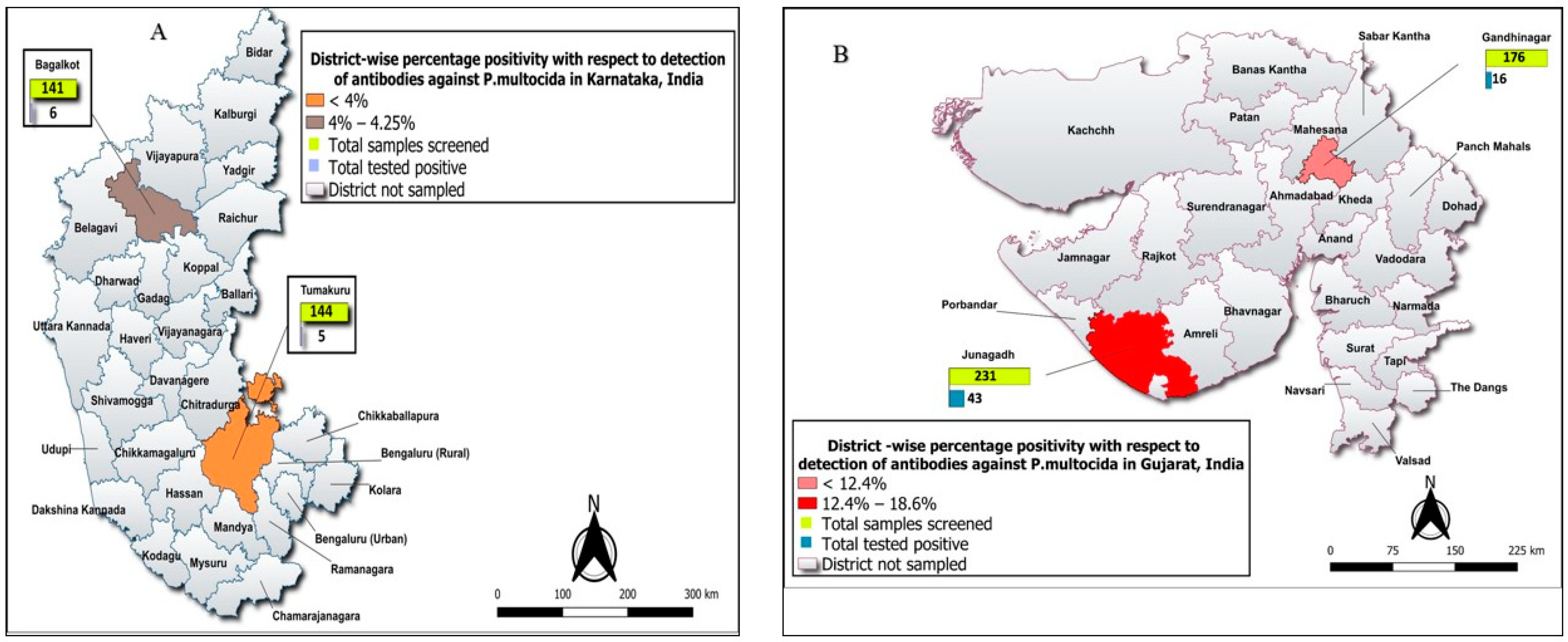
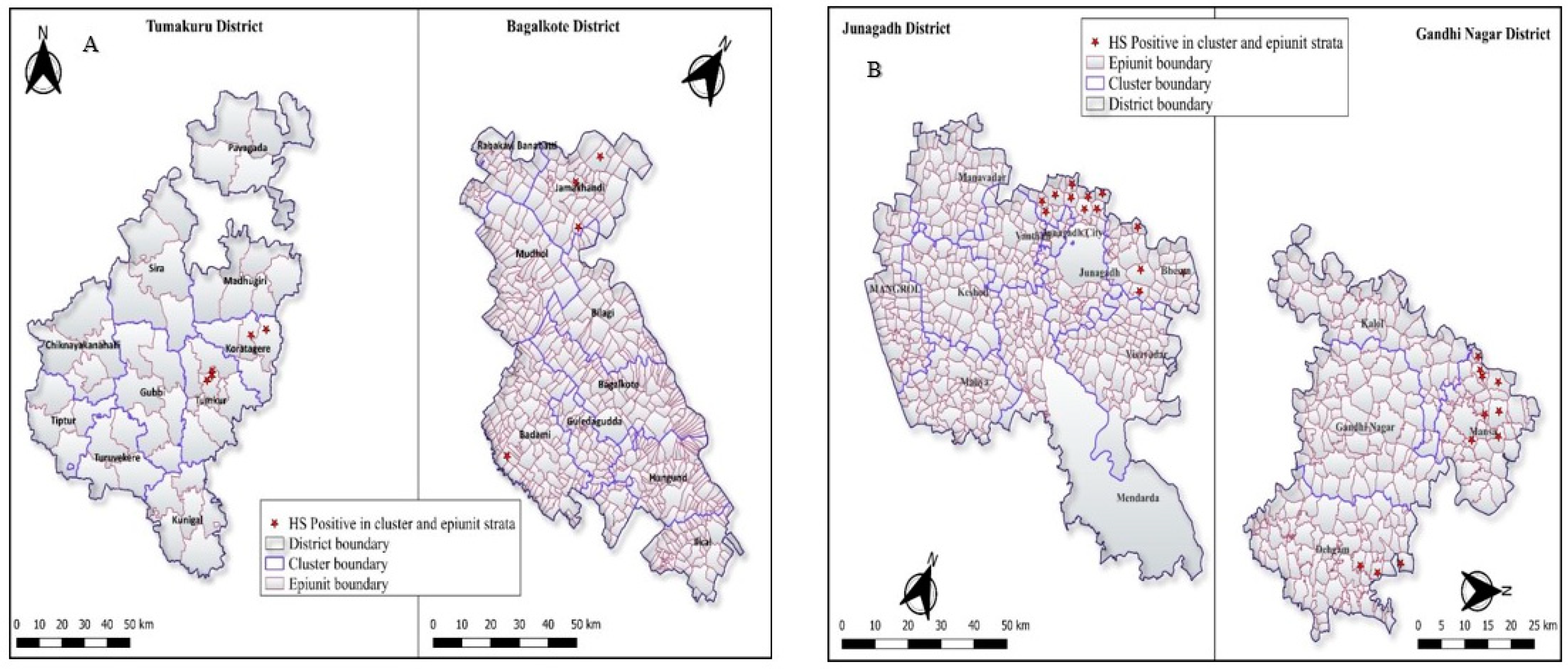
| Karnataka | 11/285 (3.85%), [1.94–6.80] | <0.0001 * | Tumakuru | 5/144 (3.47%), [1.44–7.92] | 0.97 | Tumkur | 3/83 (3.61%), [0.75–10.20] | 0.72 | 3/9 (33.33%), [7.49–70.07] | 3/42 (7.14%), [1.50–19.49] |
| Koratagere | 2/61 (3.28%), [0.40–11.35] | 2/7 (28.57%), [3.76–70.96] | 2/35 (5.71%), [0.70–19.16] | |||||||
| Bagalkote | 6/141 (4.25%), [1.58–9.03] | Jamakhandi | 4/68 (5.88%), [1.63–14.38] | 0.61 | 3/6 (50.11%), [81–88.19] | 4/22 (18.18), [5.19–40.28] | ||||
| Badami | 2/73 (2.73%), [0.33–9.55] | 1/5 (20%), [0.5–71.64] | 1/34 (2.94%), [0.07–15.33] | |||||||
| Gujarat | 59/407 (14.49%), [11.22–18.30] | Junagadh | 43/231 (18.61%), [13.81–24.24] | 0.01 * | Junagadh | 35/152 (23.02%), [16.59–30.54] | 0.03 * | 9/9 (100%), [66.37–100] | 20/58 (34.48%), [22.49–48.12] | |
| Bhesan | 8/79 (10.12%), [4.47–18.98] | 5/6 (83.33%), [35.88–99.58] | 6/28 (21.43%), [8.30–40.95] | |||||||
| Gandhinagr | 16/176 (9.09%), [5.29–14.34] | Dehgam | 3/55 (5.45%), [4.47–18.98] | 0.40 | 3/6 (50%), [11.81–88.19] | 3/25 (12%), [2.55–31.22] | ||||
| Mansa | 13/121 (10.74%), [5.85–17.67] | 7/8 (87.50%), [47.35–99.68] | 13/62 (20.97%), [11.66–33.18] |
| States | Districts | No. of HH Surveyed | No. of Animals/HH | One Animal Positive/HH | Two Animals Positive/HH | Three Animals Positive/HH | Four Animals Positive/HH | Total Number of HH Positive | p Value between HH in Two States |
|---|---|---|---|---|---|---|---|---|---|
| Karnataka | Tumakuru | 77 | 144 | 5 | − | − | − | 5 (6.49%) | 0.0002 * |
| Bagalkote | 56 | 141 | 4 | 1 | − | − | 5 (8.93%) | ||
| Total HH | 133 | 285 | 9 | 1 | − | − | 10 (7.52%) | ||
| Gujarat | Junagadh | 86 | 231 | 15 | 6 | 4 | 1 | 26 (30.23%) | |
| Gandhinagar | 87 | 176 | 16 | − | − | − | 16 (18.39%) | ||
| Total HH | 173 | 407 | 31 | 6 | 4 | 1 | 42 (24.28%) | ||
| Total | 306 | 692 | 40 | 7 | 4 | 1 | 52 | ||
| Parameters | State | Karnataka | Gujarat | Total Samples (Percentage Positivity) |
|---|---|---|---|---|
| Age (years) | 1–2 | 0/3 | 6/29 | 6/32 (18.75) |
| 2–4 | 2/132 | 16/101 | 18/233 (7.73) | |
| 4–6 | 6/118 | 14/103 | 20/221 (9.05) | |
| 6–8 | 2/25 | 16/117 | 18/142 (12.68) | |
| >8 | 1/7 | 7/57 | 8/64 (12.50) | |
| Total | 11/285 (3.85%, CI: 1.94–6.80) | 59/407 (14.49%, CI: 11.22–18.30) | 70/692 (10.12%, CI: 7.97–12.61) | |
| χ2 /p value | 5.76/0.22 | 1.40/0.84 | 5.79/0.22 | |
| Species | Cattle | 11/259 | 37/232 | 48/491 (9.78) |
| Buffalo | 0/26 | 22/175 | 22/201 (10.95) | |
| Total | 11/285 (3.86%, CI:1.94–6.80) | 59/407 (14.50%, CI: 11.22–18.30) | 70/692 (10.12%, CI: 7.97–12.61) | |
| χ2 /p value | 0.29/0.59 | 0.67/0.41 | 0.11/0.75 | |
| Cattle breeds | Cross bred | 10/237 | 12/97 | 22/334 (6.59) |
| Indigenous | 1/22 | 25/135 | 26/157 (16.56) | |
| Total | 11/259 (4.25%,CI: 2.14–7.47) | 37/232 (15.64%, CI: 11.48–21.31) | 48/491 (9.78%, CI: 7.30–12.75) | |
| χ2 /p value | 0.23/0.63 | 1.17/0.28 | 10.94/0.0009 * | |
| Buffalo breeds | Jafarabadi | – | 8/39 | 8/39 (20.51) |
| Mehsana | 0/26 | 14/136 | 14/162 (8.64) | |
| Total | 0/26 (CI: 0–13.23) | 22/175 (12.57%, CI: 8.05–18.41) | 22/201 (10.95%, CI: 6.99–16.10) | |
| χ2 /p value | – | 0.20/0.66 | 3.41/0.065 | |
| Lactation | 1 | 0/69 | 26/147 | 26/216 (12.04) |
| 2 | 4/85 | 10/81 | 14/166 (8.43) | |
| 3 | 3/73 | 7/78 | 10/151 (6.62) | |
| 4 | 2/26 | 7/62 | 9/88 (10.23) | |
| >5 | 2/32 | 9/39 | 11/71 (15.49) | |
| Total | 11/285 (3.85%, CI: 1.94–6.80) | 59/407 (14.49%, CI: 11.22–18.30) | 70/692 (10.12%, CI: 7.97–12.61) | |
| χ2 /p value | 4.47/0.35 | 6.26/0.18 | 5.68/0.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shome, R.; Kanani, A.; Gurrappanaidu, G.; Subbanna, N.K.G.; Mohandoss, N.; Prajapati, A.; Baskar, K.; Skariah, S.; Shanmugam, G.; Maharana, S.M.; et al. Sero-Prevalence of Hemorrhagic Septicaemia in Cattle and Buffalo Population of Indian States Karnataka and Gujarat. Vet. Sci. 2024, 11, 386. https://doi.org/10.3390/vetsci11080386
Shome R, Kanani A, Gurrappanaidu G, Subbanna NKG, Mohandoss N, Prajapati A, Baskar K, Skariah S, Shanmugam G, Maharana SM, et al. Sero-Prevalence of Hemorrhagic Septicaemia in Cattle and Buffalo Population of Indian States Karnataka and Gujarat. Veterinary Sciences. 2024; 11(8):386. https://doi.org/10.3390/vetsci11080386
Chicago/Turabian StyleShome, Rajeswari, Amit Kanani, Govindraj Gurrappanaidu, Naveen Kumar Gajalavarahalli Subbanna, Nagalingam Mohandoss, Awadesh Prajapati, Kanaka Baskar, Somy Skariah, G. Shanmugam, Snigdha Madhaba Maharana, and et al. 2024. "Sero-Prevalence of Hemorrhagic Septicaemia in Cattle and Buffalo Population of Indian States Karnataka and Gujarat" Veterinary Sciences 11, no. 8: 386. https://doi.org/10.3390/vetsci11080386
APA StyleShome, R., Kanani, A., Gurrappanaidu, G., Subbanna, N. K. G., Mohandoss, N., Prajapati, A., Baskar, K., Skariah, S., Shanmugam, G., Maharana, S. M., Vijayalakshmy, K., & Habibur, R. (2024). Sero-Prevalence of Hemorrhagic Septicaemia in Cattle and Buffalo Population of Indian States Karnataka and Gujarat. Veterinary Sciences, 11(8), 386. https://doi.org/10.3390/vetsci11080386








