Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi
Abstract
Simple Summary
Abstract
1. Introduction
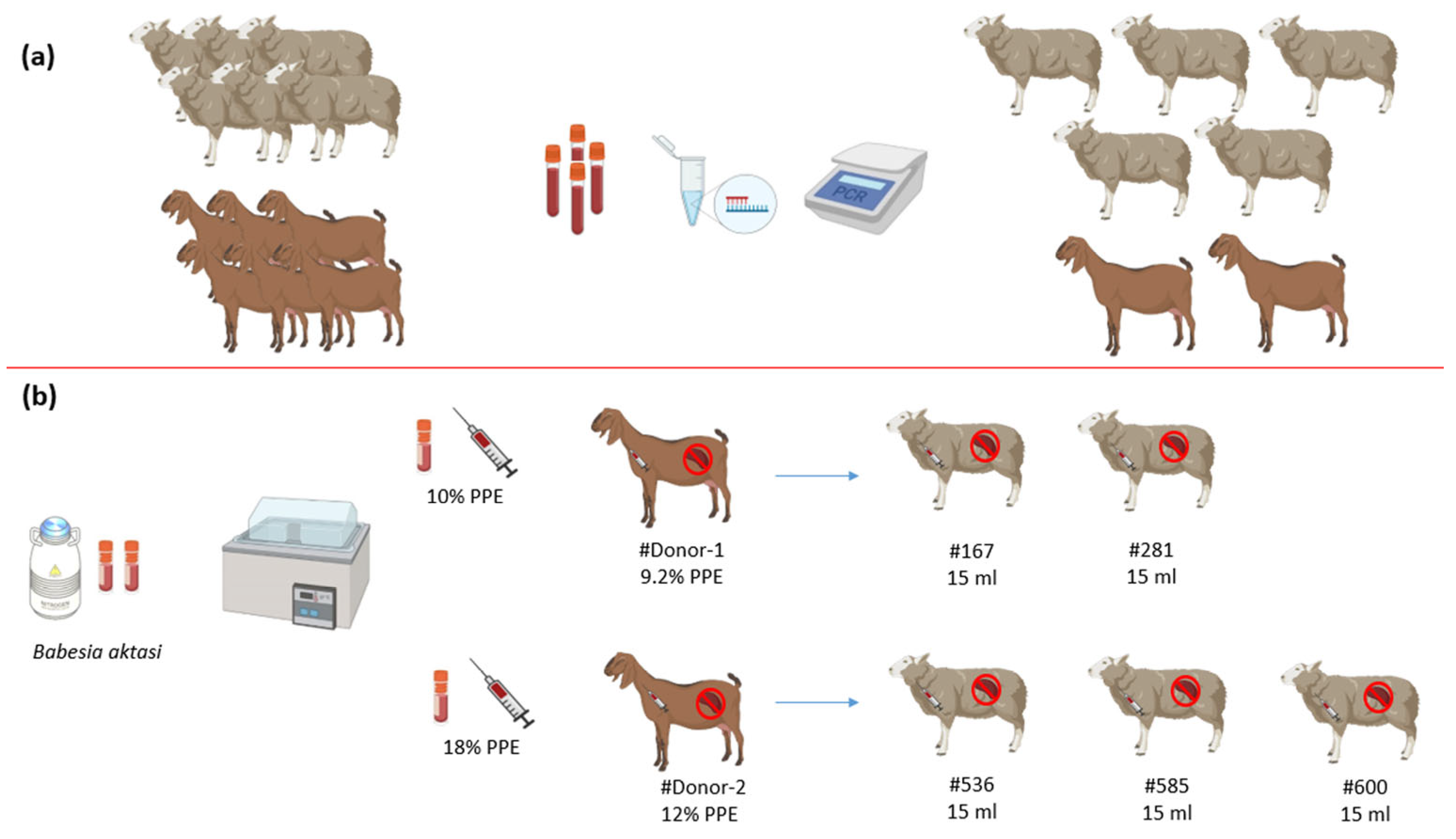
2. Materials and Methods
2.1. Parasite (Babesia aktasi Stabilate)
2.2. Selection of Experimental Animals
2.3. Splenectomy and Post-Operative Care
2.4. Experimental Infections
2.5. Inoculation of B. aktasi Stabilate in Donor Goats
2.6. Inoculation of Fresh Blood Infected with B. aktasi in Recipient Lambs
2.7. Microscopic Detection
2.8. DNA Isolation, PCR and DNA Sequencing
3. Results
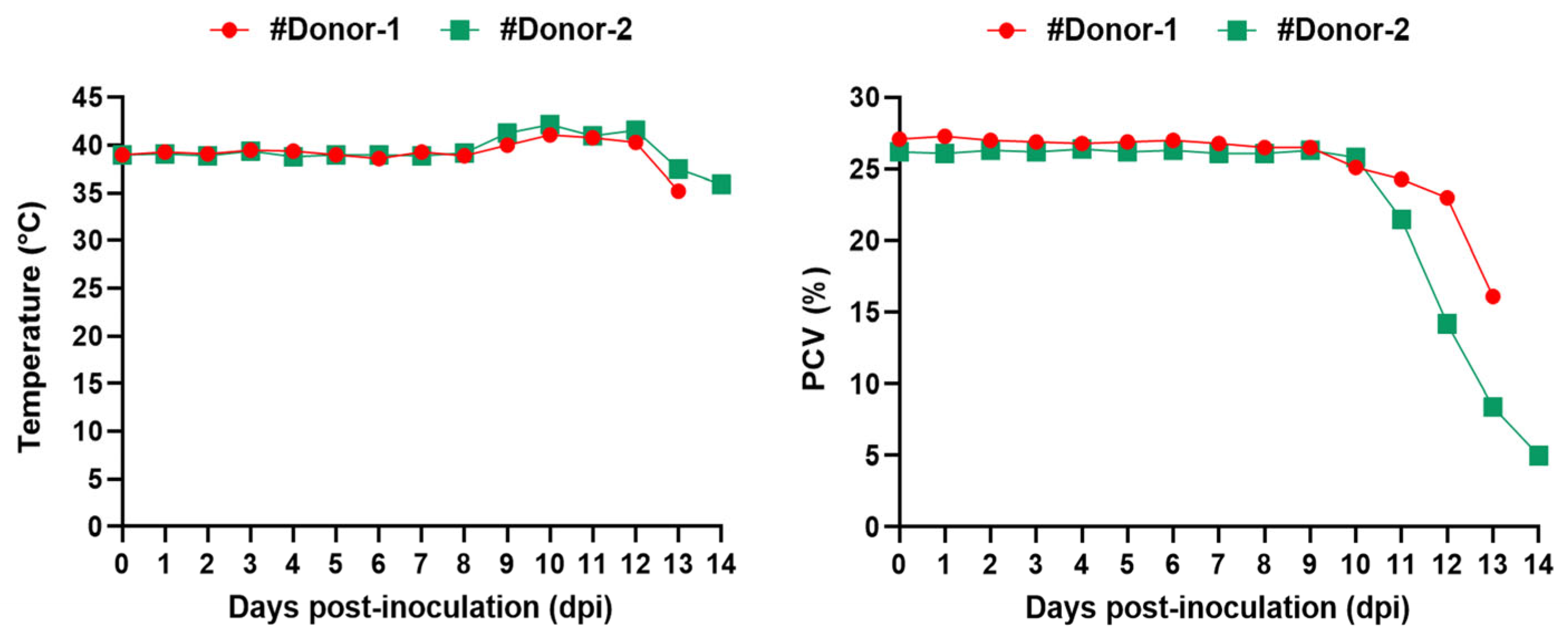
3.1. Babesia aktasi is Highly Virulent to Immune-Suppressed Indigenous Goats
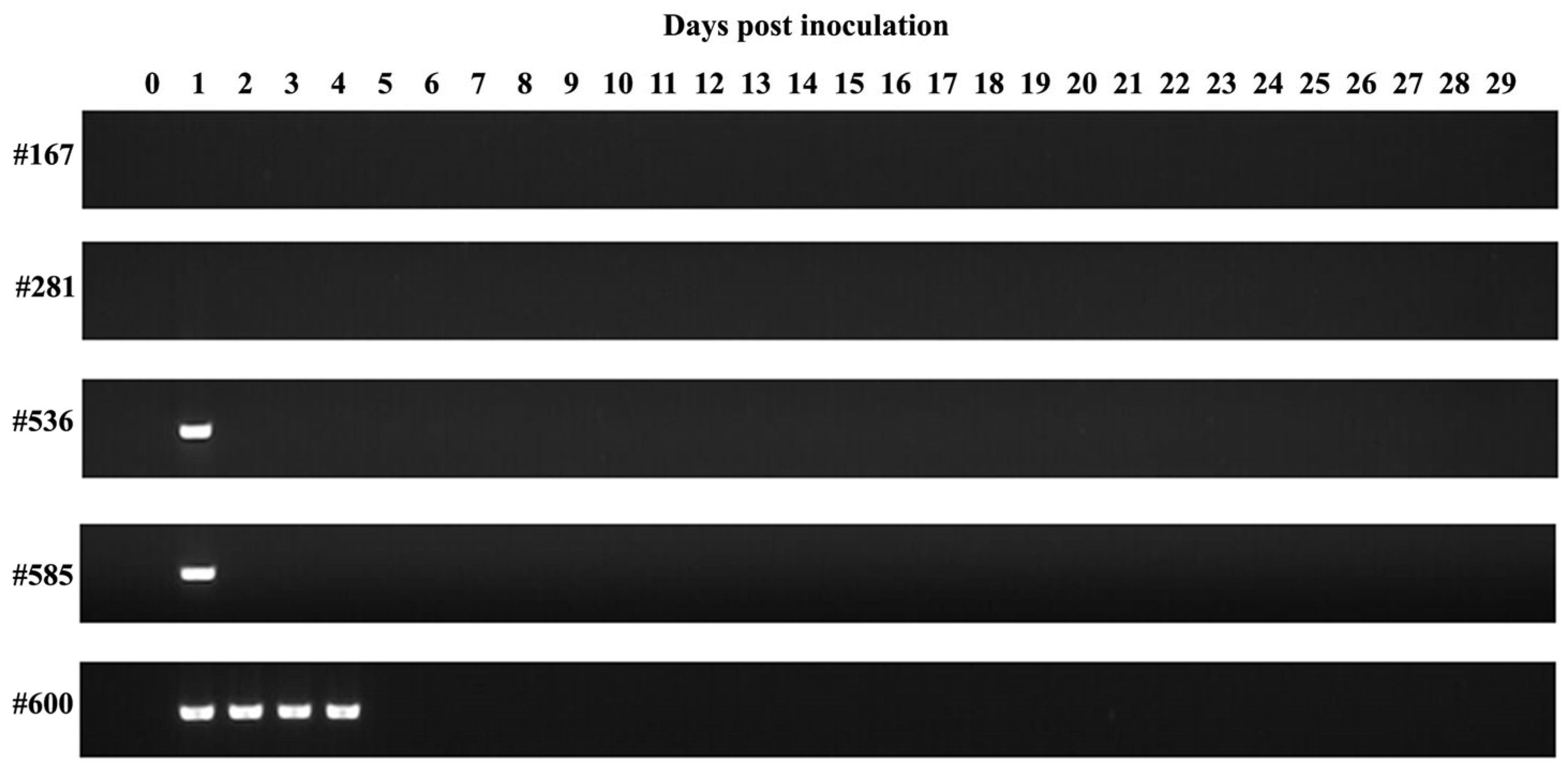
3.2. Babesia aktasi Failed to Infect Immune-Suppressed Sheep
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kappes, A.; Tozooneyi, T.; Shakil, G.; Railey, A.F.; McIntyre, K.M.; Mayberry, D.E.; Rushton, J.; Pendell, D.L.; Marsh, T.L. Livestock health and disease economics: A scoping review of selected literature. Front. Vet. Sci. 2023, 10, 1168649. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Estrada-Peña, A.; Zintl, A. Vectors of babesiosis. Annu. Rev. Entomol. 2019, 64, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Ganzinelli, S.; Bhoora, R.; Omondi, D.; Nijhof, A.M.; Florin-Christensen, M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022, 121, 1207–1245. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, G.; Santamaría-Espinosa, R.M.; Lira-Amaya, J.J.; Figueroa, J.V. Challenges in tick-borne pathogen detection: The case for Babesia spp. identification in the tick vector. Pathogens 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Sevinc, M.; Ekici, O.D.; Yildiz, R.; Isik, N.; Aydogdu, U. Babesia ovis infections: Detailed clinical and laboratory observations in the pre- and post-treatment periods of 97 field cases. Vet. Parasitol. 2013, 191, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S. Haemoparasites—Challenging and wasting infections in small ruminants: A review. Animals 2020, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Galon, E.M.; Zafar, I.; Ji, S.; Li, H.; Ma, Z.; Xuan, X. Molecular reports of ruminant Babesia in Southeast Asia. Pathogens 2022, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Yeruham, I.; Hadani, A.; Galker, F. Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis—A review. Vet. Parasitol. 1998, 74, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yeruham, I.; Hadani, A.; Galker, F. The Effect of the ovine host parasitaemia on the development of Babesia ovis (Babes, 1892) in the tick Rhipicephalus bursa (Canestrini and Fanzago, 1877). Vet. Parasitol. 2001, 96, 195–202. [Google Scholar] [CrossRef]
- Liu, A.H.; Yin, H.; Guan, G.Q.; Schnittger, L.; Liu, Z.J.; Ma, M.L.; Dang, Z.S.; Liu, J.L.; Ren, Q.Y.; Bai, Q.; et al. At least two genetically distinct large Babesia species infective to sheep and goats in China. Vet. Parasitol. 2007, 147, 246–251. [Google Scholar] [CrossRef]
- Iqbal, F.; Fatima, M.; Shahnawaz, S.; Naeem, M.; Shaikh, R.S.; Ali, M.; Shaikh, A.S.; Aktas, M.; Ali, M. A study on the determination of risk factors associated with babesiosis and prevalence of Babesia sp., by PCR amplification, in small ruminants from Southern Punjab (Pakistan). Parasite 2011, 18, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Liu, Z.; Yang, J.; Yu, P.; Pan, Y.; Zhai, B.; Luo, J.; Yin, H. Genetic diversity and molecular characterization of Babesia motasi-like in small ruminants and ixodid ticks from China. Infect. Genet. Evol. 2016, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.-M.; Oosthuizen, M.C.; Peirce, M.A.; Venter, E.H.; Penzhorn, B.L. Babesia Lengau sp. nov., a novel Babesia species in cheetah (Acinonyx Jubatus, Schreber, 1775) populations in South Africa. J. Clin. Microbiol. 2010, 48, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Ozubek, S.; Ulucesme, M.C.; Aktas, M. Discovery of a novel species infecting goats: Morphological and molecular characterization of Babesia aktasi n. sp. Pathogens 2023, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Ozubek, S.; Ulucesme, M.C.; Bastos, R.G.; Alzan, H.F.; Laughery, J.M.; Suarez, C.E.; Aktas, M. Experimental infection of non-immunosuppressed and immunosuppressed goats reveals differential pathogenesis of Babesia aktasi n. sp. Front. Cell. Infect. Microbiol. 2023, 13, 1277956. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Liu, G.; Liu, D.; Ren, J.; Li, X. Isolation and preliminary characterization of a large Babesia sp. from sheep and goats in the eastern part of Gansu province, China. Parasitol. Res. 2002, 88, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Luo, J.; Guan, G.; Liu, Z.; Ma, M.; Liu, A.; Gao, J.; Ren, Q.; Li, Y.; Qiu, J. Differentiation of two ovine Babesia based on the ribosomal DNA Internal Transcribed Spacer (ITS) sequences. Exp. Parasitol. 2009, 121, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ulucesme, M.C.; Ozubek, S.; Karoglu, A.; Turk, Z.I.; Olmus, I.; Irehan, B.; Aktas, M. Small ruminant piroplasmosis: High prevalence of Babesia aktasi n. sp. in goats in Türkiye. Pathogens 2023, 12, 514. [Google Scholar] [CrossRef]
- Aydin, M.F.; Aktas, M.; Dumanli, N. Molecular identification of Theileria and Babesia in sheep and goats in the Black Sea Region in Turkey. Parasitol. Res. 2013, 112, 2817–2824. [Google Scholar] [CrossRef]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. App. Environ. Microbiol. 2006, 72, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.P.J.; de Vos, S.; Taoufik, A.; Sparagano, O.A.E.; Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002, 89, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, M.C.; Zweygarth, E.; Collins, N.E.; Troskie, M.; Penzhorn, B.L. Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838). J. Clin. Microbiol. 2008, 46, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Sevinc, F.; Cao, S.; Xuan, X.; Sevinc, M.; Ceylan, O. Identification and expression of Babesia ovis secreted antigen 1 and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2015, 53, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Turgut, K.; Sevinc, M.; Ekici, O.D.; Coskun, A.; Koc, Y.; Erol, M.; Ica, A. Therapeutic and prophylactic efficacy of imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 2007, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Ma, M.; Moreau, E.; Liu, J.; Lu, B.; Bai, Q.; Luo, J.; Jorgensen, W.; Chauvin, A.; Yin, H. A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum. Exp. Parasitol. 2009, 122, 261–267. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Transmission of Babesia. In Babesiosis of Domestic Animals and Man; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-1-351-07002-7. [Google Scholar]
- Liu, Q.; Zhao, J.L.; Zhou, Y.Q.; Liu, E.Y.; Yao, B.A.; Fu, Y. Study on some molecular characterization of Babesia orientalis. Vet. Parasitol. 2005, 130, 191–198. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997, 39, 99–109. [Google Scholar]
- Lewis, D.; Holman, M.R.; Purnell, R.E.; Young, E.R.; Herbert, I.V.; Bevan, W.J. Investigations on Babesia motasi isolated from wales. Res. Vet. Sci. 1981, 31, 239–243. [Google Scholar] [CrossRef]
- Guan, G.; Moreau, E.; Liu, J.; Hao, X.; Ma, M.; Luo, J.; Chauvin, A.; Yin, H. Babesia sp. BQ1 (Lintan): Molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 2010, 59, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Persinger, K.A.; Taus, N.S.; Davis, S.K.; Poh, K.C.; Kappmeyer, L.S.; Laughery, J.M.; Capelli-Peixoto, J.; Lohmeyer, K.H.; Ueti, M.W.; et al. Nilgai antelope display no signs of infection upon experimental challenge with a virulent Babesia bovis strain. Parasit Vectors 2024, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Olafson, P.U.; Freeman, J.M.; Johnson, W.C.; Scoles, G.A. A Virulent Babesia bovis strain failed to infect white-tailed deer (Odocoileus virginianus). PLoS ONE 2015, 10, e0131018. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Moreau, E.; Brisseau, N.; Luo, J.; Yin, H.; Chauvin, A. Determination of erythrocyte susceptibility of Chinese sheep (Tan Mutton Breed) and French sheep (Vendéen Breed) to Babesia sp. BQ1 (Lintan) by in vitro culture. Vet. Parasitol. 2010, 170, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.; Mesplet, M.; Echaide, I.; de Echaide, S.T.; Schnittger, L.; Florin-Christensen, M. Mitigated clinical disease in water buffaloes experimentally infected with Babesia bovis. Ticks Tick Borne Dis. 2018, 9, 1358–1363. [Google Scholar] [CrossRef]
| Donor ID | Cryopreserved Stabliate | Prepatent Period | Max. Fever | Max. PPE | Clinical Signs | Response to Treatment | Death |
|---|---|---|---|---|---|---|---|
| #Donor-1 | B. aktasi 10% PPE | 4 dpi | 41.1 °C | 9.2% | + * | - | + |
| #Donor-2 | B. aktasi 12% PPE | 5 dpi | 42.2 °C | 35% | + * | - | + |
| Lamb ID | Source of Infection and Parasitemia (%) | Inoculum Amount and Route | Clinical Findings | Microscopic Examination | Nested PCR |
|---|---|---|---|---|---|
| #281 | #Donor-1 (9.2%) | 15 mL (iv) | - | - | - |
| #167 | #Donor-1 (9.2%) | 15 mL (iv) | - | - | - |
| #536 | #Donor-2 (12%) | 15 mL (iv) | - | - | + |
| #585 | #Donor-2 (12%) | 15 mL (iv) | - | - | + |
| #600 | #Donor-2 (12%) | 15 mL (iv) | - | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulucesme, M.C.; Ozubek, S.; Aktas, M. Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi. Vet. Sci. 2024, 11, 359. https://doi.org/10.3390/vetsci11080359
Ulucesme MC, Ozubek S, Aktas M. Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi. Veterinary Sciences. 2024; 11(8):359. https://doi.org/10.3390/vetsci11080359
Chicago/Turabian StyleUlucesme, Mehmet Can, Sezayi Ozubek, and Munir Aktas. 2024. "Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi" Veterinary Sciences 11, no. 8: 359. https://doi.org/10.3390/vetsci11080359
APA StyleUlucesme, M. C., Ozubek, S., & Aktas, M. (2024). Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi. Veterinary Sciences, 11(8), 359. https://doi.org/10.3390/vetsci11080359








