Post-Chemotherapy Canine Lymphomatous Lymph Node Observations on B-Mode and Strain Elastographic Ultrasound
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analysis of Ultrasonographic Images
2.3. Statistical Analysis
3. Results
3.1. Clinical Demographic Data
3.2. Comparison of US Parameters between NLNs and LLNs
3.3. Comparison of US Parameters among LLN at Different Time Points of Chemotherapy
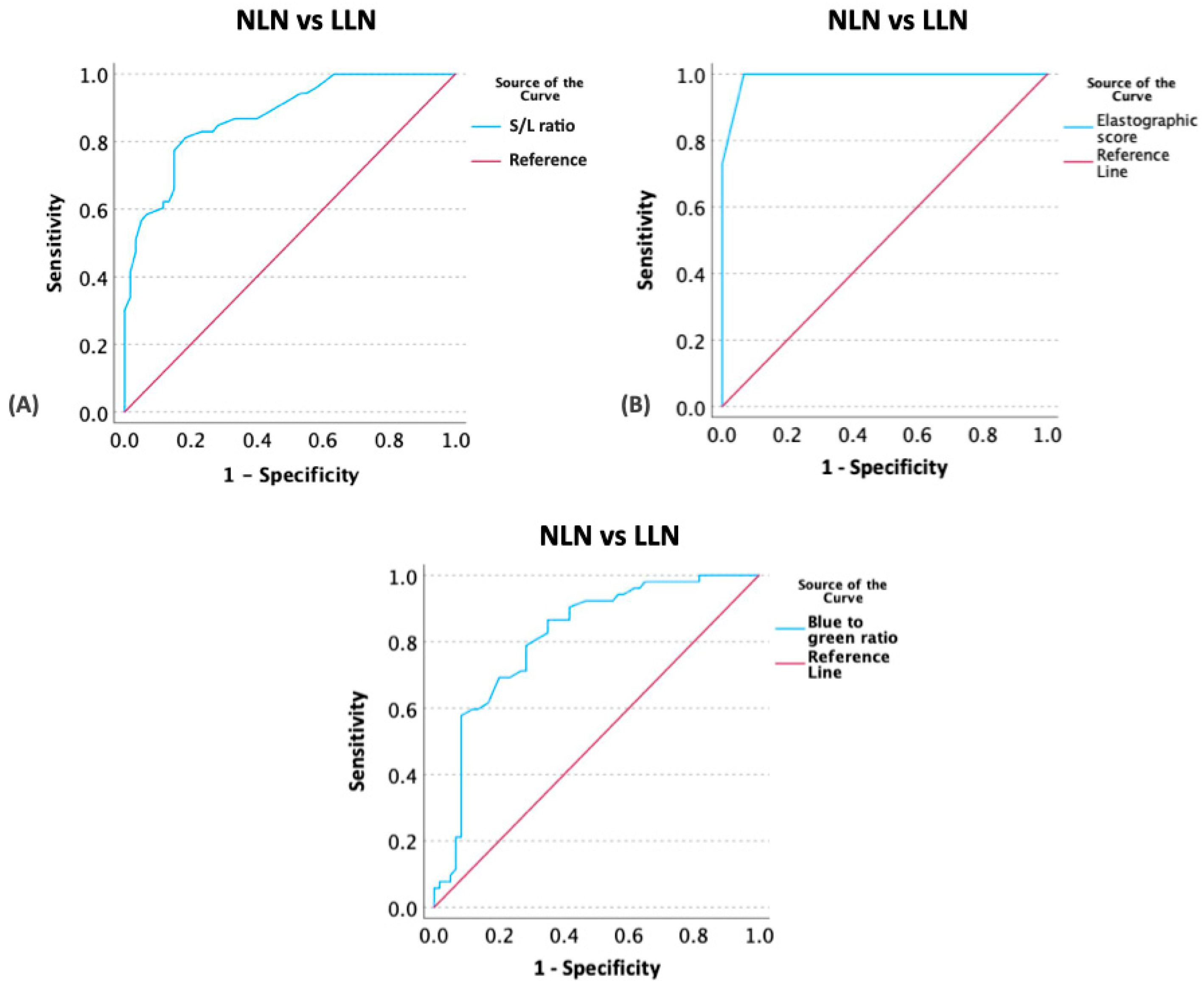
3.4. Determination of Optimal Cut-Off Values of US Parameters between NLNs and LLNs
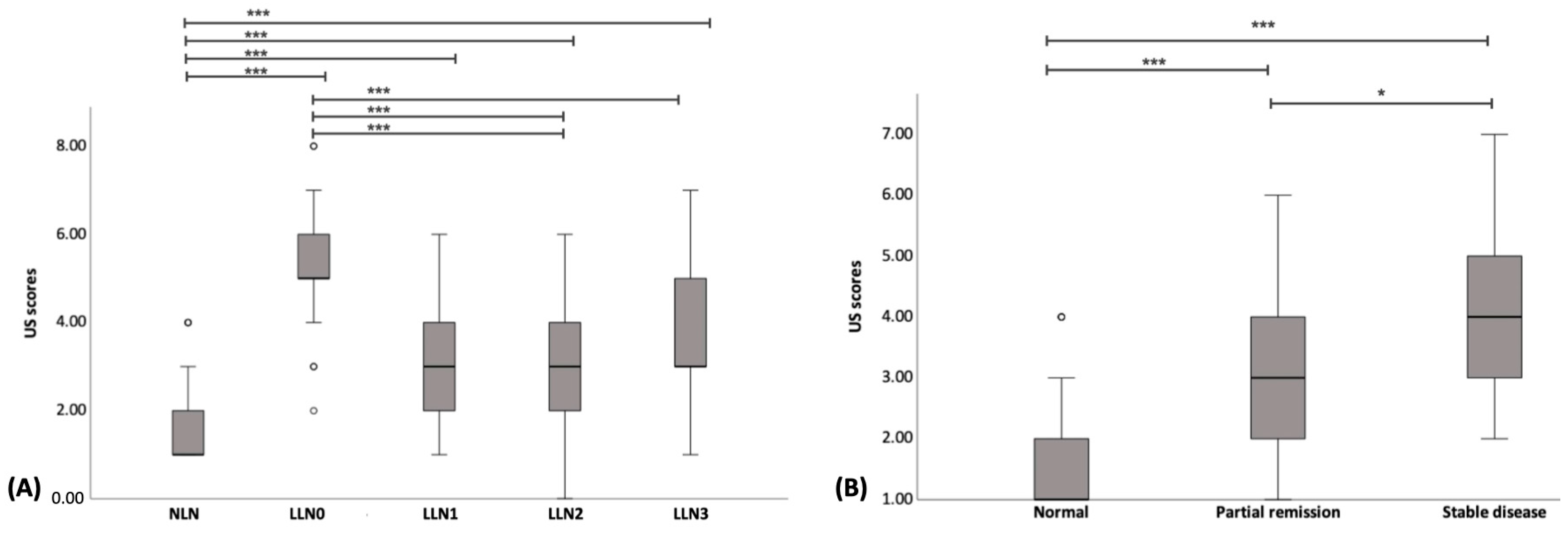
3.5. Comparison of US Scores between NLNs and LLNs
3.6. Comparison of US Scores with Conventional Treatment Response Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Hematopoietic Tumors. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: St. Louis, MO, USA, 2019; pp. 688–772. [Google Scholar]
- Montaner-Angoiti, E.; Marín-García, P.J.; Llobat, L. Epigenetic Alterations in Canine Malignant Lymphoma: Future and Clinical Outcomes. Animals 2023, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.; Williamson, P.; Taylor, R. Review of Canine Lymphoma Treated with Chemotherapy-Outcomes and Prognostic Factors. Vet. Sci. 2023, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Michels, G.M.; Khanna, C.; Selting, K.A.; London, C.A. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2010, 8, 28–37. [Google Scholar] [CrossRef]
- Alexandrakis, I.; Tuli, R.; Ractliffe, S.C.; Tappin, S.W.; Foale, R.D.; Roos, A.; Slater, K.J. Utility of a multiple serum biomarker test to monitor remission status and relapse in dogs with lymphoma undergoing treatment with chemotherapy. Vet. Comp. Oncol. 2017, 15, 6–17. [Google Scholar] [CrossRef]
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J. Vet. Intern. Med. 2019, 33, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Febo, E.; Del Signore, F.; Bernabò, N.; Paolini, A.; Simeoni, F.; De Bonis, A.; Rosto, M.; Canal, S.; Vignoli, M. Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Animals 2023, 13, 2664. [Google Scholar] [CrossRef]
- Choi, M.; Yoon, J.; Choi, M. Semi-quantitative strain elastography may facilitate pre-surgical prediction of mandibular lymph nodes malignancy in dogs. J. Vet. Sci. 2019, 20, e62. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; de Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci. Rep. 2018, 8, 17708. [Google Scholar] [CrossRef] [PubMed]
- Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals 2022, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Prieto, S.; Gomez-Ochoa, P.; De Blas, I.; Gascón, M.; Aceña, C.; Corda, A.; Sosa, I.; Gregori, T.; Couto, G. Pathologic correlation of resistive and pulsatility indices in canine abdominal lymph nodes. Vet. Radiol. Ultrasound 2009, 50, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Salwei, R.M.; O’Brien, R.T.; Matheson, J.S. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and Power Doppler ultrasound. Vet. Radiol. Ultrasound 2005, 46, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Menzel, L.; Höpken, U.E.; Rehm, A. Angiogenesis in Lymph Nodes Is a Critical Regulator of Immune Response and Lymphoma Growth. Front. Immunol. 2020, 11, 591741. [Google Scholar] [CrossRef]
- Seiler, G.S.; Griffith, E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet. Radiol. Ultrasound 2018, 59, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.H.; Baek, J.H. Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N.; World Health Organization. Veterinary Public Health Unit & WHO Collaborating Center for Comparative Oncology. TNM Classification of Tumours in Domestic Animals; Owen, L.N., Ed.; WHO: Geneva, Switzerland, 1980. [Google Scholar]
- Vineela, E.; Sakalecha, A.K.; Suresh, T.N.; Reddy, V.; Sakalecha, A.K.; Suresh, T. Role of sonoelastography in differentiating benign from malignant cervical lymph nodes and correlating with pathology. Cureus 2022, 14, e22984. [Google Scholar] [CrossRef] [PubMed]
- Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. [Google Scholar] [CrossRef]
- Mayer, M.N.; Lawaon, J.A.; Silver, T.I. Sonographic characteristics of presumptively normal canine medial iliac and superficial inguinal lymph nodes. Vet. Radiol. Ultrasound 2010, 51, 638–641. [Google Scholar] [CrossRef]
- Davé, A.C.; Zekas, L.J.; Auld, D.M. Correlation of cytologic and histopathologic findings with perinodal echogenicity of abdominal lymph nodes in dogs and cats. Vet. Radiol. Ultrasound 2017, 58, 463–470. [Google Scholar] [CrossRef]
- Nyman, H.T.; Kristensen, A.T.; Skovgaard, I.M.; McEvoy, F.J. Characterization of normal and abnormal canine superficial lymph nodes using gray-scale B-mode, color flow mapping, power, and spectral Doppler ultrasonography: A multivariate study. Vet. Radiol. Ultrasound 2005, 46, 404–410. [Google Scholar] [CrossRef]
- Kinns, J.; Mai, W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet. Radiol. Ultrasound 2007, 48, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Gödde, D.; Degener, S.; Walles, C.; Keller, R.; Graf, K.; Tosch, M.; Krege, S.; Musch, M.; Kvasnicka, H.M.; Ackermann, M.; et al. Degenerative Changes in Aging Human Pelvic Lymph Nodes-A Reason to Rethink Staging and Therapy of Regional Malignancies? Cancers 2023, 15, 4754. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.; Medovich, S.C.; Silva-Junior, I.A.; Brown, E.M.; Haug, J.C.; Barrios, M.R.; Morris, K.A.; Lancaster, J.N. Age-Associated Changes to Lymph Node Fibroblastic Reticular Cells. Front. Aging 2022, 3, 838943. [Google Scholar] [CrossRef]
- Nyman, H.T.; O’Brien, R.T. The Sonographic Evaluation of Lymph Nodes. Clin. Tech. Small Anim. Pract. 2007, 22, 128–137. [Google Scholar] [CrossRef]
- Silver, T.I.; Lawson, J.A.; Mayer, M.N. Sonographic characteristics of presumptively normal main axillary and superficial cervical lymph nodes in dogs. Am. J. Vet. Res. 2012, 73, 1200–1206. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Q.; Wang, J.-Y.; Yu, Y.; Yi, A.-J.; Cui, X.-W.; Dietrich, C.F. Ultrasound Elastography for the Evaluation of Lymph Nodes. Front. Oncol. 2021, 11, 714660. [Google Scholar] [CrossRef]
- Cè, M.; Amico, N.C.; Danesini, G.M.; Foschini, C.; Oliva, C.; Martinenghi, C.; Cellina, M. Ultrasound Elastography: Basic Principles and Examples of Clinical Applications with Artificial Intelligence—A Review. BioMedInformatics 2023, 3, 17–43. [Google Scholar] [CrossRef]
- Tamura, M.; Ohta, H.; Shimbo, G.; Osuga, T.; Sasaki, N.; Morishita, K.; Kagawa, Y.; Takiguchi, M. Usefulness of noninvasive shear wave elastography for the assessment of hepatic fibrosis in dogs with hepatic disease. J. Vet. Intern. Med. 2019, 33, 2067–2074. [Google Scholar] [CrossRef]
- Huaijantug, S.; Yatmark, P.; Phophug, P.; Worapakdee, M.; Phutrakul, A.; Julapanthong, P.; Chuaychoo, K. Quantitative ultrasound elastography and serum ferritin level in dogs with liver tumors. J. Adv. Vet. Anim. Res. 2020, 7, 575–584. [Google Scholar] [CrossRef]
- Thanaboonnipat, C.; Sutayatram, S.; Buranakarl, C.; Choisunirachon, N. Renal ultrasonographic shear-wave elastography and urinary procollagen type III amino-terminal propeptide in chronic kidney disease dogs. Vet. World 2020, 13, 1955. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Gloria, A.; Romanucci, M.; Della Salda, L.; Di Francesco, L.; Contri, A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Vet. Sci. 2022, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Brizzi, G.; Crepaldi, P.; Roccabianca, P.; Morabito, S.; Zini, E.; Auriemma, E.; Zanna, G. Strain elastography for the assessment of skin nodules in dogs. Vet. Dermatol. 2021, 32, e272–e275. [Google Scholar] [CrossRef] [PubMed]
- Secchi, V.; Masala, G.; Corda, A.; Corda, F.; Potop, E.; Barbero Fernandez, A.; Pinna Parpaglia, M.L.; Sanna Passino, E. Strain Elastography of Injured Equine Superficial Digital Flexor Tendons: A Reliability Study of Manual Measurements. Animals 2021, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.F.; Ewertsen, C.; Săftoiu, A.; Lönn, L.; Nielsen, M.B. Accuracy of visual scoring and semi-quantification of ultrasound strain elastography—A phantom study. PLoS ONE 2014, 9, e88699. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.; Chan, J. Fine-needle-aspiration-induced histologic changes. Curr. Diagn. Pathol. 2003, 9, 78–88. [Google Scholar] [CrossRef]
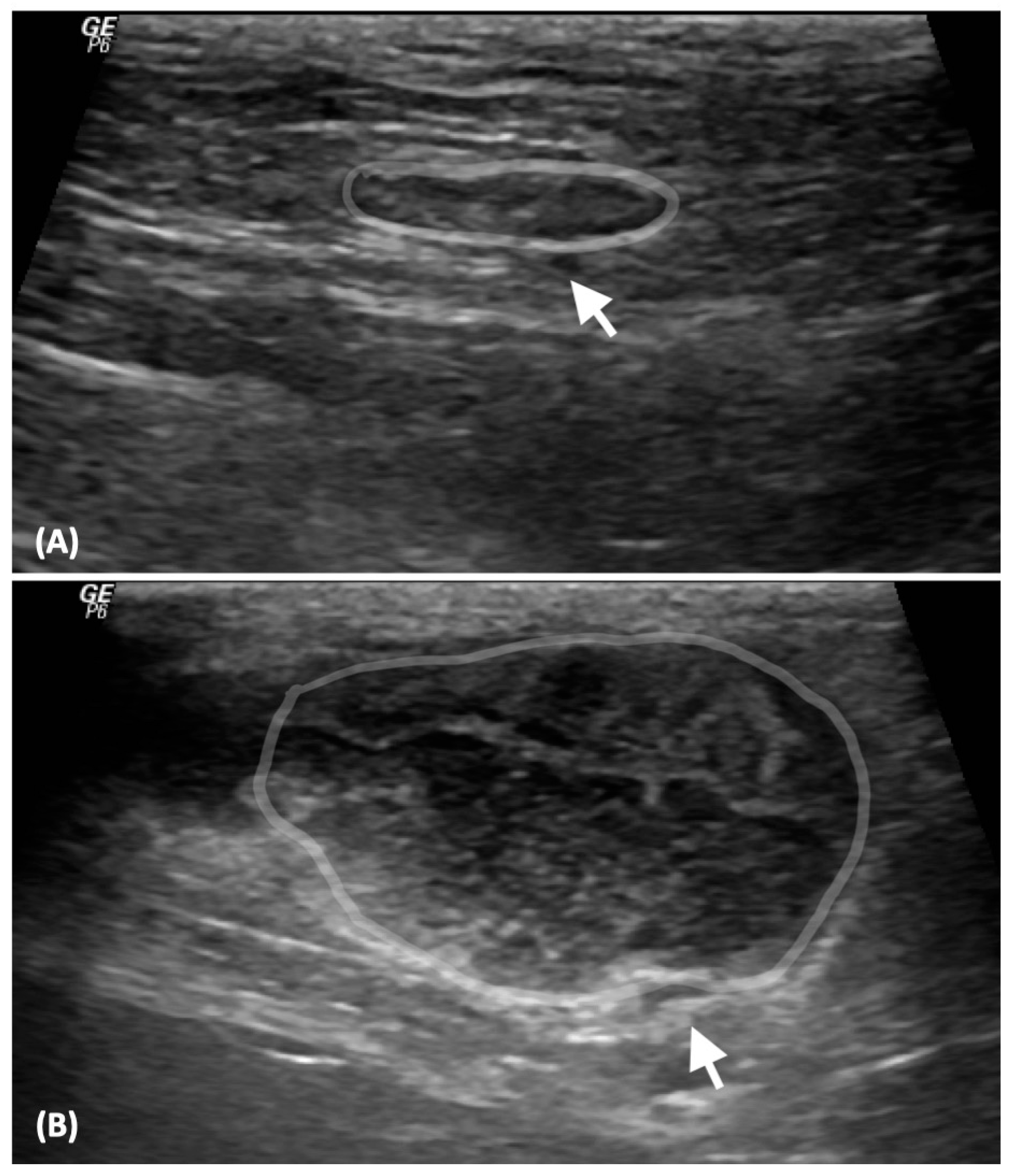
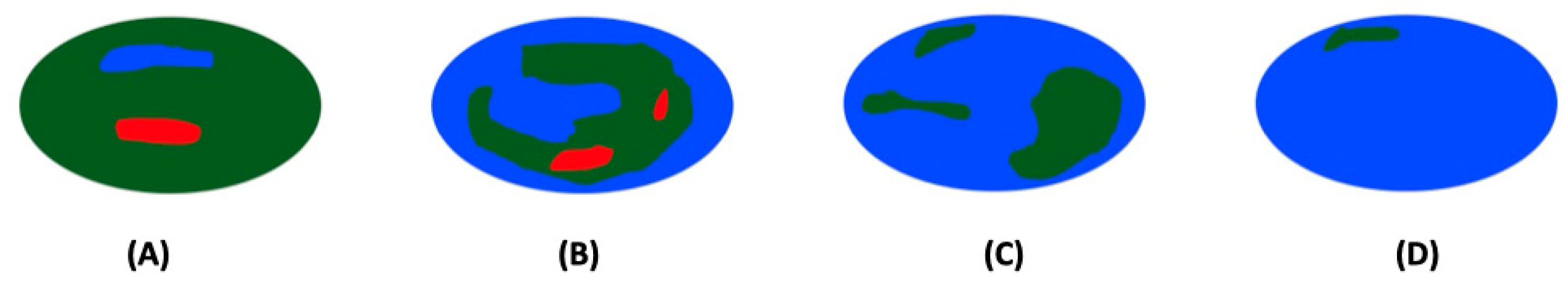





| Criteria | Score |
|---|---|
| 1. Short to length axis ratio | |
| ≤0.345 | 0 |
| >0.345 | 1 |
| 2. Contour regularity | |
| Regular | 0 |
| Irregular | 1 |
| 3. Margin definition | |
| Well-defined margin | 0 |
| Ill-defined margin | 1 |
| 4. Parenchymal uniformity | |
| Homogeneous | 0 |
| Heterogeneous | 1 |
| 5. Nodal hilum | |
| Presence | 0 |
| Absence | 1 |
| 6. Perinodal fat echogenicity compare with lymph node | |
| Isoechoic | 0 |
| Hyperechoic | 1 |
| 7. Elastrographic scores | |
| ≤2 | 0 |
| >2 | 1 |
| 8. The blue to green ratio | |
| <0.745 | 0 |
| ≥0.745 | 1 |
| Characteristics | CML Group n = 15 | Normal Group n = 15 | p-Value |
|---|---|---|---|
| Age (years) | 9.07 ± 0.74 | 10.86 ± 0.76 | 0.102 |
| Gender | <0.001 | ||
| Male | 11 (73.33%) | 9 (60%) | |
| Female | 4 (26.67%) | 6 (40%) | |
| Body weight (kg) | 9.91 ± 1.39 | 8.31 ± 2.13 | 0.536 |
| Characteristics | CML Group n = 15 |
|---|---|
| WHO stage and substage | |
| Iva | 2 (13.33%) |
| IVb | 11 (73.33%) |
| Va | 0 (0%) |
| Vb | 2 (13.34%) |
| Treatment protocol | |
| COP | 3 (20%) |
| L-COP | 12 (80%) |
| Lymph Node | LLN | NLN |
|---|---|---|
| Medial iliac lymph nodes | 27 (23.28%) | 30 (50.00%) |
| Popliteal lymph nodes | 26 (22.41%) | - |
| Superficial inguinal lymph nodes | 26 (22.41%) | 30 (50.00%) |
| Prescapular lymph nodes | 16 (13.79%) | - |
| NLN (n = 60) | LLN (n = 53) | ||||
|---|---|---|---|---|---|
| LLN0 | LLN1 | LLN2 | LLN3 | ||
| B-mode ultrasound | |||||
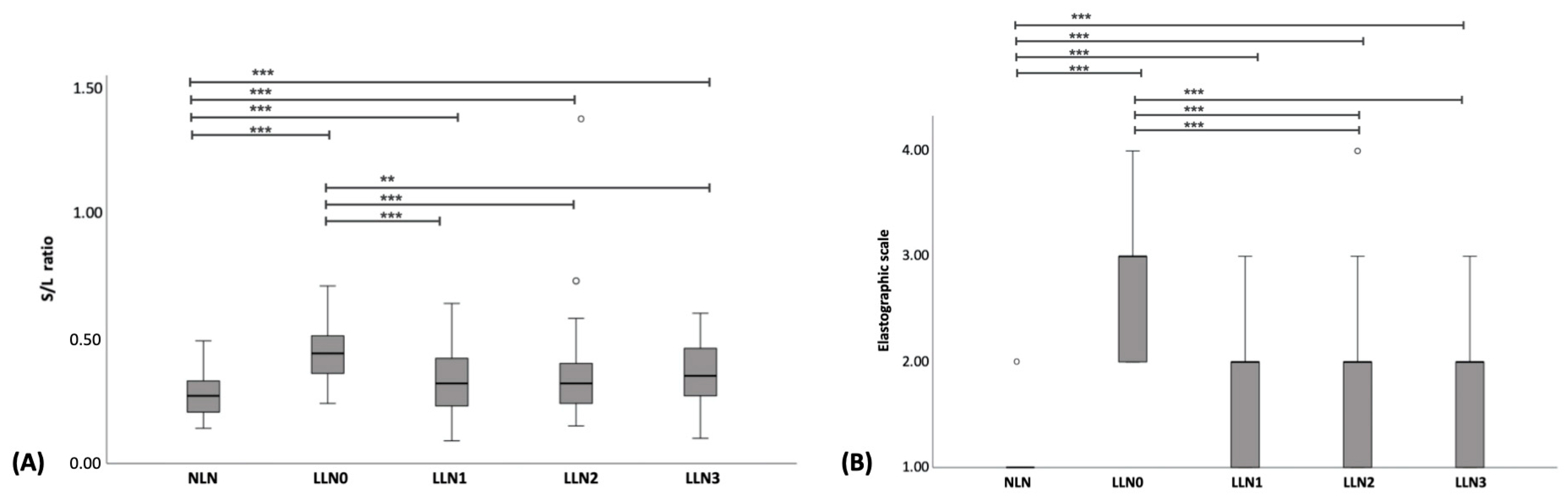
| Short to long axis ratio | 0.28 ± 0.01 | 0.44 ± 0.02 ** | 0.33 ± 0.02 ##,** | 0.36 ± 0.03 ##,** | 0.36 ± 0.02 #,** |
| Contour regularity | |||||
| Regular | 60 (100.00) | 28 (52.83) | 41 (77.36) | 43 (81.13) | 40 (75.47) |
| Irregular | 0 (0.00) | 25 (47.17) | 12 (22.64) | 6 (11.32) | 13 (24.53) |
| Nodal border definition | |||||
| Well-defined | 60 (100.00) | 50 (94.34) | 53 (100.00) | 49 (92.45) | 53 (100.00) |
| Ill-defined | 0 (0.00) | 3 (5.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Parenchymal uniformity | |||||
| Homogeneous | 50 (83.33) | 20 (37.74) | 34 (64.15) | 38 (71.70) | 35 (66.04) |
| Heterogeneous | 10 (16.77) | 33 (62.26) | 19 (35.85) | 11 (20.75) | 18 (33.96) |
| Nodal hilum definition | |||||
| Present | 1 (1.67) | 4 (7.55) | 4 (7.55) | 6 (11.32) | 1 (1.89) |
| Absent | 59 (98.33) | 49 (92.45) | 49 (92.45) | 43 (81.13) | 52 (98.11) |
| Perinodal fat echogenicity | |||||
| Isoechoic | 60 (100.00) | 13 (24.53) | 33 (62.26) | 40 (75.47) | 35 (66.04) |
| Hyperehoic | 0 (0.00) | 40 (75.47) | 20 (37.74) | 9 (16.98) | 18 (33.96) |
| Strain elastography | |||||
| Elastrographic scales | 1 (1–2) | 3 (2–4) ** | 2 (1–3) ##,** | 2 (1–4) ##,** | 2 (1–3) ##,** |
| Color histogram | |||||
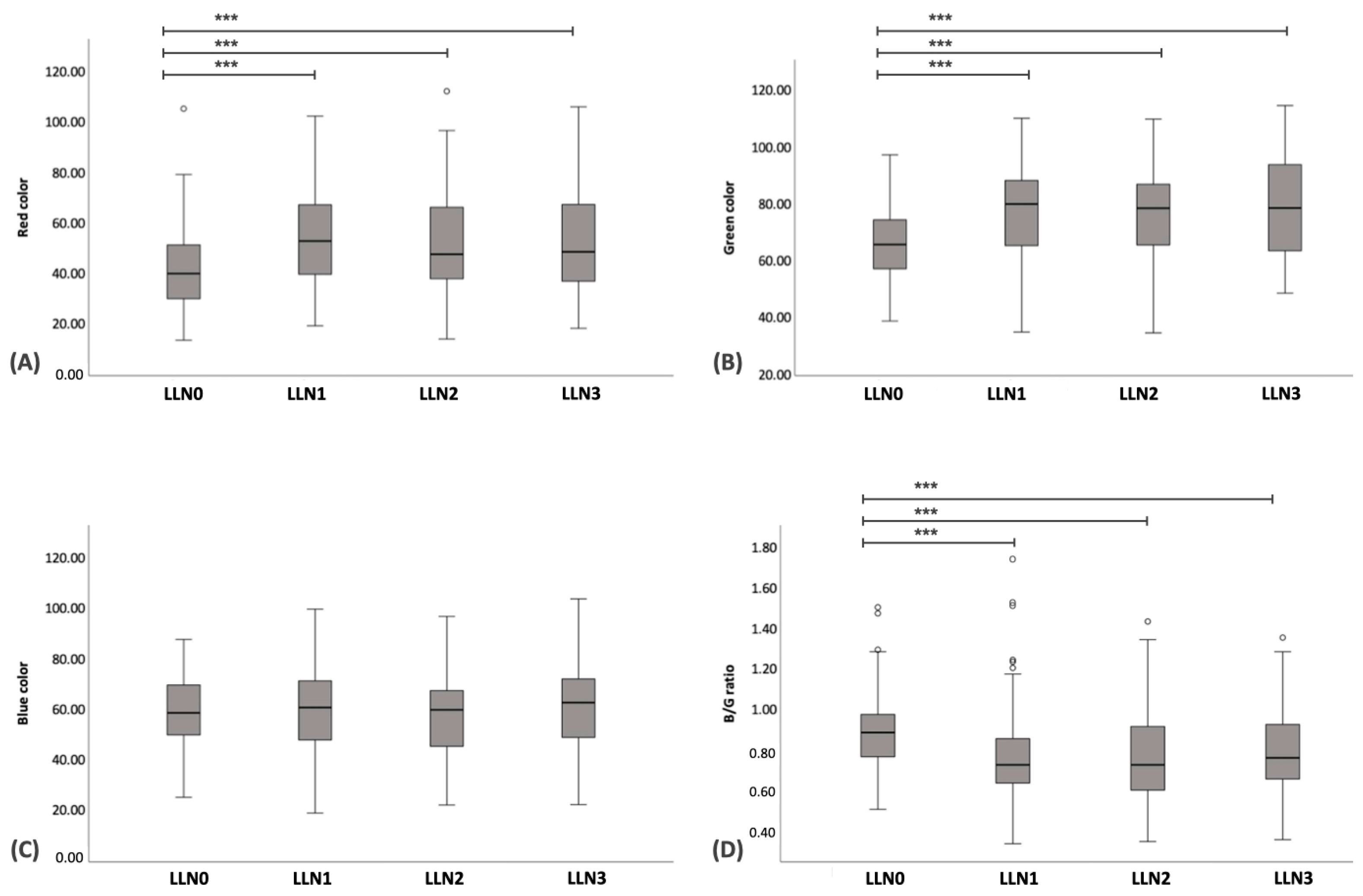
| Red | 71.30 ± 2.52 | 79.49 ± 1.85 ** | 60.10 ± 2.91 #,* | 57.53 ± 3.48 #,** | 58.59 ± 3.14 #,** |
| Green | 84.86 ± 2.70 | 71.68 ± 1.83 ** | 81.24 ± 2.22 * | 81.34 ± 2.61 * | 81.74 ± 2.31 * |
| Blue | 56.43 ± 2.59 | 62.20 ± 1.99 | 59.94 ± 2.19 | 61.41 ± 2.30 | 61.88 ± 2.28 |
| Blue/green ratio | 0.66 ± 0.22 | 0.87 ± 0.23 ** | 0.74 ± 0.02 ##,* | 0.79 ± 0.32 #,* | 0.76 ± 0.02 #,* |
| LLN0 | LLN1 | LLN2 | LLN3 | |
|---|---|---|---|---|
| B-mode ultrasound | ||||
| Short to long axis ratio | 0.54 ± 0.02 | 0.44 ± 0.02 * | 0.46 ± 0.02 * | 0.46 ± 0.02 * |
| Contour regularity | ||||
| Regular | 63 (66.32) | 81 (85.26) | 80 (84.21) | 77 (81.05) |
| Irregular | 32 (33.68) | 14 (14.74) | 7 (15.79) | 18 (18.95) |
| Nodal border definition | ||||
| Well-defined | 92 (96.84) | 94 (98.95) | 87 (91.58) | 95 (100.00) |
| Ill-defined | 3 (3.16) | 1 (1.05) | 0 (8.42) | 0 (0.00) |
| Parenchyma uniformity | ||||
| Homogeneous | 31 (32.63) | 45 (47.47) | 58 (61.05) | 54 (56.84) |
| Heterogeneous | 64 (67.37) | 50 (52.63) | 29 (38.95) | 41 (43.16) |
| Nodal hilum definition | ||||
| Present | 7 (7.37) | 5 (5.26) | 7 (7.37) | 3 (3.16) |
| Absent | 88 (92.63) | 90 (94.74) | 80 (92.63) | 92 (96.84) |
| Perinodal fat echogenicity (n, %) | ||||
| Isoechoic | 18 (18.95) | 47 (49.47) | 66 (69.47) | 58 (61.05) |
| Hyperechoic | 77 (81.05) | 48 (50.53) | 21 (30.53) | 37 (38.95) |
| Strain elastography | ||||
| Elastrographic score (median, range) | 3 (1–4) | 2 (1–4) * | 2 (1–4) * | 2 (1–4) * |
| Color histogram (mean ± SD) | ||||
| Red | 41.33 ± 1.53 | 55.33 ± 2.09 * | 53.13 ± 2.28 * | 53.65 ± 2.23 * |
| Green | 67.30 ± 1.31 | 78.11 ± 1.71 * | 76.72 ± 1.75 * | 78.90 ± 1.71 * |
| Blue | 59.76 ± 1.45 | 59.96 ± 1.79 | 58.06 ± 1.74 | 62.24 ± 1.74 |
| Blue/green ratio | 0.89 ± 0.02 | 0.78 ± 0.03 * | 0.78 ± 0.03 * | 0.80 ± 0.02 * |
| Ultrasonographic Parameters | Cut-Off | p | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Short to long axis ratio | 0.345 | <0.001 | 0.876 | 81.10% | 81.70% |
| Elastographic scale | 1.5 | <0.001 | 0.991 | 100% | 93.33% |
| Blue-to-green ratio | 0.745 | <0.001 | 0.815 | 78.80% | 71.70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutthigran, S.; Saisawart, P.; Soeratanapant, S.; Teewasutrakul, P.; Sirivisoot, S.; Thanaboonnipat, C.; Rungsipipat, A.; Choisunirachon, N. Post-Chemotherapy Canine Lymphomatous Lymph Node Observations on B-Mode and Strain Elastographic Ultrasound. Vet. Sci. 2024, 11, 352. https://doi.org/10.3390/vetsci11080352
Sutthigran S, Saisawart P, Soeratanapant S, Teewasutrakul P, Sirivisoot S, Thanaboonnipat C, Rungsipipat A, Choisunirachon N. Post-Chemotherapy Canine Lymphomatous Lymph Node Observations on B-Mode and Strain Elastographic Ultrasound. Veterinary Sciences. 2024; 11(8):352. https://doi.org/10.3390/vetsci11080352
Chicago/Turabian StyleSutthigran, Somchin, Phasamon Saisawart, Suphat Soeratanapant, Patharakrit Teewasutrakul, Sirintra Sirivisoot, Chutimon Thanaboonnipat, Anudep Rungsipipat, and Nan Choisunirachon. 2024. "Post-Chemotherapy Canine Lymphomatous Lymph Node Observations on B-Mode and Strain Elastographic Ultrasound" Veterinary Sciences 11, no. 8: 352. https://doi.org/10.3390/vetsci11080352
APA StyleSutthigran, S., Saisawart, P., Soeratanapant, S., Teewasutrakul, P., Sirivisoot, S., Thanaboonnipat, C., Rungsipipat, A., & Choisunirachon, N. (2024). Post-Chemotherapy Canine Lymphomatous Lymph Node Observations on B-Mode and Strain Elastographic Ultrasound. Veterinary Sciences, 11(8), 352. https://doi.org/10.3390/vetsci11080352






