Investigation of the Infection of Enterocytozoon bieneusi in Sheep and Goats in Jiangsu, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples Collection
2.2. Extraction of DNA and PCR Analysis
2.3. Phylogenetic Analysis and Statistical Analysis
3. Results
3.1. PCR Amplification Results
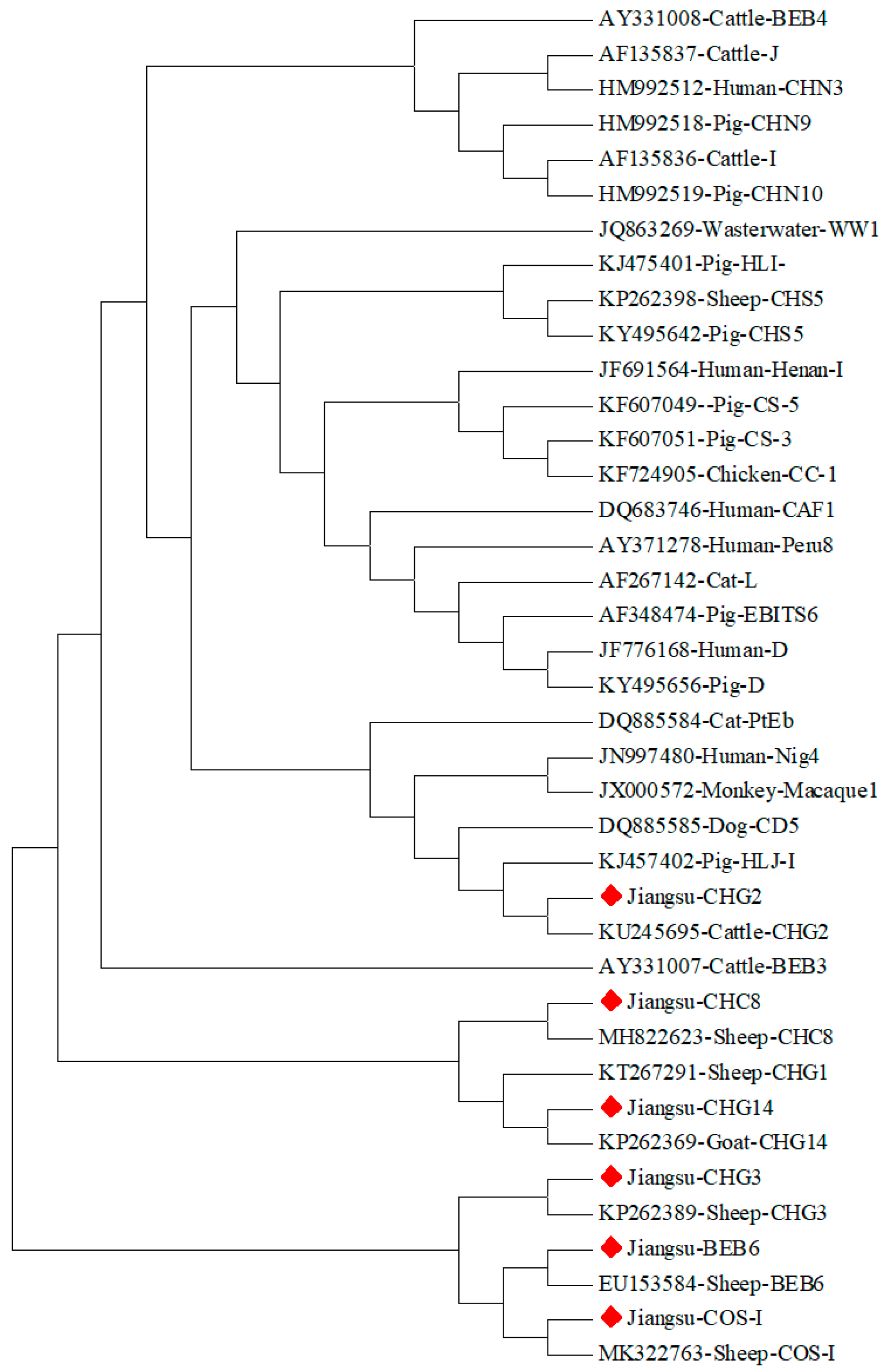
3.2. Sequencing and Phylogenetic Tree Construction
3.3. Infection Status of E. bieneusi in Sheep and Goats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenčáková, A.; Danišová, O. Molecular Characterization of New Genotypes Enterocytozoon bieneusi in Slovakia. Acta Trop. 2019, 191, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in Domesticated and Wild Animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef]
- Didier, E.S.; Stovall, M.E.; Green, L.C.; Brindley, P.J.; Sestak, K.; Didier, P.J. Epidemiology of Microsporidiosis: Sources and Modes of Transmission. Vet. Parasitol. 2004, 126, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Zhang, Y.; Gasser, R.B. A Perspective on the Molecular Identification, Classification, and Epidemiology of Enterocytozoon bieneusi of Animals. Exp. Suppl. 2022, 114, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Koehler, A.V.; Wang, T.; Gasser, R.B. Enterocytozoon bieneusi of Animals—With an ‘Australian Twist’. Adv. Parasitol. 2021, 111, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, J.; Li, P.; Wang, L.; Guo, Y.; Li, C.; Lei, M.; Feng, Y.; Xiao, L. Enterocytozoon bieneusi Genotypes in Tibetan Sheep and Yaks. Parasitol. Res. 2018, 117, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Wang, K.; Gu, Y.F. Detection and Genotyping Study of Enterocytozoon bieneusi in Sheep and Goats in East-Central China. Acta Parasitol. 2019, 64, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Fayer, R.; Lal, A.A.; Trout, J.M.; Schaefer, F.W.; Xiao, L. Molecular Characterization of Microsporidia Indicates That Wild Mammals Harbor Host-Adapted Enterocytozoon spp. as Well as Human-Pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 2003, 69, 4495. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Y.; Santin, M. Host Specificity of Enterocytozoon bieneusi and Public Health Implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, Z.; Cheng, D.; Tao, J. Prevalence of Cryptosporidium spp. in Sheep and Goats in Jiangsu, China. Vet. Sci. 2024, 11, 144. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Therapeutic Targets for the Treatment of Microsporidiosis in Humans. Expert. Opin. Ther. Targets 2018, 22, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Current Status. Curr. Opin. Infect. Dis. 2006, 19, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Buckholt, M.A.; Lee, J.H.; Tzipori, S. Prevalence of Enterocytozoon bieneusi in Swine: An 18-Month Survey at a Slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 2002, 68, 2595. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S. Microsporidiosis: An Emerging and Opportunistic Infection in Humans and Animals. Acta Trop. 2005, 94, 61–76. [Google Scholar] [CrossRef]
- Peng, X.Q.; Tian, G.R.; Ren, G.J.; Yu, Z.Q.; Lok, J.B.; Zhang, L.X.; Wang, X.T.; Song, J.K.; Zhao, G.H. Infection Rate of Giardia Duodenalis, Cryptosporidium spp. and Enterocytozoon bieneusi in Cashmere, Dairy and Meat Goats in China. Infect. Genet. Evol. 2016, 41, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, M.; Wang, X.; Li, J.; Karim, M.R.; Wang, R.; Zhang, L.; Jian, F.; Ning, C. Molecular Survey of Enterocytozoon bieneusi in Sheep and Goats in China. Parasit. Vectors 2016, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, R.; Yang, J.; Wang, J.; Gong, H.; Huang, Y.; Wang, X.; Han, X.; Zhou, H.; Chen, Z. Enterocytozoon bieneusi Genotypes in Farmed Goats and Sheep in Ningxia, China. Infect. Genet. Evol. 2020, 85, 104559. [Google Scholar] [CrossRef]
- Zhou, H.H.; Zheng, X.L.; Ma, T.M.; Qi, M.; Cao, Z.X.; Chao, Z.; Wei, L.M.; Liu, Q.W.; Sun, R.P.; Wang, F.; et al. Genotype Identification and Phylogenetic Analysis of Enterocytozoon bieneusi in Farmed Black Goats (Capra Hircus) from China’s Hainan Province. Parasite 2019, 26, 62. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Ai, S.; Wang, X.; Zhang, R.; Duan, Z. Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis from Animal Sources in the Qinghai-Tibetan Plateau Area (QTPA) in China. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101346. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, Y.; Wu, Y.; Niu, Z.; Li, J.; Zhang, S.; Wang, R.; Jian, F.; Ning, C.; Zhang, L. Molecular Characterization of Giardia duodenalis and Enterocytozoon bieneusi Isolated from Tibetan Sheep and Tibetan Goats Under Natural Grazing Conditions in Tibet. J. Eukaryot. Microbiol. 2020, 67, 100–106. [Google Scholar] [CrossRef]
- Xie, S.C.; Zou, Y.; Li, Z.; Yang, J.F.; Zhu, X.Q.; Zou, F.C. Molecular Detection and Genotyping of Enterocytozoon bieneusi in Black Goats (Capra Hircus) in Yunnan Province, Southwestern China. Animals 2021, 11, 3387. [Google Scholar] [CrossRef]
- Fiuza, V.R.d.S.; Lopes, C.W.G.; Cosendey, R.I.J.; de Oliveira, F.C.R.; Fayer, R.; Santín, M. Zoonotic Enterocytozoon bieneusi Genotypes Found in Brazilian Sheep. Res. Vet. Sci. 2016, 107, 196–201. [Google Scholar] [CrossRef]
- Qin, R.-L.; Liu, Y.-Y.; Mei, J.-J.; Zou, Y.; Zhang, Z.-H.; Zheng, W.-B.; Liu, Q.; Gao, W.-W.; Xie, S.-C.; Ma, G.; et al. Molecular Identification and Genotyping of Enterocytozoon bieneusi in Sheep in Shanxi Province, North China. Animals 2022, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chang, Y.; Chen, Y.; Zhang, X.; Li, D.; Zheng, S.; Wang, L.; Li, J.; Ning, C.; Zhang, L. Occurrence and Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan Sheep in Gansu, China. Infect. Genet. Evol. 2018, 64, 46–51. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.; Yang, D.; Zhang, L.; Wang, R.; Liu, A. Prevalence of Enterocytozoon bieneusi and Genetic Diversity of ITS Genotypes in Sheep and Goats in China. Infect. Genet. Evol. 2015, 32, 265–270. [Google Scholar] [CrossRef]
- Yang, H.; Mi, R.; Cheng, L.; Huang, Y.; An, R.; Zhang, Y.; Jia, H.; Zhang, X.; Wang, X.; Han, X.; et al. Prevalence and Genetic Diversity of Enterocytozoon bieneusi in Sheep in China. Parasit. Vectors 2018, 11, 587. [Google Scholar] [CrossRef]
- Tuo, H.; Zhang, B.; He, Y.; Zhao, A.; Zhang, Z.; Qi, M.; Yu, F. Molecular Characterization of Enterocytozoon bieneusi Genotypes in Wild Altai Marmot (Marmota baibacina) in Xinjiang, China: Host Specificity and Adaptation. Parasitol. Res. 2024, 123, 7. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, J.; Zhao, A.; Cui, Z.; Wei, Z.; Jing, B.; Zhang, L. Host Specificity of Enterocytozoon bieneusi Genotypes in Bactrian Camels (Camelus bactrianus) in China. Parasit. Vectors 2018, 11, 219. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Yang, Z.; Liu, A. Dominance of the Enterocytozoon bieneusi Genotype BEB6 in Red Deer (Cervus elaphus) and Siberian Roe Deer (Capreolus pygargus) in China and a Brief Literature Review. Parasite 2017, 24, 54. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cong, W.; Liu, G.H.; Ni, X.T.; Ma, J.G.; Zheng, W.B.; Zhao, Q.; Zhu, X.Q. Prevalence and Genotypes of Enterocytozoon bieneusi in Sika Deer in Jilin Province, Northeastern China. Acta Parasitol. 2016, 61, 382–388. [Google Scholar] [CrossRef]
| Region | 0~2 Month | 2~6 Month | 6~10 Month | Breed |
|---|---|---|---|---|
| Suzhou | 90 | 90 | 0 | Goats |
| Nantong | 80 | 80 | 118 | Sheep and goats |
| Taizhou | 0 | 0 | 60 | Sheep |
| Suqian | 20 | 40 | 88 | Sheep and goats |
| Huaian | 60 | 0 | 60 | Sheep and goats |
| Total | 250 | 210 | 326 |
| Gene Locus | Primer | Sequence | Amplified Product |
|---|---|---|---|
| ITS | NEBF1 | 5′-GGTCATAGGGATGAAGAG-3 | 410 bp |
| NEBR1 | 5′-TTCGAGTTCTTTCGCGCTC-3′ | ||
| NEBF2 | 5′-GCTCTGAATATCTATGGCT-3′ | 392 bp | |
| NEBR2 | 5′-ATCGCCGACGGATCCAAGTG-3′ |
| Region | Farm | Sample Size | No of Positive | % of Positive | Average Positive % |
|---|---|---|---|---|---|
| Suzhou | 1 | 60 | 20 | 33.33 | 38.33 |
| 2 | 60 | 19 | 31.67 | ||
| 3 | 60 | 30 | 50 | ||
| Nantong | 4 | 80 | 24 | 30 | 42.81 |
| 5 | 60 | 17 | 28.33 | ||
| 6 | 37 | 8 | 21.62 | ||
| 7 | 30 | 20 | 66.67 | ||
| 8 | 21 | 12 | 57.14 | ||
| 9 | 30 | 23 | 76.77 | ||
| 10 | 20 | 15 | 75 | ||
| Taizhou | 11 | 30 | 16 | 53.33 | 28.33 |
| 12 | 30 | 1 | 3.33 | ||
| Suqian | 13 | 48 | 5 | 10.42 | 23.65 |
| 14 | 40 | 15 | 37.5 | ||
| 15 | 60 | 15 | 25 | ||
| Huaian | 16 | 40 | 26 | 65 | 39.17 |
| 17 | 40 | 17 | 42.5 | ||
| 18 | 40 | 4 | 10 | ||
| Total | 786 | 287 | 36.51 |
| Breed | Sample Size | No of Positive | % of Positive |
|---|---|---|---|
| Goat | 588 | 209 | 35.54 |
| Sheep | 198 | 77 | 38.89 |
| total | 786 | 287 | 36.51 |
| Breed | Age (Month) | Sample Size | No of Positive | % of Positive |
|---|---|---|---|---|
| Goat | 0–2 | 230 | 86 | 37.39 |
| 2–6 | 242 | 95 | 39.26 | |
| 6–10 | 116 | 29 | 25 | |
| Sheep | 0–2 | 20 | 8 | 40 |
| 2–6 | 20 | 15 | 75 | |
| 6–10 | 158 | 54 | 34.18 |
| Breed | Symptom | Sample Size | No of Positive | % of Positive |
|---|---|---|---|---|
| Goat | Diarrheic | 140 | 62 | 44.29 |
| Healthy | 448 | 148 | 33.04 | |
| Sheep | Diarrheic | 38 | 8 | 21.05 |
| Healthy | 160 | 69 | 43.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Cai, Y.; Xing, H.; Tao, J.; Cheng, D. Investigation of the Infection of Enterocytozoon bieneusi in Sheep and Goats in Jiangsu, China. Vet. Sci. 2024, 11, 327. https://doi.org/10.3390/vetsci11070327
Cheng C, Cai Y, Xing H, Tao J, Cheng D. Investigation of the Infection of Enterocytozoon bieneusi in Sheep and Goats in Jiangsu, China. Veterinary Sciences. 2024; 11(7):327. https://doi.org/10.3390/vetsci11070327
Chicago/Turabian StyleCheng, Cheng, Yuan Cai, Hua Xing, Jianping Tao, and Darong Cheng. 2024. "Investigation of the Infection of Enterocytozoon bieneusi in Sheep and Goats in Jiangsu, China" Veterinary Sciences 11, no. 7: 327. https://doi.org/10.3390/vetsci11070327
APA StyleCheng, C., Cai, Y., Xing, H., Tao, J., & Cheng, D. (2024). Investigation of the Infection of Enterocytozoon bieneusi in Sheep and Goats in Jiangsu, China. Veterinary Sciences, 11(7), 327. https://doi.org/10.3390/vetsci11070327








