Simple Summary
Domestic cats kept in modern household environments are susceptible to various oral health issues, including halitosis, tartar accumulation, periodontal disease, and gingivitis. While these issues may initially go unnoticed, they can become challenging to manage as they progress. Proactive measures akin to human oral care, such as tooth brushing and dietary modifications, are crucial for preventing oral diseases in cats. Probiotics are widely employed in the prevention and treatment of oral diseases in humans, providing pet owners with a viable long-term preventive approach compared to brushing. Within this context, our study found that supplementing a composite probiotic formulation can inhibit potential pathogens in the oral cavity of cats and promote the growth of beneficial or commensal bacteria, potentially enhancing oral health in cats.
Abstract
Probiotics demonstrated effectiveness in modulating oral microbiota and improving oral health in humans and rodents. However, its effects and applications on the oral microbiota of cats remain underexplored. Twelve healthy cats were randomly assigned to a control group (CON) and a composite probiotic group (CPG) for a 42-day trial. The CPG diet included additional supplementation of Bifidobacterium animalis subsp. lactis HN019, Lactobacillus acidophilus NCFM, and Lactobacillus casei LC-11, each at approximately 1 × 1010 CFU/kg. On days 0 and 42, microbial samples were collected from the gingiva, tooth surfaces, and tongue of all cats for 16S rRNA gene sequencing. Bacteroidetes, Firmicutes, and Proteobacteria were the dominant phyla across all oral sites. The CPG treatment enriched seven genera, such as Moraxella, Actinomyces, and Frederiksenia in the gingiva. Meanwhile, Bergeyella and Streptococcus were enriched on the tooth surfaces, while Bergeyella, Flavobacterium, and Luteimonas were enriched on the tongue. Furthermore, the composite probiotic effectively suppressed eight genera, such as Bacteroides, Desulfovibrio, and Filifactor in the gingiva of CPG cats, as well as Helcococcus, Lentimicrobium, and Campylobacter on tooth surfaces, and Porphyromonas, Treponema, and Fusibacter on the tongue. These findings suggest that the composite probiotic used in this study modulates the feline oral microbiota by supporting beneficial or commensal bacteria and inhibiting oral pathogens, demonstrating potential to improve oral health in cats.
1. Introduction
Oral health plays a crucial role in animal well-being, serving as the primary gateway for immune and digestive systems. In modern household management, cats are frequently diagnosed with oral health issues such as halitosis, dental calculus, and periodontal disease [1]. Mild oral problems such as halitosis can impact emotional bonding between cats and their owners, while severe conditions such as gingivitis and periodontitis lead to inflammation and pain in cats’ mouths, affecting their ability to eat and overall health [2]. The oral microbiota, the second-largest microbial reservoir in animals after the gut, is closely linked to the occurrence and progression of feline oral diseases [3,4]. Microorganisms play a pivotal role in the formation of dental plaque, which can trigger serious oral diseases, including periodontitis and gingivitis [5]. Typically, dysbiosis of the oral microbiota promotes proliferation and colonization of microorganisms within biofilms, thereby facilitating the formation of dental plaque and persistent dental calculus [3,6]. Moreover, if dental calculus forms above and below the gum line, the accumulation of dental plaque and its metabolites can irritate and induce tissue inflammation, leading to gingivitis and periodontitis [7].
Once dental calculus forms, professional periodontal treatment such as scaling is necessary to remove oral biofilm [8]. Therefore, preventive measures against dental plaque and calculus formation through daily oral care are crucial. However, maintaining regular physical brushing or chemical adjunct rinsing for cats may pose a challenge for owners. Hence, dietary interventions to modulate the balance of oral microbiota on a daily basis might offer a long-term effective approach for preventing oral issues in household cats. Probiotics may influence oral microbiota through mechanisms such as competitive inhibition, antimicrobial substance production, and immunoregulation [9,10], proving to be a promising strategy in alleviating periodontal diseases in humans and rodents [11,12]. Probiotics, including Bifidobacterium animalis subsp. lactis HN019 [11], Lactobacillus acidophilus NCFM [13], and Lactobacillus casei LC-11 [14] were demonstrated to improve the oral environment by inhibiting pathogenic strains’ growth, creating an acidic environment, and suppressing biofilm formation. Therefore, supplementing composite probiotics in diets may have potential benefits for promoting oral health in cats. However, current research on the effects of probiotics on cats primarily focused on gastrointestinal health, leaving a gap in understanding their impact on feline oral microbiota.
The oral cavity exhibits a complex structure, where varied microbial characteristics are determined by the growth conditions of different sites [15]. Generally, aerobic microorganisms tend to colonize areas more exposed to air, such as the tongue, oral mucosa, and tooth surfaces, while gingival crevices and subgingival areas favor anaerobic bacterial growth [16]. A study indicated that seven distinct sites within the human oral cavity harbor three types of bacterial communities, with significant differences observed between the microbial communities in subgingival and supragingival regions compared to other sites (hard palate, keratinized gingiva, buccal mucosa, tongue, and saliva) [17]. In addition, comparable research was conducted with dogs. A series of studies demonstrated that the oral microbial community of dogs exhibits different diversity and composition influenced by factors such as habitat surface type, pH, and oxygen tension [18,19]. Moreover, bacterial communities in supragingival and subgingival sites are most closely associated with oral diseases in dogs [20]. However, previous studies on the sequencing of feline oral microbiota mainly relied on samples collected from a single site or a mixture of multiple sites, with little focus on the simultaneous assessment of microbial variations across different sites within the feline oral cavity in a single study.
Therefore, this study supplemented three probiotic strains known for their beneficial effects on oral health in humans and rodents into the daily diet of cats. Utilizing high-throughput sequencing techniques, we analyzed the microbial composition changes across three oral sites (gingiva, tooth surfaces, and tongue) in cats to evaluate the potential effects of oral administration of the composite probiotic on modulating microbial compositions and improving the oral environment. This research aims to support the maintenance of feline oral microbiota balance and proposes novel nutritional strategies for promoting oral health in cats.
2. Materials and Methods
2.1. Animals
The study was approved by the Institutional Animal Care and Use Committee of China Agricultural University (AW50503202-2-6). Twelve neutered cats, consisting of six Chinese domestic cats and six British Shorthair cats, with an equal distribution of males and females, were recruited for the study. These cats had not received any oral care previously, but were found to be free from any oral diseases. Before the start of the research, a medical examination was performed on the experimental cats, which included blood and serum analyses, appetite, body condition, fecal score, and parasites. The results indicate that all cats were in good health. During the three months preceding the experiment, the cats were not administered antibiotics, therapeutic drugs, or any diets targeting the studied function. The cats had a median weight of 4.35 kg (with a range of 3.62–5.15 kg) and a median age of 3 years (with a range of 2–4 years). Based on a 9-point body condition score scale, these cats had a similar body condition score of 5.50 ± 0.50 points. All cats were housed at the Pet Feeding Center, Ministry of Agriculture and Rural Affairs Feed Industry Centre, China Agricultural University, and received care in accordance with the National Research Council’s guidelines.
2.2. Study Design
This study randomly assigned twelve cats into two groups, with six cats in each group evenly distributed by gender and breed. The control group (CON) was fed a basal diet, while the composite probiotic group (CPG) received the basal diet with a post-sprayed composite probiotic formulation. The CPG diet contained Bifidobacterium animalis subsp. lactis HN019, Lactobacillus acidophilus NCFM, and Lactobacillus casei LC-11, each at approximately 1 × 1010 CFU/kg. The composite probiotic preparation was purchased from Aikoyou Health Technology Co., Ltd. (Suzhou, China). The probiotic supplement is a powder formulated with maltodextrin as a carrier and a mixture of Bifidobacterium animalis subsp. lactis HN019, Lactobacillus acidophilus NCFM, and Lactobacillus casei LC-11, each at a concentration of approximately 2 × 1010 CFU/g. The experimental diets for cats in both CON and CPG groups were prepared by Hangzhou Wangmiao Biotechnology Co., Ltd. (Hangzhou, China). The nutritional level and composition of the basal diet are presented in Table 1. According to the Official Methods of Analysis of AOAC International [21], feed ingredients were analyzed in terms of moisture, crude protein, crude fat, crude fiber, and ash. All chemical compositions were analyzed in duplicate. The study lasted for 42 days, during which each cat had ad libitum access to food and water. Before the formal trial, all cats were fed with the same basal diet for 30 days. The feeding rate and all nutritional requirements met the NRC (2006) recommendations for adult cats.

Table 1.
Dietary composition and nutritional level of basal diet.
2.3. Sample Collection
On days 0 and 42 of the experiment, microbial samples were collected from the gums, tooth surfaces, and tongue of all cats. To minimize food residue in the oral cavity, the cats’ diet was removed 8 h prior to sampling, while water was freely available. All procedures were conducted using sterile gloves and standard aseptic techniques. Sterile swabs were gently brushed over the upper and lower gums, as well as the dorsal and ventral surfaces of the tongue to collect microbial samples, avoiding contact with the teeth. Each swab was wiped three times on each side, for 5 s each time. Tooth surface microbes were collected using sterile swabs from teeth labeled as 104, 204, 108, and 208 in the upper jaw and 304, 404, 309, and 409 in the lower jaw, according to the modified Triadan system [22]. Three swab samples were taken from each site of each cat, individually placed in cryotubes, and immediately stored at −80 °C.
2.4. Oral Microbiota Analysis
Total microbial genomic DNA was extracted from the swab samples using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA). Amplification of 16S rRNA from the V3-V4 regions was performed using primer 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The purified PCR products were combined in equimolar proportions and subjected to paired-end sequencing using the Illumina MiSeq platform (San Diego, CA, USA). The demultiplexed sequencing reads were subjected to quality control using fastp software (v0.19.6) and spliced with Flash software (v1.2.11) following the method described in our previous study [23]. Subsequently, the feature table containing operational taxonomic units (OTUs) was generated employing UPARSE (v11) with a 97% similarity. To annotate the sequences of OTUs, the RDP classifier Bayesian algorithm (v2.13), with a confidence threshold of 0.7, was applied based on the Silva138/16S_bacteria database. The community composition of each sample was analyzed at different taxonomic levels. Mothur (v1.30.2) was utilized to assess the α-diversity via Faith’s phylogenetic diversity (Faith’s PD) as well as Ace, Chao, Simpson, Shannon, and Sobs indexes. β-diversity was evaluated by calculating the weighted UniFrac distance with ANOSIM examination and then visualized through principal coordinate analysis (PCoA). Linear discriminant analysis effect size (LEfSe) was employed to identify distinct differences in bacterial taxa at the phylum, family, and genus levels between the two treatments (LDA score > 3 or 4, p < 0.05).
2.5. Statistical Analysis
Statistical analysis was conducted on all data using IBM SPSS 26.0 (Chicago, IL, USA), and graphical representation of α-diversity was generated using GraphPad Prism 9.0 (San Diego, CA, USA). The raw sequencing data of microbiomes were analyzed using R tools to generate visualizations. Bar plots for the microbiota composition were created using the R ggplot package, and community heatmap analysis was generated using the R vegan package. The Wilcoxon rank-sum test was applied to assess intergroup differences in diversity indices and microbiota abundance. Multiple testing correction was performed using the false discovery rate method. Bootstrap resampling was employed to calculate the confidence intervals with a 95% confidence level. p < 0.05 was deemed statistically significant. The results are presented as the means ± SEMs.
3. Results
3.1. Oral Microbial Composition in Cats at Baseline
Illumina sequencing yielded an average of 58,846 16S rRNA amplicon sequences per sample post quality filtering. Subsequent analyses were performed on all samples rarified to a depth of 18,583 sequences. The α-diversity indicators comprised Ace, Chao, Faith’s PD, Simpson, Shannon, and Sobs. At the experiment’s baseline, no significant differences in microbial α-diversity were observed between the CON and CPG groups on the gingiva, tooth surfaces, and tongue (p ≥ 0.05, Figure 1A–F). Furthermore, employing weighted-UniFrac distances, PCoA analysis demonstrated no significant separation among the microbiota communities of the gingiva, tooth surfaces, and tongue in the CON and CPG groups at the baseline (p ≥ 0.05, Figure 1G–I). The relative abundance of feline oral microbiota with distinct differences at the phylum, family, and genus levels is shown in Table 2. In the gingival region, the abundance of Desulfomicrobiaceae, Caulobacteraceae, Desulfomicrobium, and norank_f_Propionibacteriaceae was significantly increased in the CPG group compared with the CON group (p < 0.05). Additionally, the relative abundance of Parabacteroides, Granulicatella, and unclassified_f_Anaerovoracaceae was lower in the CPG group than that in the CON group (p < 0.05). On the tooth surfaces, a significant increase in the relative abundance of Campilobacterota, unclassified_c_Gammaproteobacteria, Frederiksenia, unclassified_c_Gammaproteobacteria, and norank_f_Pasteurellaceae was observed in the CPG group compared to the CON group (p < 0.05). At the tongue site, the abundance of Synergistota, Synergistaceae, Fretibacterium, and Prevotellaceae_UCG-003 was markedly decreased in the CPG group compared with the CON group (p < 0.05). Moreover, the abundance of unclassified_f_Lachnospiraceae was higher in the CPG group than that in the CON group (p < 0.05).
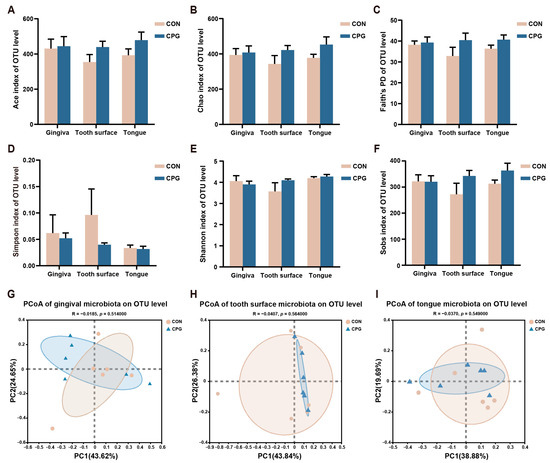
Figure 1.
Differences in α- and β-diversity of oral microbiota in cats at baseline. (A–F) Ace index, Chao index, Faith’s phylogenetic diversity, Simpson index, Shannon index, and Sobs index at the OTU level. (G–I) Principal coordinate analysis (PCoA) based on weighted UniFrac distance at the OTU level of cat gum, tooth surface, and tongue microbiota. CON, cats fed with a basal diet; and CPG, cats received the basal diet with a post-sprayed composite probiotic formulation. Values are mean ± SEM (n = 6).

Table 2.
Differences of oral microbial composition in cats at baseline.
3.2. Effects of Composite Probiotics on α- and β-Diversity of Oral Microbiota in Cats
The oral microbial diversity of cats in different treatments is illustrated in Figure 2. On day 42, the CON and CPG treatments did not exhibit any significant differences in terms of microbial community diversity (Faith’s PD, Simpson, and Shannon), nor in community richness (Ace, Chao, and Sobs indexes), across the gingival, tooth surface, and tongue regions of cats (p ≥ 0.05, Figure 2A–F). However, the PCoA result using weighted-UniFrac distances reveals a discernible segregation of microbial communities between the two experimental treatments, with distinct clustering observed in both the gingival and tongue samples (p < 0.05, Figure 2G,I). In addition, no significant clustering of microbial communities on the tooth surfaces was observed between the CON and CPG groups in the weighted PCoA plots (p ≥ 0.05, Figure 2H).
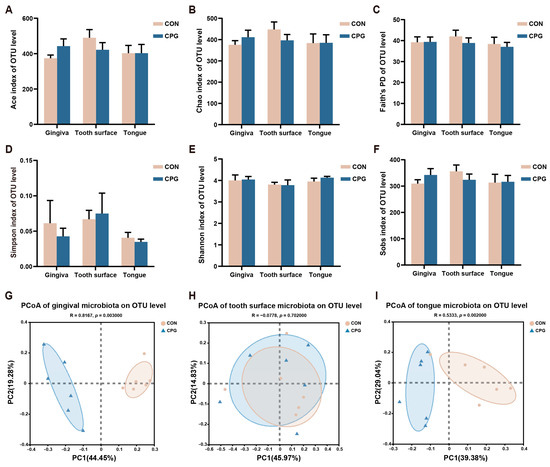
Figure 2.
Differences in α- and β-diversity of oral microbiota in different parts of cats on day 42. (A–F) Ace index, Chao index, Faith’s phylogenetic diversity, Simpson index, Shannon index, and Sobs index at the OTU level. (G–I) Principal coordinate analysis (PCoA) based on weighted UniFrac distance at the OTU level of cat gum, tooth surface, and tongue microbiota. CON, cats fed with a basal diet; and CPG, cats received the basal diet with a post-sprayed composite probiotic formulation. Values are mean ± SEM (n = 6).
3.3. Effects of Composite Probiotics on the Composition of Gingival Microbiota in Cats
For gingival microbiota in cats, a total of 407 shared OTUs were identified, alongside 173 (CON) and 427 (CPG) specific OTUs. The gingival microbiota was categorized into 17 phyla, 34 classes, 83 orders, 143 families, 255 genera, and 480 species. The top 3 of the gingival microbial abundance in each group at the phylum level were both: Bacteroidota (CON: 26.59%; CPG: 30.27%), Firmicutes (CON: 39.07%; CPG: 15.50%), and Proteobacteria (CON: 7.18%; CPG: 32.97%), comprising over 70% of the total sequences (Figure 3A). Furthermore, the abundance of Firmicutes, Patescibacteria, Desulfobacterota and Spirochaetota was markedly reduced in the CPG treatment compared with those in the CON treatment (p < 0.05, Table 3). The abundances of Proteobacteria and Actinobacteriota were higher in the CPG treatment than those in the CON treatment (p < 0.05, Table 3).
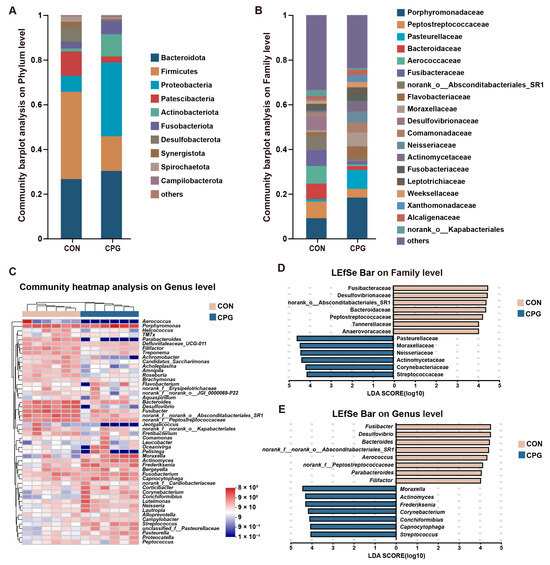
Figure 3.
Effects of different treatments on the composition of gingival microbiota in cats on day 42. (A,B) Community barplot analysis on the phylum and family levels. (C) Community heatmap analysis on the genus level. (D,E) Linear discriminant analysis effect size (LEfSe) analysis on the family and genus levels. CON, cats fed with a basal diet; and CPG, cats received the basal diet with a post-sprayed composite probiotic formulation.

Table 3.
Differential composition of gingival microbiota in cats on day 42.
The most abundant families of gingival microbiota (Figure 3B) included Porphyromonadaceae (CON: 9.08%; CPG: 18.32%), Peptostreptococcaceae (CON: 7.41%; CPG: 3.93%), Pasteurellaceae (CON: 1.11%; CPG: 8.41%), and Bacteroidaceae (CON: 6.97%; CPG: 1.73%). In terms of genus (Figure 3C), the dominant bacteria were Porphyromonas (CON: 9.08%; CPG: 18.32%), Bacteroides (CON: 6.97%; CPG: 1.73%), and Fusibacter (CON: 7.24%; CPG: 1.37%). Further, using the LEfSe (LDA score > 4) in conjunction with the Wilcoxon rank-sum test for differential analysis, our results indicate significant enrichment of Fusibacteraceae, Desulfovibrionaceae, norank_o_Absconditabacteriales_SR1, Bacteroidaceae, Peptostreptococcaceae, Anaerovoracaceae, Tannerellaceae, Fusibacter, Desulfovibrio, Bacteroides, norank_f_norank_o_Absconditabacteriales_SR1, Aerococcus, norank_f_Peptostreptococcaceae, Parabacteroides, and Filifactor in the gingival samples of the CON group (p < 0.05), while Pasteurellaceae, Moraxellaceae, Neisseriaceae, Actinomycetaceae, Corynebacteriaceae, Streptococcaceae, Moraxella, Actinomyces, Frederiksenia, Capnocytophaga, Corynebacterium, Conchiformibius, and Streptococcus were notably enriched in the gingival microbiota of the CPG group (p < 0.05, Figure 3D,E and Table 3).
3.4. Effects of Composite Probiotics on the Composition of Tooth Surface Microbiota in Cats
For tooth surface microbiota in cats, a total of 524 shared OTUs were identified, alongside 360 (CON) and 308 (CPG) specific OTUs. The taxonomic classification of the microbiota encompassed 17 phyla, 32 classes, 74 orders, 129 families, 233 genera, and 445 species. In terms of phylum (Figure 4A), the dominant bacteria were Bacteroidota (CON: 32.90%; CPG: 30.43%), Firmicutes (CON: 26.23%; CPG: 25.52%), and Proteobacteria (CON: 21.06%; CPG: 24.67%), accounting for approximately 80% of all bacterial phyla. In terms of family (Figure 4B), the Porphyromonadaceae (CON: 20.97%; CPG: 12.54%), Staphylococcaceae (CON: 8.52%; CPG: 11.41%), Pasteurellaceae (CON: 6.55%; CPG: 8.69%), and Moraxellaceae (CON: 7.56%; CPG: 7.19%) were dominated. In addition, the most abundant genera of tooth surface bacteria (Figure 4C) included Porphyromonas (CON: 20.97%; CPG: 12.54%), Moraxella (CON: 7.56%; CPG: 7.19%), and Staphylococcus (CON: 2.94%; CPG: 9.23%). Although PCoA results indicate no significant impact of dietary supplementation with the composite probiotic on the bacterial structure of cat tooth surfaces, the LEfSe analysis (LDA score > 3) identified enrichment of five families and six genera on the tooth surfaces of both treatments. In detail, the CPG treatments exhibited a significantly elevated abundance of Weeksellaceae, Streptococcaceae, Bergeyella, and Streptococcus compared to the CON treatments (p < 0.05, Figure 4D,E and Table 4). Meanwhile, dietary supplementation with the composite probiotic markedly decreased the abundance of Lachnospiraceae, Campylobacteraceae, Lentimicrobiaceae, Catonella, Helcococcus, Campylobacter, and Lentimicrobium on the tooth surfaces of cats compared with the CON treatment (p < 0.05, Figure 4D,E and Table 4).
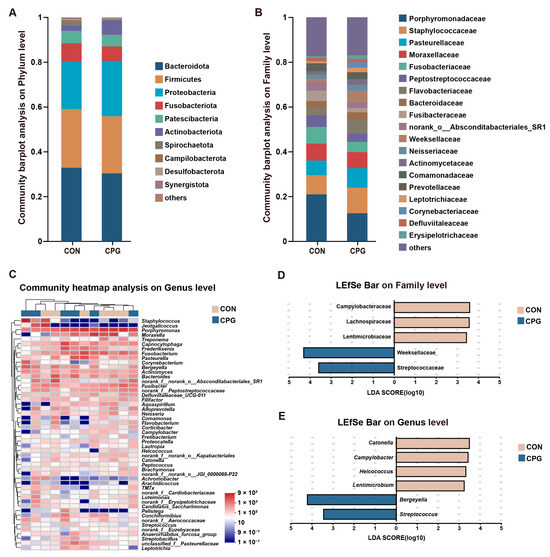
Figure 4.
Effects of different treatments on the composition of tooth surface microbiota in cats on day 42. (A,B) Community barplot analysis on the phylum and family levels. (C) Community heatmap analysis on the genus level. (D,E) Linear discriminant analysis effect size (LEfSe) analysis on the family and genus levels. CON, cats fed with a basal diet; and CPG, cats received the basal diet with a post-sprayed composite probiotic formulation.

Table 4.
Differential composition of tooth surface microbiota in cats on day 42.
3.5. Effects of Composite Probiotics on the Composition of Tongue Microbiota in Cats
For tongue microbiota in cats, a total of 463 shared OTUs were identified, alongside 342 (CON) and 259 (CPG) specific OTUs. The tongue microbiota was categorized into 18 phyla, 35 classes, 81 orders, 139 families, 246 genera, and 449 species. In terms of phylum (Figure 5A), the Bacteroidota (CON: 37.87%; CPG: 29.72%), Proteobacteria (CON: 13.90%; CPG: 34.36%), and Firmicutes (CON: 23.28%; CPG: 17.26%) were dominated. The results of the Wilcoxon rank-sum test indicate a distinct increase in the abundance of Proteobacteria (p < 0.05) and a significant decrease in the abundance of Spirochaetota (p < 0.05) in the tongue microbiota of the CPG group compared to the CON group (Table 5). At the family level (Figure 5B), the most abundant bacteria were Porphyromonadaceae (CON: 25.33%; CPG: 10.67%), Pasteurellaceae (CON: 6.42%; CPG: 8.70%), Moraxellaceae (CON: 3.67%; CPG: 8.41%), and Flavobacteriaceae (CON: 2.46%; CPG: 8.79%). The dominant genera (Figure 5C) included Porphyromonas (CON: 25.33%; CPG: 10.67%), Moraxella (CON: 3.66%; CPG: 8.40%), and Fusobacterium (CON: 5.46%; CPG: 2.82%). LEfSe analysis (LDA score > 4) identified enrichment of eight families and six genera in the two treatments. Among them, four families (Porphyromonadaceae, Spirochaetaceae, Fusibacteraceae, and Peptostreptococcaceae) were enriched in the CON group (p < 0.05), while four families (Flavobacteriaceae, Xanthomonadaceae, Weeksellaceae, and Neisseriaceae) were enriched in the CPG group (p < 0.05, Figure 5D and Table 5). Additionally, three genera (Porphyromonas, Treponema, and Fusibacter) were significantly enriched in the CON group (p < 0.05), while three genera (Luteimonas, Bergeyella, and Flavobacterium) were markedly enriched in the CPG group (p < 0.05, Figure 5E and Table 5).
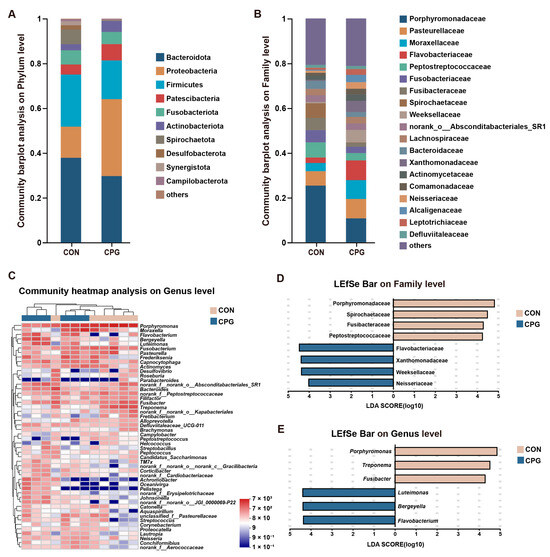
Figure 5.
Effects of different treatments on the composition of tongue microbiota in cats on day 42. (A,B) Community barplot analysis on the phylum and family levels. (C) Community heatmap analysis on the genus level. (D,E) Linear discriminant analysis effect size (LEfSe) analysis on the family and genus levels. CON, cats fed with a basal diet; and CPG, cats received the basal diet with a post-sprayed composite probiotic formulation.

Table 5.
Differential composition of tongue microbiota in cats on day 42.
4. Discussion
The oral cavity harbors a diverse microbiota that plays a crucial role in the occurrence and progression of oral diseases [24]. Dietary interventions aimed at modulating the structure and composition of the oral microbiota may offer approaches for improving oral health. Studies indicated that supplementing oral probiotics in daily diets can regulate the oral microbiome, effectively alleviating oral issues in humans and rodents [11,12]. However, there is currently a lack of research documenting the effects of oral probiotics on the oral microbiota of cats. Therefore, this study selected a composite probiotic preparation to investigate its regulatory effects on the oral microbiota of cats. The current results suggest that supplementing with the composite probiotic may alter the composition of the oral microbiota in different oral niches of cats. This is primarily manifested by an increase in the abundance of potentially beneficial or commensal bacteria such as Moraxella, Actinomyces, and Frederiksenia in the gingiva, Bergeyella, and Streptococcus on tooth surfaces, and Bergeyella, Flavobacterium, and Luteimonas on the tongue. Additionally, there is a decrease in the abundance of taxa associated with oral diseases, including Bacteroides, Desulfovibrio, and Filifactor in the gingiva, Helcococcus and Campylobacter on tooth surfaces, and Porphyromonas and Treponema on the tongue.
This study utilized a composite probiotic preparation composed of Bifidobacterium animalis subsp. lactis HN019, Lactobacillus acidophilus NCFM, and Lactobacillus casei LC-11. A previous in vitro study demonstrated that Bifidobacterium can inhibit the growth of periodontal pathogens, particularly Porphyromonas gingivalis, without inhibiting the beneficial oral bacterium Streptococcus [25]. Additionally, a human clinical study indicated that Bifidobacterium animalis subsp. lactis HN019 alleviated systemic inflammation in patients with advanced chronic periodontitis, reduced periodontal pathogen colonization, and potentially colonized subgingival biofilms [12]. The beneficial effects of Lactobacillus were extensively documented, including anti-inflammatory properties [26], reduction in halitosis [27], inhibition of periodontal pathogens [28], and decrease in biofilm formation through its metabolites, such as organic acids, hydrogen peroxide, and bacteriocins [29,30]. Furthermore, studies indicated that Lactobacillus acidophilus and Lactobacillus casei can ameliorate periodontal inflammation and modulate oral microbiota composition [13,14,31]. This study selected 12 healthy adult cats for a 42-day randomized controlled trial. Considering the individual variations among cats, we also compared the differences in oral microbial composition between the two groups at baseline. Baseline results indicate no significant differences in the α- and β-diversity of microbial communities on the gingiva, tooth surfaces, and tongue between the two groups before the start of the trial. Studies suggested that cats with gingivostomatitis and periodontitis tend to exhibit higher bacterial α-diversity indices (Shannon and Chao1) compared to healthy cats [3], although differences are not always observed [32]. Moreover, oral diseases in humans are associated with increased microbial diversity and abundance [33]. In this study, supplementation with the composite probiotic for 42 days did not result in significant changes in bacterial diversity and richness on the gingiva, tooth surfaces, and tongue, which is considered reasonable in the oral cavity of healthy cats. Furthermore, previous research demonstrated that diseased cats exhibit microbial dysbiosis in the oral cavity, as evidenced by β-diversity results showing significant separation of bacterial communities from healthy cats [3]. Dietary interventions can modulate the microbial structure and composition in the oral cavity of cats [34], and notably, by day 42 of the trial, supplementation with the composite probiotic preparation significantly altered the bacterial structure on the gingiva and tongue.
Further analysis of the oral microbiota composition revealed a consistent dominance of the bacterial phyla Bacteroidota, Firmicutes, and Proteobacteria, whether on the cat’s gums, the tooth surfaces, or tongues, aligning with the microbial composition observed in the oral cavity of healthy cats [35]. Previous reports indicated higher abundance of bacterial phyla such as Bacteroidota, Firmicutes, Synergistota, Chloroflexi, Fusobacteria, and Spirochaetota in cats suffering from periodontitis and gingivostomatitis compared to healthy counterparts, whereas Proteobacteria are more abundant in the oral cavity of healthy cats [3,36]. In this study, supplementation with probiotics resulted in a decrease in Spirochaetota on the gingiva and tongue of cats, accompanied by an increase in Proteobacteria, while Firmicutes decreased on the gingiva. Further, a significant reduction in Treponema, which is considered a marker of periodontal disease, was observed on the tongue. Treponema, belonging to the phylum Spirochaetota, amplifies the inflammatory response of host normal tissues by metabolizing cytotoxic products and utilizing its own structure and specific adhesion [37]. Moreover, Actinobacteriota, serving as the initial colonizers of the oral cavity, are compatible with periodontal health and ecological succession [36,38]. Actinobacteriota, particularly Actinomyces and Corynebacterium, exhibited significant enrichment at the gingiva of healthy cats supplemented with probiotics, which is consistent with previous studies [3,4]. Desulfovibrio, belonging to the phylum Desulfobacterota, was identified as a potential biomarker of periodontitis, with a significant decrease in abundance observed at the gingiva after probiotic supplementation in cats [39].
In this study, both Porphyromonadaceae and Pasteurellaceae consistently ranked among the top three most abundant bacterial families in the three oral sites of cats. Indeed, Porphyromonadaceae and Pasteurellaceae were described as the most prevalent bacterial taxa in the oral cavity of healthy cats [35]. Notably, Porphyromonas, although among the most abundant genera in the oral cavity of cats, is highly correlated with the prevalence of periodontal disease [40]. Some of these pathogenic bacteria can selectively inhibit bactericidal activity by evading host immune responses, thereby leading to microbiota dysbiosis and inflammatory microenvironments [41,42]. Additionally, Porphyromonas secretes lipopolysaccharides and extracellular proteases that degrade the soft tissues surrounding the teeth [42]. Conversely, studies demonstrated that the relative abundance of Frederiksenia, a genus within the Pasteurellaceae family, is increased in multiple oral sites of healthy cats compared to cats with gingivitis [4]. Our study found a decrease in Porphyromonas on the tongue and an increase in Frederiksenia on the gingiva of cats following supplementation with a composite probiotic, suggesting a potential protective effect on feline oral health. Furthermore, clinical research showed that, in addition to Porphyromonas, Moraxella and Fusobacterium are also dominant genera in the oral cavity of healthy cats [3], consistent with our findings. Interestingly, we observed differences in the abundance and order of dominant bacteria at the phylum, family, and genus levels across the gingiva, tooth surfaces, and tongue. Oral bacteria exhibit high variability, and microbial communities in different habitats may vary due to differences in host oral pH, oxygen supply, cell types, mucosal characteristics, and immune factors [17,43]. Additionally, it is noteworthy that microbial communities in the gingiva, tooth surfaces, and tongue exhibited different responses to probiotic supplementation, suggesting specific effects of the composite probiotic on the oral microbiota.
This study also found that supplementation with a composite probiotic for 42 days resulted in increased abundance of Streptococcus on the gingiva and tooth surfaces of cats, along with an elevated abundance of Bergeyella on the tongue and tooth surfaces. Previous research indicated that the abundance of Streptococcus and Bergeyella, as oral symbiotic bacteria, is negatively correlated with the development of dental calculus, plaque, and oral diseases [19,35]. Moreover, it was theorized in previous studies that the subgingival area is anaerobic and rich in peptides, and changes in the oral environment may promote overgrowth of Gram-negative and anaerobic bacteria, thereby driving the progression of oral diseases [44,45]. Consequently, cats with periodontal and gingival diseases showed a significant increase in Gram-negative and anaerobic bacteria, as reported in previous studies [3,32]. In addition to the bacteria mentioned above, this study also observed a significant decrease in the abundance of Gram-negative bacteria such as Bacteroides and Parabacteroides at the gingival, and Campylobacter and Lentimicrobium on the tooth surfaces in cats orally administered probiotics. Meanwhile, the CPG group also exhibited a reduction in several bacterial genera previously associated with oral diseases, including Filifactor [3], Aerococcus [46], and Helcococcus [47], and an increase in beneficial or commensal bacteria of healthy cats in the subgingival and tongue regions, including Capnocytophaga [48], Conchiformibius [4], Flavobacterium [4], and Luteimonas [49]. These findings suggest that the composite probiotic used in this study may potentially offer protective effects on feline oral health by balancing the oral microbiota and inhibiting the proliferation of bacteria associated with periodontal diseases.
This study has certain limitations. Firstly, the experimental period lasted for 42 days, and to observe more significant effects, longer-term feeding studies may be necessary. Secondly, this study focused on relatively small sample sizes of orally healthy cats, aiming to provide support for the use of composite probiotics in preventing oral diseases. Further research should involve larger cohorts of cats with existing oral diseases and explore the therapeutic effects of composite probiotics. Additionally, while this study offers valuable insights into the impact of probiotics on the oral microbiota of cats, further research is needed to elucidate the underlying mechanisms driving these observed changes, thus providing a more comprehensive understanding of the actions and applications of composite probiotics.
5. Conclusions
In this study, supplementation of the composite probiotic (Bifidobacterium animalis subsp. lactis HN019, Lactobacillus acidophilus NCFM, and Lactobacillus casei LC-11) was associated with changes in the oral microbiota of healthy cats. Microbial communities at different oral sampling sites responded differently to the probiotic formulation, with a decrease in potential pathogen abundance observed, including Bacteroides, Desulfovibrio, and Filifactor in the gingiva, Helcococcus and Campylobacter on tooth surfaces, and Porphyromonas and Treponema on the tongue. Additionally, the abundance of commensal or beneficial microbiota increased in the cat’s oral cavity after probiotic supplementation, such as Moraxella, Actinomyces, and Frederiksenia in the gingiva, Bergeyella and Streptococcus on tooth surfaces, and Bergeyella, Flavobacterium, and Luteimonas on the tongue. Therefore, the composite probiotic studied may contribute to promoting oral health in cats, but further research is needed to validate its potential for preventing or assisting in the treatment of oral diseases.
Author Contributions
Methodology, M.Z., X.M. and Y.C.; conceptualization, M.Z., X.M. and L.L.; software and data curation, H.W.; resources, Y.C. and X.M.; formal analysis, M.Z. and L.L.; investigation, Y.L. and L.L.; validation and visualization, X.M., M.Z. and Y.C.; writing—original draft preparation, M.Z.; writing—review and editing, Y.W. and Y.C.; project administration, supervision and funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Young Scientists Fund of the National Natural Science Foundation of China (32302752), Key Research and Development Program of Shaanxi (2022QCY-LL-52) and China National Postdoctoral Program for Innovation Talents (BX20230420).
Institutional Review Board Statement
The animal research protocol was approved by the Institutional Animal Care and Use Committee of China Agricultural University (protocol code AW50503202-2-6).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the findings in this research are available by contacting the corresponding author on request.
Acknowledgments
The authors would thank the faculty and staff at the Ministry of Agriculture and Rural Affairs Feed Industry Centre (Beijing, China) for their valuable assistance in conducting this research.
Conflicts of Interest
The authors declare no conflicts of interest. Xiaoying Mei and Longxian Li are employees of Wangmiao Biotechnology Co., Ltd. (Hangzhou, China). The paper reflects the views of the scientists, and not the company.
References
- Perry, R.; Tutt, C. Periodontal disease in cats: Back to basics—With an eye on the future. J. Feline Med. Surg. 2015, 17, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.B.; Verstraete, F.J.M.; Arzi, B. An Update on Feline Chronic Gingivostomatitis. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: Characterization and comparison between diseased and healthy cats. Sci. Rep. 2019, 9, 12340. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Rojas, C.A.; Scarsella, E.; Entrolezo, Z.; Jospin, G.; Hoffman, S.L.; Force, J.; MacLellan, R.H.; Peak, M.; Shope, B.H.; et al. The Oral Microbiome across Oral Sites in Cats with Chronic Gingivostomatitis, Periodontal Disease, and Tooth Resorption Compared with Healthy Cats. Animals 2023, 13, 3544. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef] [PubMed]
- Logan, E.I. Dietary influences on periodontal health in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.Y.; Steinbach-Rankins, J.M.; Demuth, D.R. Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J. Control. Release 2019, 297, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Wan, X.; Tao, H.; Yang, Q.; Zhao, X.; Liu, H.; Hu, J.; Luo, Y.; Shu, T.; Geng, R.; et al. Multi-function screening of probiotics to improve oral health and evaluating their efficacy in a rat periodontitis model. Front. Cell. Infect. Microbiol. 2023, 13, 1261189. [Google Scholar] [CrossRef]
- Van Holm, W.; Carvalho, R.; Delanghe, L.; Eilers, T.; Zayed, N.; Mermans, F.; Bernaerts, K.; Boon, N.; Claes, I.; Lebeer, S.; et al. Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms. NPJ Biofilms Microbiomes 2023, 9, 3. [Google Scholar] [CrossRef]
- Araujo, L.D.C.; Furlaneto, F.A.C.; da Silva, L.A.B.; Kapila, Y.L. Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases. Int. J. Mol. Sci. 2022, 23, 9334. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Dos Santos, V.R.; Duque, C.; Ervolino, E.; Mogami Bomfim, S.; Gomes-Filho, J.E. Systemic administration of probiotics reduces the severity of apical periodontitis. Int. Endod. J. 2019, 52, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Korte, F.; Dörfer, C.E.; Kneist, S.; Fawzy El-Sayed, K.; Paris, S. Inhibition of Streptococcus mutans Growth and Biofilm Formation by Probiotics in vitro. Caries Res. 2017, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.L.; Ramírez-Puebla, S.T.; Borisy, G.G. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe 2020, 28, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Ruparell, A.; Inui, T.; Staunton, R.; Wallis, C.; Deusch, O.; Holcombe, L.J. The canine oral microbiome: Variation in bacterial populations across different niches. BMC Microbiol. 2020, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.M.; Carroll, M.Q.; Alexander, C.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; Swanson, K.S. Dental chews positively shift the oral microbiota of adult dogs. J. Anim. Sci. 2021, 99, skab100. [Google Scholar] [CrossRef]
- Rober, M.; Quirynen, M.; Haffajee, A.D.; Schepers, E.; Teughels, W. Intra-oral microbial profiles of beagle dogs assessed by checkerboard DNA-DNA hybridization using human probes. Vet. Microbiol. 2008, 127, 79–88. [Google Scholar] [CrossRef][Green Version]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Floyd, M.R. The modified Triadan system: Nomenclature for veterinary dentistry. J. Vet. Dent. 1991, 8, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, R.; Wang, H.; Liu, T.; Zhang, G.; Wu, Y. Grape seed proanthocyanidin improves intestinal inflammation in canine through regulating gut microbiota and bile acid compositions. FASEB J. 2023, 37, e23285. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.G.B.; de Oliveira, A.M.; Tsute Chen, G.; Colombo, A.P.V. Oral-gut bacterial profiles discriminate between periodontal health and diseases. J. Periodontal Res. 2022, 57, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiao, L.; Shen, D.; Hao, Y. Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontol. Scand. 2010, 68, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Ervolino, E.; Plazza, F.; Mogami Bomfim, S.; Duarte, P.C.T.; Junior, V.; Gomes-Filho, J.E. Reduced bone resorption and inflammation in apical periodontitis evoked by dietary supplementation with probiotics in rats. Int. Endod. J. 2020, 53, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.G.; Carvalho, E.B.; Tinoco, E.M.B. Clinical effect of Lactobacillus on the treatment of severe periodontitis and halitosis: A double-blinded, placebo-controlled, randomized clinical trial. Am. J. Dent. 2019, 32, 9–13. [Google Scholar] [PubMed]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2009, 36, 506–513. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef]
- Imran, F.; Das, S.; Padmanabhan, S.; Rao, R.; Suresh, A.; Bharath, D. Evaluation of the efficacy of a probiotic drink containing Lactobacillus casei on the levels of periodontopathic bacteria in periodontitis: A clinico-microbiologic study. Indian J. Dent. Res. 2015, 26, 462–468. [Google Scholar] [CrossRef]
- Older, C.E.; Gomes, M.O.S.; Hoffmann, A.R.; Policano, M.D.; Reis, C.; Carregaro, A.B.; Ambrósio, C.E.; Carregaro, V.M.L. Influence of the FIV Status and Chronic Gingivitis on Feline Oral Microbiota. Pathogens 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Pyysalo, M.J.; Mishra, P.P.; Sundström, K.; Lehtimäki, T.; Karhunen, P.J.; Pessi, T. Increased tooth brushing frequency is associated with reduced gingival pocket bacterial diversity in patients with intracranial aneurysms. PeerJ 2019, 7, e6316. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.J.; Malik, R.; Browne, G.V.; Norris, J.M. Diet may influence the oral microbiome composition in cats. Microbiome 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, L.; Leylabadlo, H.E.; Pourlak, T.; Eslami, H.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Kafil, H.S. Oral spirochetes: Pathogenic mechanisms in periodontal disease. Microb. Pathog. 2020, 144, 104193. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D.; Qin, Y.; Tu, Q.; Jorgensen, M.; He, Z.; Wu, L.; et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018, 12, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, G.; Chen, M.; He, Y.; Yu, W.; Chen, X.; Mao, W.; Liu, N.; Zhang, Y.; Chang, Q.; et al. Oral microbial dysbiosis in patients with periodontitis and chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2023, 13, 1121399. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Salcedo, L.; Herrera, D.; Esteban-Saltiveri, D.; León, R.; Jeusette, I.; Torre, C.; O’Connor, A.; González, I.; González, I. Isolation and identification of Porphyromonas spp. and other putative pathogens from cats with periodontal disease. J. Vet. Dent. 2013, 30, 208–213. [Google Scholar] [CrossRef]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community-Rebels with a Cause. Curr. Oral Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sisk-Hackworth, L.; Ortiz-Velez, A.; Reed, M.B.; Kelley, S.T. Compositional Data Analysis of Periodontal Disease Microbial Communities. Front. Microbiol. 2021, 12, 617949. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.M.; Sieja, K.M.; Schauwecker, A.; Somrak, A.J.; Hristova, T.S.; Keating, S.C.J.; Swanson, K.S. Effects of a novel dental chew on oral health outcomes, halitosis, and microbiota of adult dogs. J. Anim. Sci. 2024, 102, skae071. [Google Scholar] [CrossRef] [PubMed]
- Dolieslager, S.M.; Riggio, M.P.; Lennon, A.; Lappin, D.F.; Johnston, N.; Taylor, D.; Bennett, D. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet. Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef]
- Thomas, S.; Lappin, D.F.; Nile, C.J.; Spears, J.; Bennett, D.; Brandt, B.W.; Riggio, M.P. Microbiome analysis of feline odontoclastic resorptive lesion (FORL) and feline oral health. J. Med. Microbiol. 2021, 70, 001353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).