Effect of Breed and Season in Buck Semen Cryopreservation: The Portuguese Animal Germplasm Bank
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Semen Collection, Evaluation, and Processing
2.3. Breeding and Non-Breeding Season
2.4. Statistical Analysis
3. Results
3.1. Ejaculate Traits (Volume, Spermatozoa Concentration, and Total Sperm)
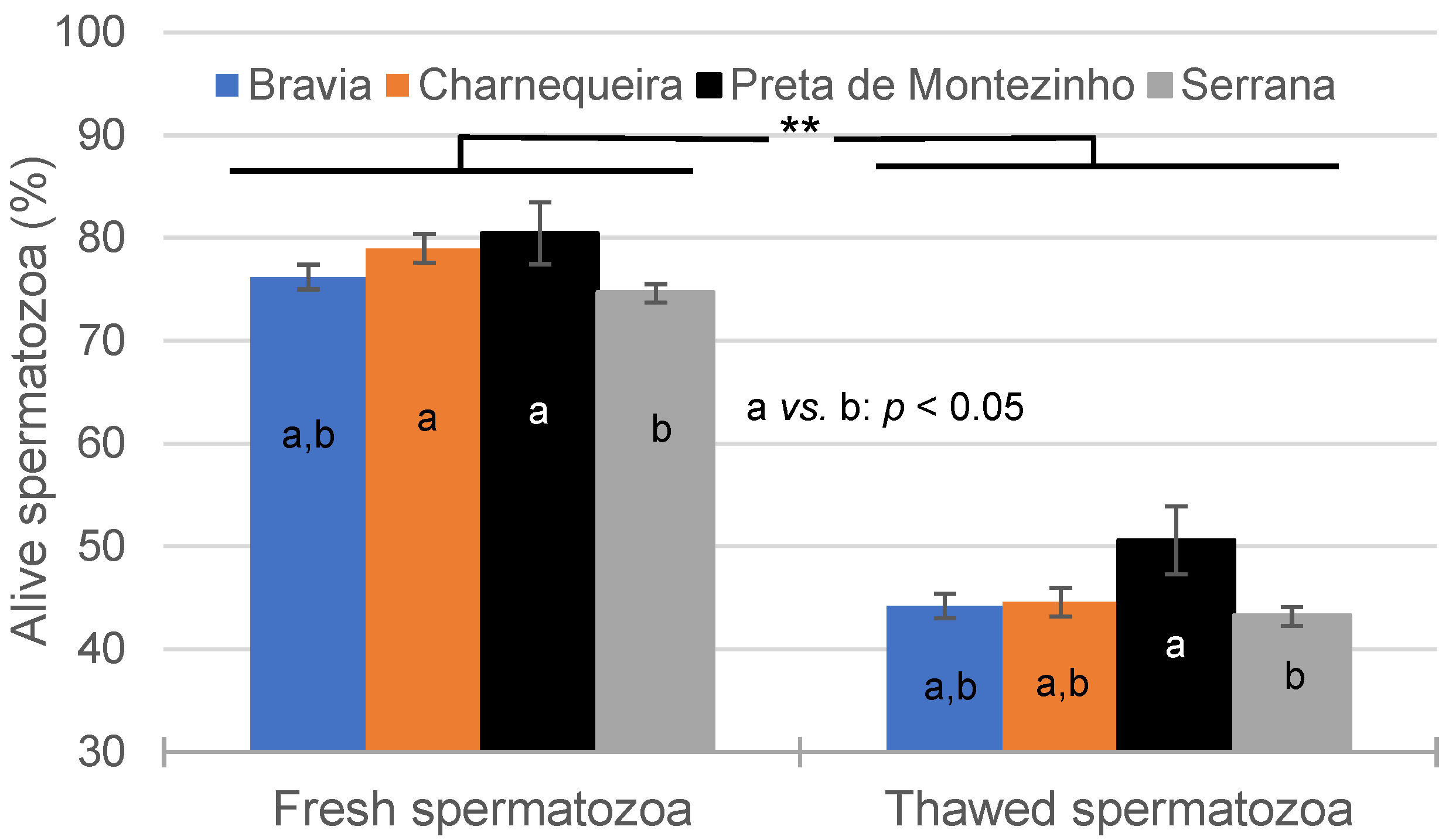
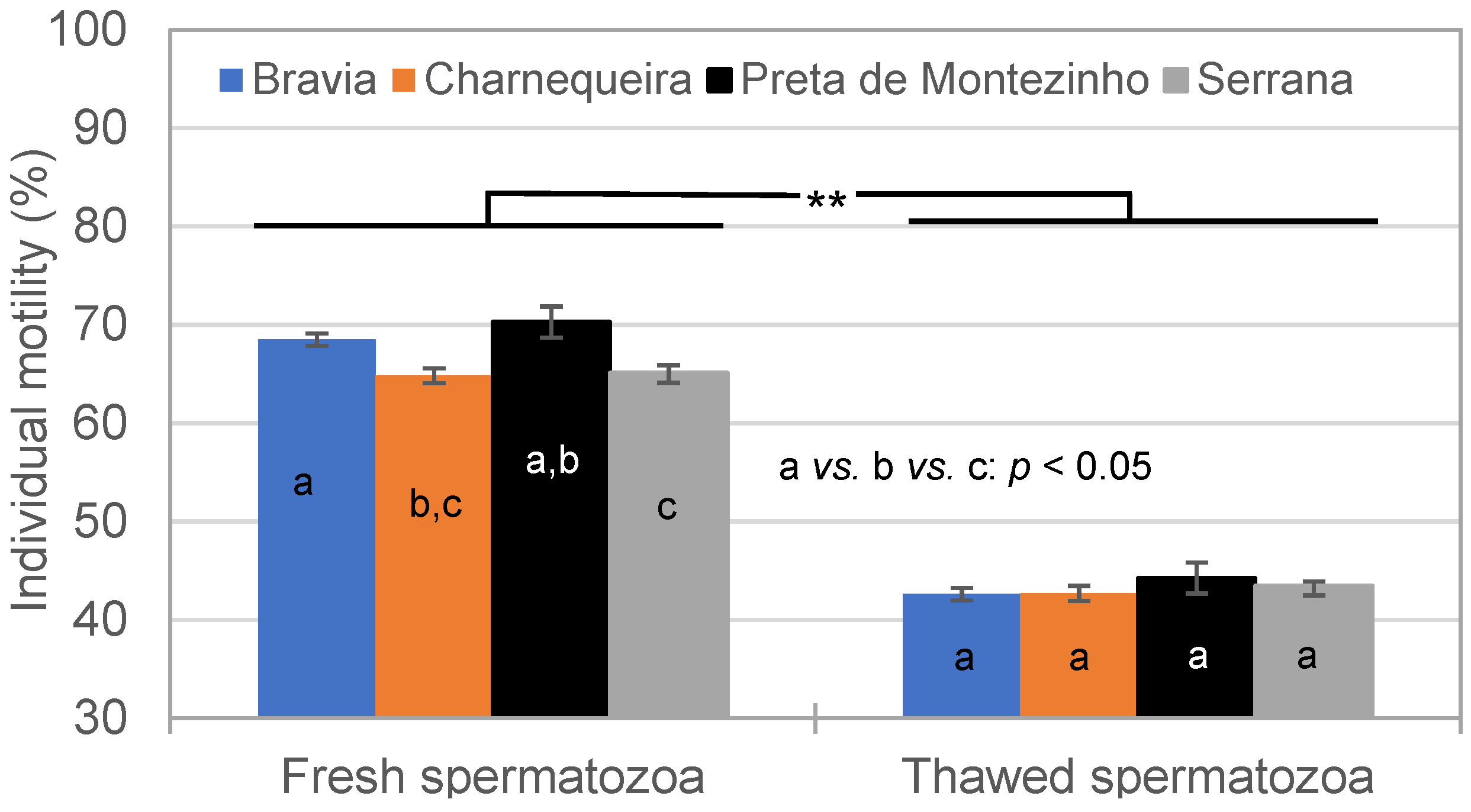
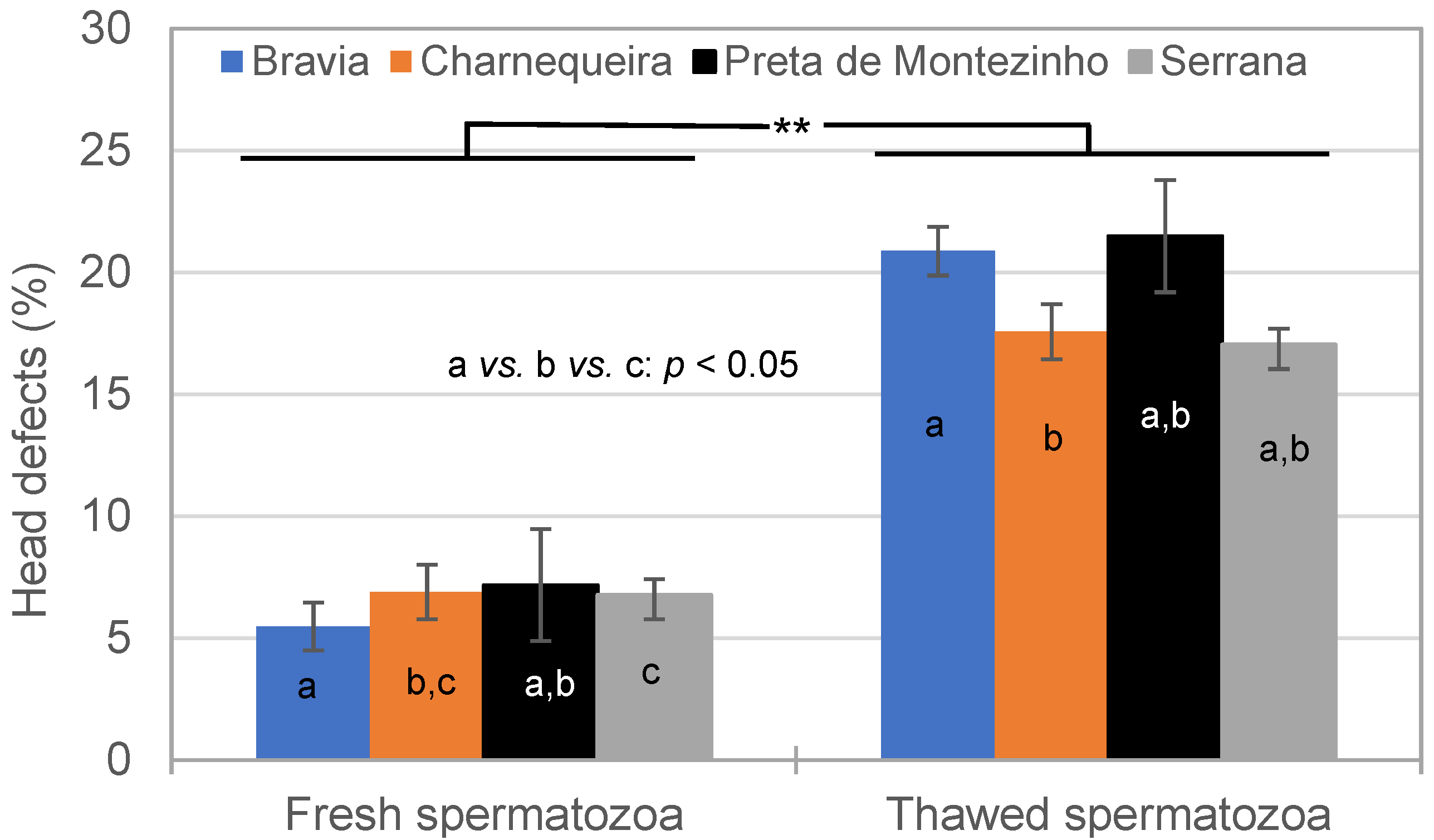
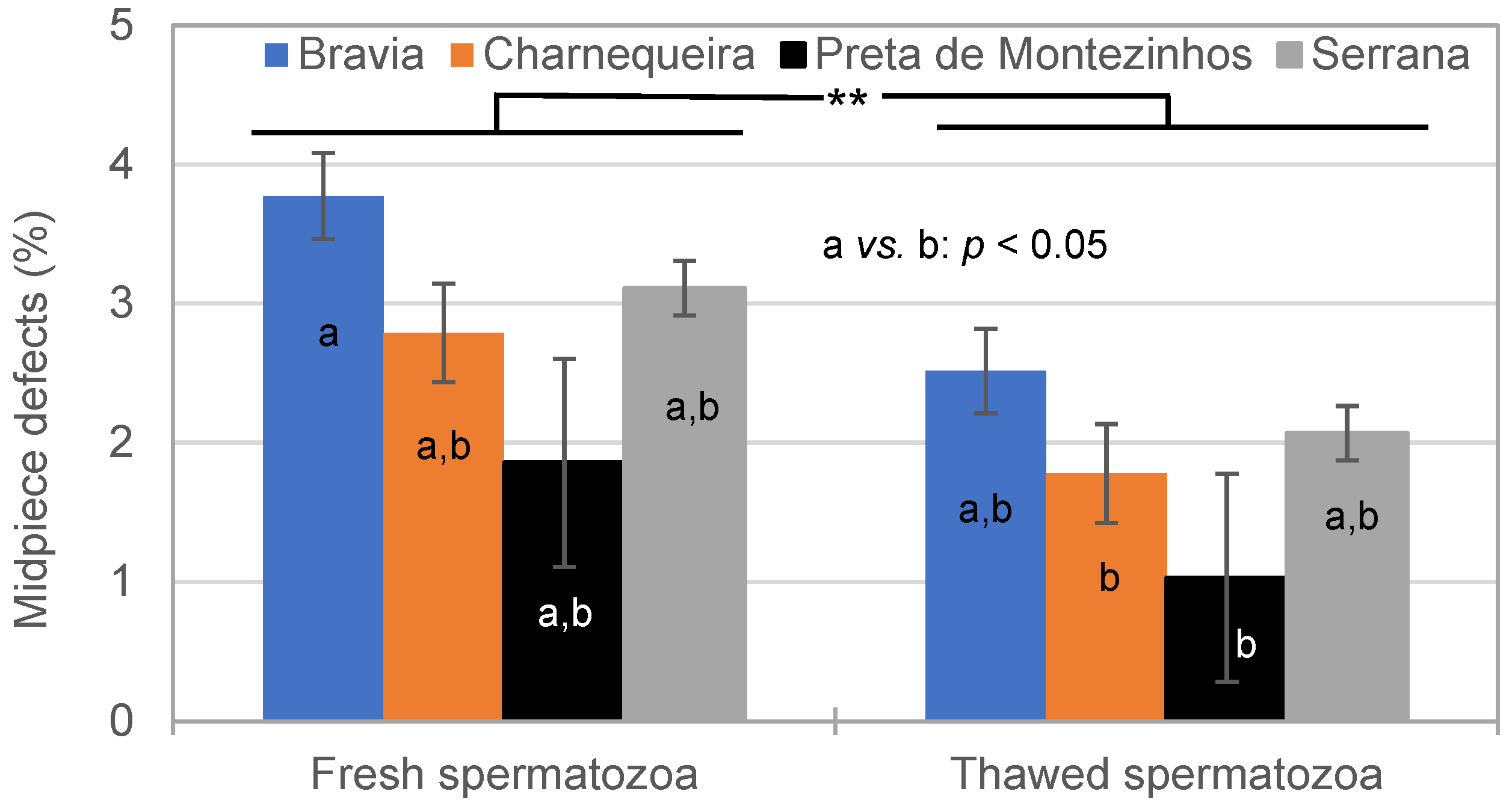
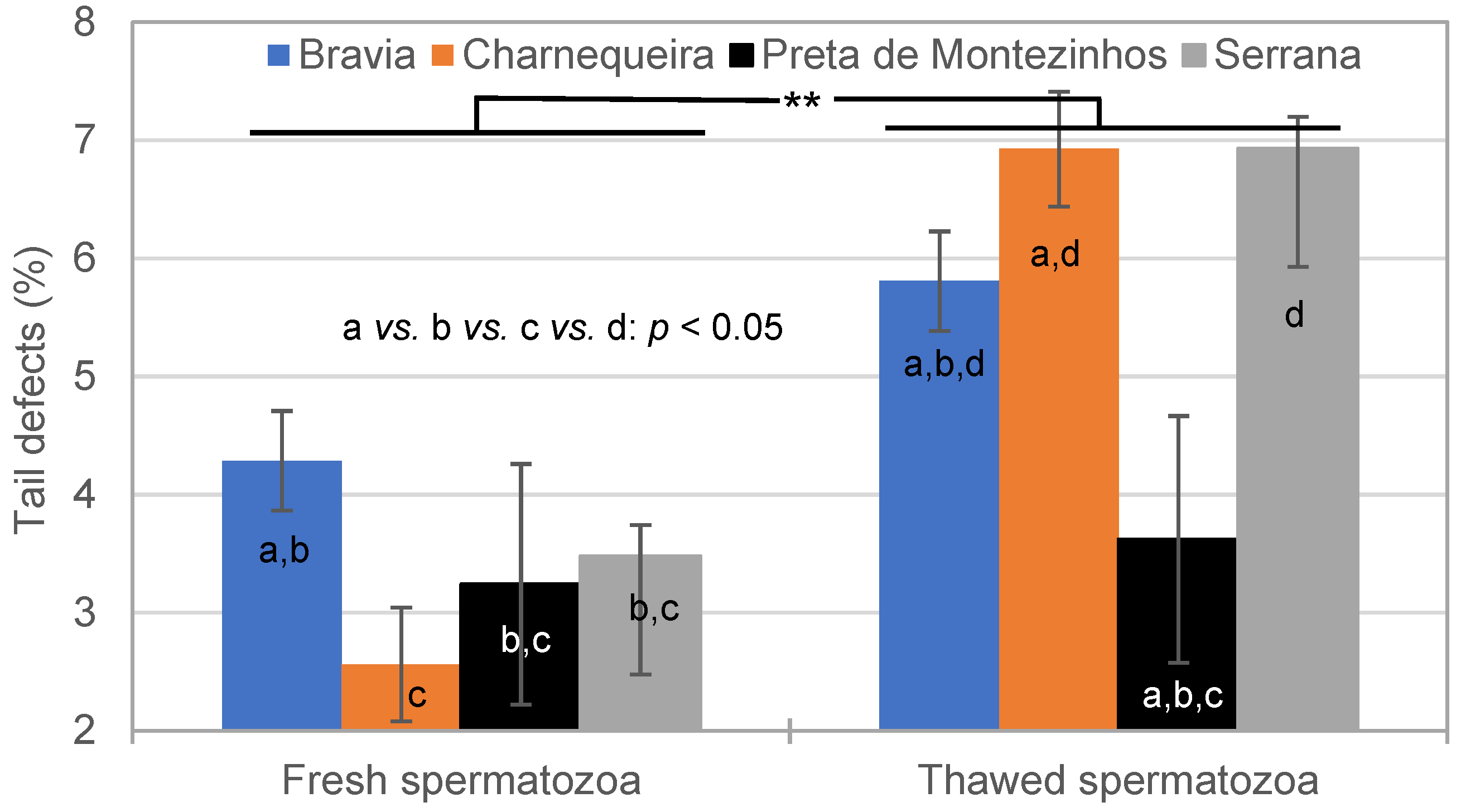
3.2. Spermatozoa Traits in Fresh and Thawed Semen
4. Discussion
4.1. Semen Production
4.2. Spermatozoa Traits in Fresh Semen
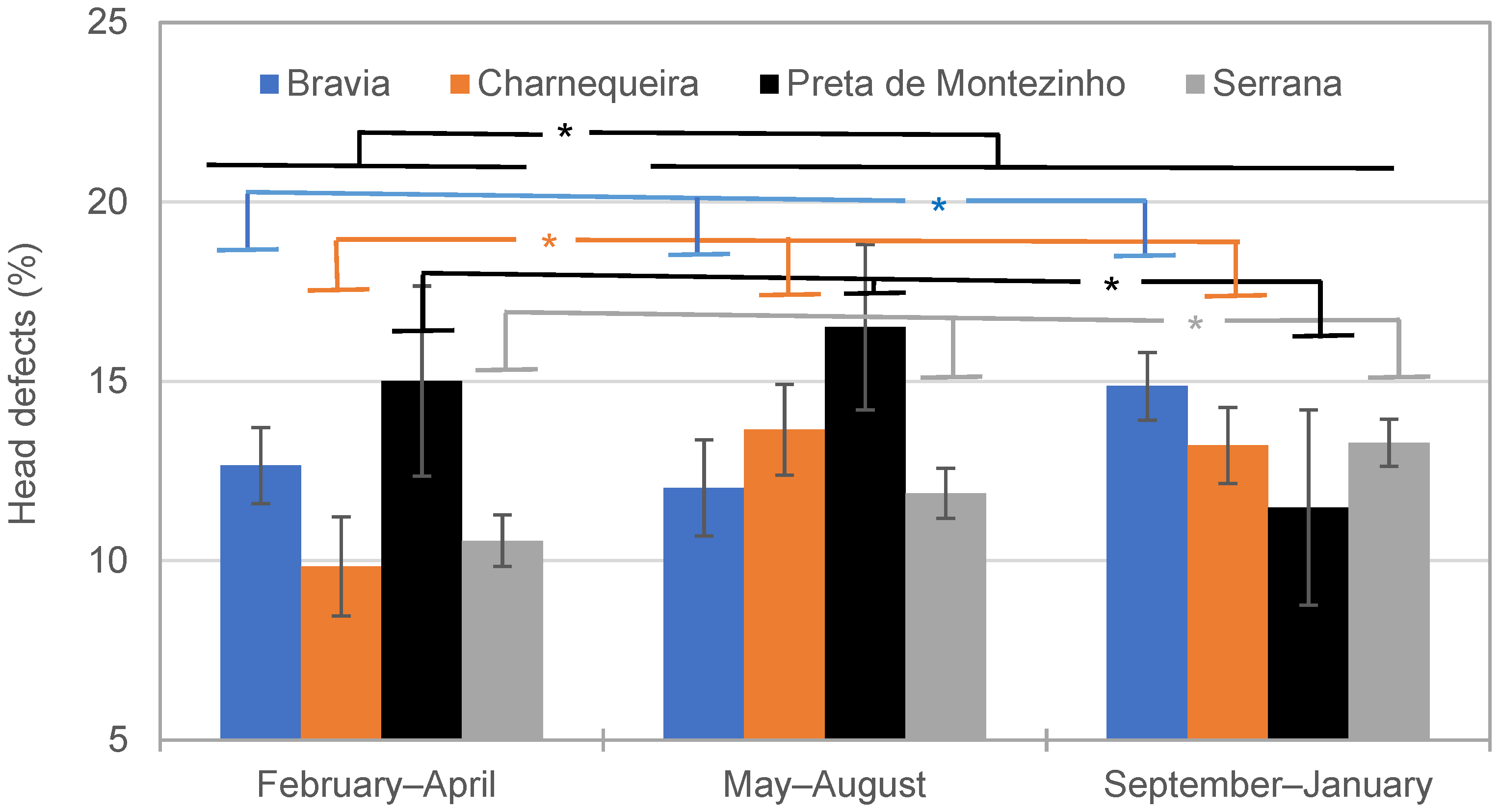
4.2.1. Effect of Season on Spermatozoa Traits
4.2.2. Effect of Freezing–Thawing Cycle on Spermatozoa Traits
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carolino, N.; Sousa, C.; Carolino, I.; Santos-Silva, F.; Sousa, C.; Vicente, A.; Ginja, C.; Gama, L.T. Biodiversidade caprina em Portugal. In Biodiversidad Caprina Iberoamericana; Universidad Cooperativa de Colombia: Medellín, Columbia, 2017; pp. 57–74. [Google Scholar]
- SPREGA. Caprinos–Raça Serrana. Available online: http://www.sprega.com.pt/conteudo.php?idesp=caprinos&idraca=Serrana (accessed on 4 June 2024).
- DGAV Official Catalog of Portuguese Autochthonous Breeds. 2021. Available online: https://www.dgav.pt/wp-content/uploads/2021/04/Catalogo-Oficial-Racas-Autoctones-Portuguesas.pdf (accessed on 11 June 2024).
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Bauer, A. Reproductive and Milk Production Profiles in Serrana Goats. In Sustainable Goat Production in Adverse Environments: Volume II; Simões, J., Gutiérrez, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 233–243. ISBN 978-3-319-71293-2. [Google Scholar]
- Barbas, J.P.; Pimenta, J.; Baptista, M.C.; Marques, C.C.; Pereira, R.M.L.N.; Carolino, N.; Simões, J. Ram Semen Cryopreservation for Portuguese Native Breeds: Season and Breed Effects on Semen Quality Variation. Animals 2023, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.C.; Barbas, J.P.; Baptista, M.C.; Cannas Serra, C.; Vasques, M.I.; Pereira, R.M.; Cavaco-Gonçalves, S.; Horta, A.E.M. Reproduction in the ovine Saloia breed: Seasonal and individual factors affecting fresh and frozen semen performance, in vivo and in vitro fertility. In Animal Products from the Mediterranean Area; Ramalho Ribeiro, J.M.C., Horta, A.E.M., Mosconi, C., Rosati, A., Eds.; Brill—Wageningen Academic: Wageningen, The Netherlands, 2006; pp. 331–336. ISBN 978-90-8686-568-0. [Google Scholar]
- Fatet, A.; Pellicer-Rubio, M.-T.; Leboeuf, B. Reproductive cycle of goats. Anim. Reprod. Sci. 2011, 124, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, R.; Baptista, M.; Cavaco-Gonçalves, S.; Barbas, J. Caracterização da actividade reprodutiva e utilização da IA em pequenos ruminantes. In Agrorrural: Contributos Científicos; INCM e INRB: Lisbon, Portugal, 2011; pp. 1071–1082. [Google Scholar]
- Sousa, J.; Barbas, J.; Ferreira, G.; Horta, A.E.M. Variação Anual das Características Seminais em Bodes da Raça Serrana. In Revista Portuguesa de Zootecnia; Associação Portuguesa de Engenheiros Zootécnicos: Bragança, Portugal, 2001; pp. 297–311. [Google Scholar]
- Mujitaba, M.A.; Kútvölgyi, G.; Radnai Szentpáli, J.; Debnár, V.J.; Tokár, A.; Vass, N.; Bodó, S. The Influence of Three Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Quality of Pre-Freeze and Post-Thaw Ram Epididymal Spermatozoa. Animals 2024, 14, 1237. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Padilla, A.J.; Jimenez-Trejo, F.; Cerbon, M.; Chavez-Garcia, A.; Cruz-Cano, N.B.; Martinez-Torres, M.; Alcantar-Rodriguez, A.; Medrano, A. Sperm melatonin receptors, seminal plasma melatonin and semen freezability in goats. Theriogenology 2024, 225, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, B. Insemination artificielle caprine: Etat de l´art. In Proceedings of the Livro de Resumos, III Congresso Ibérico de Reprodução Animal; Fundação Cupertino de Miranda: Porto, Portugal, 2001; pp. 387–392. [Google Scholar]
- Sharma, A.; Sood, P. Cryopreservation and fertility of frozen thawed Chegu goat semen. Indian J. Anim. Res. 2019, 53, 1414–1419. [Google Scholar] [CrossRef]
- Barbas, J.P.; Marques, C.C.; Baptista, M.C.; Vasques, M.I.; Pereira, R.M.; Cavaco-Gonçalves, S.; Mascarenhas, R.M.; Poulin, N.; Cognie, Y.; Horta, A.E.M. Reproduction in the Serrana goat breed: Seasonal and individual factors affecting fresh and frozen semen performance, in vivo and in vitro fertility. In Animal Products from the Mediterranean Area; Ramalho Ribeiro, J.M.C., Horta, A.E.M., Mosconi, C., Rosati, A., Eds.; Brill—Wageningen Academic: Wageningen, The Netherlands, 2006; pp. 337–342. ISBN 978-90-8686-568-0. [Google Scholar]
- Leboeuf, B.; Delgadillo, J.; Manfredi, E.; Piacère, A.; Clément, V.; Martin, P.; Pellicer, M.; Boué, P.; De Cremoux, R. Management of Goat Reproduction and Insemination for Genetic Improvement in France. Reprod. Domest. Anim. 2008, 43, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Malaluang, P.; Ntallaris, T.; Johannisson, A. Practical Method for Freezing Buck Semen. Animals 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Prado, V.; Orihuela, A.; Lozano, S.; Pérez-León, I. Effect on ejaculatory performance and semen parameters of sexually-satiated male goats (Capra hircus) after changing the stimulus female. Theriogenology 2003, 60, 261–267. [Google Scholar] [CrossRef]
- Arrebola, F.; Pérez-Marín, C.C.; Santiago-Moreno, J. Limitation of seasonality in reproductive parameters of Mediterranean bucks, using photoperiod treatment. Small Rumin. Res. 2010, 89, 31–35. [Google Scholar] [CrossRef]
- Chemineau, P.; Baril, G.; Leboeuf, B.; Maurel, M.C.; Roy, F.; Pellicer-Rubio, M.-T.; Malpaux, B.; Cognie, Y. Implications of recent advances in reproductive physiology for reproductive management of goats. J. Reprod. Fertil. Suppl 1999, 54, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Şogorescu, E. Seasonal variations of plasma testosterone levels and testicular volume in Carpathian bucks. Afr. J. Agric. Res. 2011, 6, 6735–6740. [Google Scholar] [CrossRef]
- Ridler, A.L.; Smith, S.L.; West, D.M. Ram and buck management. Anim. Reprod. Sci. 2012, 130, 180–183. [Google Scholar] [CrossRef]
- Delgadillo, J.A.; Leboeuf, B.; Chemineau, P. Decrease in the seasonality of sexual behavior and sperm production in bucks by exposure to short photoperiodic cycles. Theriogenology 1991, 36, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; Vélez, L.I.; Flores, J.A. Continuous light after a long-day treatment is equivalent to melatonin implants to stimulate testosterone secretion in Alpine male goats. Animal 2016, 10, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; Espinoza-Flores, L.A.; López-Magaña, D.; Hernández, H.; Keller, M.; Chesneau, D.; Lainé, A.L.; Chemineau, P. Maintenance of permanent sexual activity throughout the year in seasonal bucks using short photoperiodic cycles in open barns. Animal 2024, 18, 101041. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Martínez, E.; Sánchez-Valverde, M.A.; Ruiz, S.; Vázquez, J.M. Seasonal variations of semen quality in male goats: Study of sperm abnormalities. Theriogenology 1992, 38, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Mateos, E. Effect of photoperiod on semen production and quality in bucks of Verata and Malagueña breeds. Small Rumin. Res. 1996, 22, 163–168. [Google Scholar] [CrossRef]
- Martínez-Madrid, B.; Castaño, C.; Ureña, L.P.; Flix, E.; Velázquez, R.; López-Sebastián, A.; Ungerfeld, R.; Arrebola, F.A.; Santiago-Moreno, J. Seasonal changes in testosterone and thyroxine concentrations in Mediterranean rams and bucks and their relationship with sperm cryoresistance. Livest. Sci. 2021, 249, 104513. [Google Scholar] [CrossRef]
- Galián, S.; Peinado, B.; Almela, L.; Poto, Á.; Ruiz, S. Post-Thaw Quality of Spermatozoa Frozen with Three Different Extenders in the Murciano Granadina Goat Breed. Animals 2023, 13, 309. [Google Scholar] [CrossRef]
- El Kadili, S.; Kirschvink, N.; Raes, M.; Bister, J.L.; Archa, B.; Douaik, A.; Chentouf, M. Influence of Season and Liquid Storage at 16 °C on Beni Arouss Bucks’ Semen Quality. Animals 2020, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Tabarez, A. Optimizacion del Protocolo de Crioconservacion de Semen Caprino de la Raza Autóctone en Peligro de Extinction—Blanca de Rasquera. Ph. D. Thesis, Universidad Autonoma de Barcelona, Barcelona, Spain, 2014. [Google Scholar]
- Wang, W.; Luo, J.; Sun, S.; Xi, L.; Gao, Q.; Haile, A.; Shi, H.; Zhang, W.; Shi, H. The Effect of Season on Spermatozoa Motility, Plasma Membrane and Acrosome Integrity in Fresh and Frozen–Thawed Semen from Xinong Saanen Bucks. Reprod. Domest. Anim. 2015, 50, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Boes, J.; Boettcher, P.; Honkatukia, M. Innovations in Cryoconservation of Animal Genetic Resources Practical Guide; FAO Animal Production and Health Guidelines; Food and Agriculture Organisation: Rome, Italy, 2023; Volume 33. [Google Scholar]
- Valencia, M.; González, H.; González, G.; Trejo, G. Motilidad y daño acrosomal del semen caprino congelado en pajillas de 0.25 ml y 0.5 ml y descongelado a dos diferentes ritmos de temperatura. Vet. Mex. 1994, 25, 127–131. [Google Scholar]
- Vallecillo, A.; Wheat, P.; Thin, J.; Hair, A.; Santos, E.; Tenorio, T. Effects of cryopreservation on sperm motility in white Andalusian Serrana Goat. S. Afr. J. Anim. Sci. 2004, 34, 116–118. [Google Scholar]
- Tabarez, A.; García, W.; Palomo, M.J. Effect of the type of egg yolk, removal of seminal plasma and donor age on buck sperm cryopreservation. Small Rumin. Res. 2017, 149, 91–98. [Google Scholar] [CrossRef]
- Gangwar, C.; Kharche, S.D.; Kumar, S.; Jindal, S.K. Cryopreservation of goat semen: Status and prospects. Indian J. Small Rumin. 2016, 22, 1. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, S.; Su, J.; Zhang, J.; Bao, X.; Ding, R.; Shi, P.; Li, S.; Wu, C.; Zhao, G.; et al. The effects of cryopreservation on the acrosome structure, enzyme activity, motility, and fertility of bovine, ovine, and goat sperm. Anim. Reprod. 2020, 17, e20200219. [Google Scholar] [CrossRef] [PubMed]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kapila, R.; Gandhi, K.K.; Anand, S.R. Acrosome damage and enzyme leakage of goat spermatozoa during dilution, cooling and freezing. Andrologia 2009, 26, 21–26. [Google Scholar] [CrossRef]
- Leboeuf, B.; Restall, B.; Salamon, S. Production and storage of goat semen for artificial insemination. Anim. Reprod. Sci. 2000, 62, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Rodríguez, I.; Dorado, J.M. The effect of cryopreservation on sperm head morphometry in Florida male goat related to sperm freezability. Anim. Reprod. Sci. 2007, 100, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.; Pérez-Guzmán, M.D.; Jiménez-Rabadán, P.; Esteso, M.C.; García-Álvarez, O.; Maroto-Morales, A.; Anel-López, L.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J. Sperm Cell Population Dynamics in Ram Semen during the Cryopreservation Process. PLoS ONE 2013, 8, e59189. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Arenán, H.; Sancho, M.; Contell, J.; Yániz, J.; Fernández, A.; Soler, C. Morphometry and subpopulation structure of Holstein bull spermatozoa: Variations in ejaculates and cryopreservation straws. Asian J. Androl. 2016, 18, 851. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, G.; Xian, M.; Zhang, G.; Wen, F.; Hu, Z.; Hu, J. Proteomic Analysis of Frozen–Thawed Spermatozoa with Different Levels of Freezability in Dairy Goats. Int. J. Mol. Sci. 2023, 24, 15550. [Google Scholar] [CrossRef] [PubMed]
- Zoppino, F.C.M.; Halón, N.D.; Bustos, M.A.; Pavarotti, M.A.; Mayorga, L.S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 2012, 97, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Wang, J.Z.; Van Kessel, J.S.; Ren, F.Z.; Zeng, S.S. Effect of somatic cell count in goat milk on yield, sensory quality, and fatty acid profile of semisoft cheese. J. Dairy Sci. 2010, 93, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Van Der Horst, G. Computer Aided Sperm Analysis (CASA) in domestic animals: Current status, three D tracking and flagellar analysis. Anim. Reprod. Sci. 2020, 220, 106350. [Google Scholar] [CrossRef]
| Breed | Number of Bucks | Number of Ejaculates | Ejaculate Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume (mL) | SPZ Concentration (106/mL) | Total SPZ per Ejaculate (106) | |||||||||
| CV% | CV% | CV% | |||||||||
| Bravia | 15 | 207 | 0.50 | 0.59 | 43 | 4340 | 4333 | 34 | 2375 | 2614 | 54 |
| Charnequeira | 11 | 171 | 0.68 | 0.72 | 47 | 4540 | 4585 | 29 | 3025 | 3311 | 52 |
| Preta de Montezinho | 3 | 23 | 0.70 | 0.70 | 48 | 5550 | 5307 | 17 | 5068 | 5181 | 20 |
| Serrana | 30 | 616 | 0.70 | 0.75 | 46 | 4150 | 4268 | 30 | 2790 | 3158 | 52 |
| Total | 59 | 1017 | 0.68 | 0.71 | 47 | 4300 | 4341 | 31 | 2750 | 3099 | 53 |
| Traits/Effects | DF | Volume per Ejaculate (mL) | SPZ Concentration (106/mL) | Total SPZ per Ejaculate (106) |
|---|---|---|---|---|
| Breed | 3 | * | NS | ** |
| Season | 2 | ** | ** | * |
| Breed × Season | 3 | * | * | NS |
| N | 1017 | |||
| Breed | Season | SPZ Traits (LSmean ± SEM) | ||
|---|---|---|---|---|
| Volume per Ejaculate (mL) | SPZ Concentration (106/mL) | Total SPZ per Ejaculate (106) | ||
| Bravia | February–April | 0.47 ± 0.06 a | 5274 ± 245 a | 2480 ± 296 a |
| May–August | 0.54 ± 0.07 a,b | 5052 ± 290 a | 2967 ± 352 a | |
| September–January | 0.65 ± 0.05 b | 3824 ± 180 b | 2537 ± 210 a | |
| Charnequeira | February–April | 0.50 ± 0.08 a | 5226 ± 339 a | 2850 ± 412 a |
| May–August | 0.61 ± 0.07 a | 4855 ± 249 a | 3121 ± 333 a | |
| September–January | 0.82 ± 0.06 b | 4327 ± 219 b | 3709 ± 254 b | |
| Preta de Montezinho | February–April | 0.56 ± 0.15 a | - * | - * |
| May–August | 0.87 ± 0.13 b | 5410 ± 556 a | 5049 ± 671 a | |
| September–January | 0.64 ± 0.15 a,b | 4922 ± 958 a | 5594 ± 1179 a | |
| Serrana | February–April | 0.63 ± 0.04 a | 4723 ± 150 a | 3000 ± 178 a |
| May–August | 0.74 ± 0.04 b | 4852 ± 141 a | 3541 ± 166 b | |
| September–January | 0.84 ± 0.04 c | 4017 ± 127 b | 3322 ± 147 b | |
| Breed | Bucks (n) | Ejaculates (n) * | Alive SPZ | IM | NM | HD | MPD | Tail D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CV% | CV% | CV% | CV% | CV% | CV% | |||||||||
| Bravia | 15 | 186–207 | 75.7 | 14.8 | 68.3 | 7.8 | 84.9 | 8.6 | 6.5 | 89.7 | 3.5 | 126.3 | 4.4 | 99.4 |
| Charnequeira | 11 | 164–170 | 77.6 | 14.4 | 64.5 | 7.9 | 88.4 | 6.9 | 6.0 | 68.2 | 2.6 | 115.9 | 2.7 | 110.0 |
| Preta de Montezinho | 3 | 22–23 | 80.8 | 8.9 | 70.3 | 5.9 | 86.8 | 4.8 | 7.5 | 49.8 | 2.0 | 118.8 | 3.3 | 60.1 |
| Serrana | 30 | 598–615 | 73.8 | 17.3 | 64.7 | 8.8 | 86.9 | 7.7 | 6.7 | 69.4 | 3.0 | 103.7 | 3.2 | 117.6 |
| Total | 59 | 970–1015 | 75.0 | 16.3 | 65.5 | 8.8 | 86.8 | 7.7 | 6.5 | 73.3 | 3.0 | 112.7 | 3.4 | 112.8 |
| Breed | Bucks (n) | Ejaculates (n) | Alive SPZ (%) | IM (%) | NM (%) | HD (%) | MPD (%) | Tail D (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CV% | CV% | CV% | CV% | CV% | CV% | |||||||||
| Bravia | 15 | 174–206 | 43.6 | 24.0 | 42.4 | 12.9 | 71.4 | 16.1 | 22.2 | 48.5 | 2.3 | 107.3 | 5.9 | 64.8 |
| Charnequeira | 11 | 155–168 | 43.0 | 23.9 | 42.4 | 11.9 | 75.2 | 12.6 | 16.8 | 57.2 | 1.6 | 104.5 | 7.0 | 60.4 |
| Preta de Montezinho | 3 | 18–23 | 51.2 | 18.0 | 44.3 | 10.3 | 72.8 | 12.8 | 21.8 | 40.9 | 1.1 | 136.8 | 3.7 | 53.3 |
| Serrana | 30 | 553–612 | 42.3 | 25.7 | 43.1 | 13.2 | 74.9 | 11.7 | 17.0 | 50.0 | 2.0 | 113.2 | 6.7 | 60.7 |
| Total | 59 | 944–1009 | 42.8 | 25.0 | 42.9 | 12.9 | 74.3 | 12.9 | 18.0 | 52.0 | 1.9 | 112.1 | 6.5 | 62.0 |
| Traits/Effects | DF | Alive SPZ | IM | NM | HD | MPD | Tail D |
|---|---|---|---|---|---|---|---|
| Breed | 3 | * | NS | NS | NS | * | NS |
| Season | 2 | NS | NS | NS | * | NS | NS |
| Semen | 1 | ** | ** | ** | ** | ** | ** |
| Breed × Season | 6 | NS | NS | NS | * | ** | NS |
| Breed × Semen | 3 | * | ** | NS | ** | NS | ** |
| Season × Semen | 2 | NS | NS | NS | NS | NS | NS |
| Breed × Season × Semen | 6 | NS | NS | NS | NS | NS | NS |
| Traits | n | r | R-Squared (%) |
|---|---|---|---|
| Alive (%) | 925 | 0.269 ** | 7.2 |
| Individual motility (%) | 1009 | 0.210 ** | 4.4 |
| Normal morphology (%) | 938 | 0.274 ** | 7.5 |
| Head defects (%) | 931 | 0.290 ** | 8.4 |
| Intermediate piece defects (%) | 983 | 0.330 ** | 10.9 |
| Tail defects (%) | 892 | 0.066 NS | 0.4 |
| Total R-Squared | Partial R-Squared (p < 0.05) | ||||
|---|---|---|---|---|---|
| Traits | Trait in Fresh SPZ | Volume per Ejaculate | SPZ Concentration | Total SPZ | |
| Live (%) | 0.099 | 0.060 | 0.030 | 0.009 | - |
| Individual motility (%) | 0.091 | 0.030 | 0.061 | - | - |
| Normal morphology (%) | 0.089 | 0.081 | - | 0.008 | - |
| Head defects (%) | 0.112 | 0.106 | - | 0.006 | - |
| Midpiece defects (%) | 0.136 | 0.115 | 0.012 | 0.008 | - |
| Tail defects (%) | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbas, J.P.; Baptista, M.C.; Carolino, N.; Simões, J.; Margatho, G.; Pimenta, J.; Claudino, F.; Ferreira, F.C.; Grilo, F.; Pereira, R.M.L.N. Effect of Breed and Season in Buck Semen Cryopreservation: The Portuguese Animal Germplasm Bank. Vet. Sci. 2024, 11, 326. https://doi.org/10.3390/vetsci11070326
Barbas JP, Baptista MC, Carolino N, Simões J, Margatho G, Pimenta J, Claudino F, Ferreira FC, Grilo F, Pereira RMLN. Effect of Breed and Season in Buck Semen Cryopreservation: The Portuguese Animal Germplasm Bank. Veterinary Sciences. 2024; 11(7):326. https://doi.org/10.3390/vetsci11070326
Chicago/Turabian StyleBarbas, João Pedro, Maria Conceição Baptista, Nuno Carolino, João Simões, Gisele Margatho, Jorge Pimenta, Francisca Claudino, Filipa Costa Ferreira, Francisco Grilo, and Rosa Maria Lino Neto Pereira. 2024. "Effect of Breed and Season in Buck Semen Cryopreservation: The Portuguese Animal Germplasm Bank" Veterinary Sciences 11, no. 7: 326. https://doi.org/10.3390/vetsci11070326
APA StyleBarbas, J. P., Baptista, M. C., Carolino, N., Simões, J., Margatho, G., Pimenta, J., Claudino, F., Ferreira, F. C., Grilo, F., & Pereira, R. M. L. N. (2024). Effect of Breed and Season in Buck Semen Cryopreservation: The Portuguese Animal Germplasm Bank. Veterinary Sciences, 11(7), 326. https://doi.org/10.3390/vetsci11070326








