Exploring the Molecular Characteristics and Role of PDGFB in Testis and Epididymis Development of Tibetan Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Primer Design and Synthesis
2.4. Cloning of PDGFB Gene
2.5. Bioinformatics Analysis
2.6. qRT-PCR Analysis
2.7. Hematoxylin and Eosin and Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
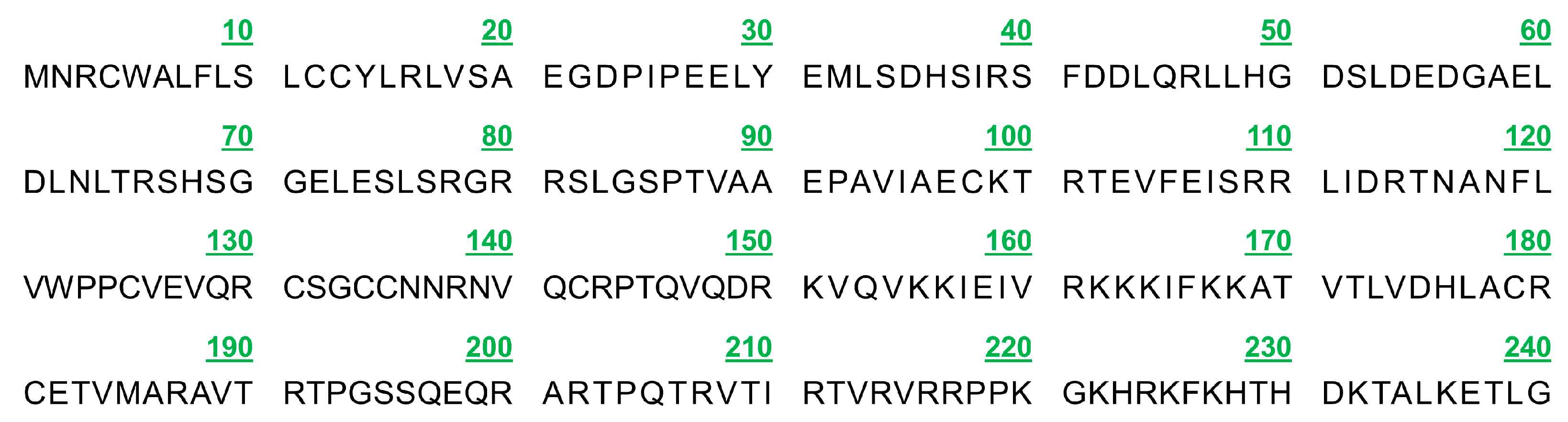
3.1. Sequence Characteristics of Complete CDS Region of PDGFB in Tibetan Sheep
3.2. Homology Comparison of PDGFB between Tibetan Sheep and Other Mammals
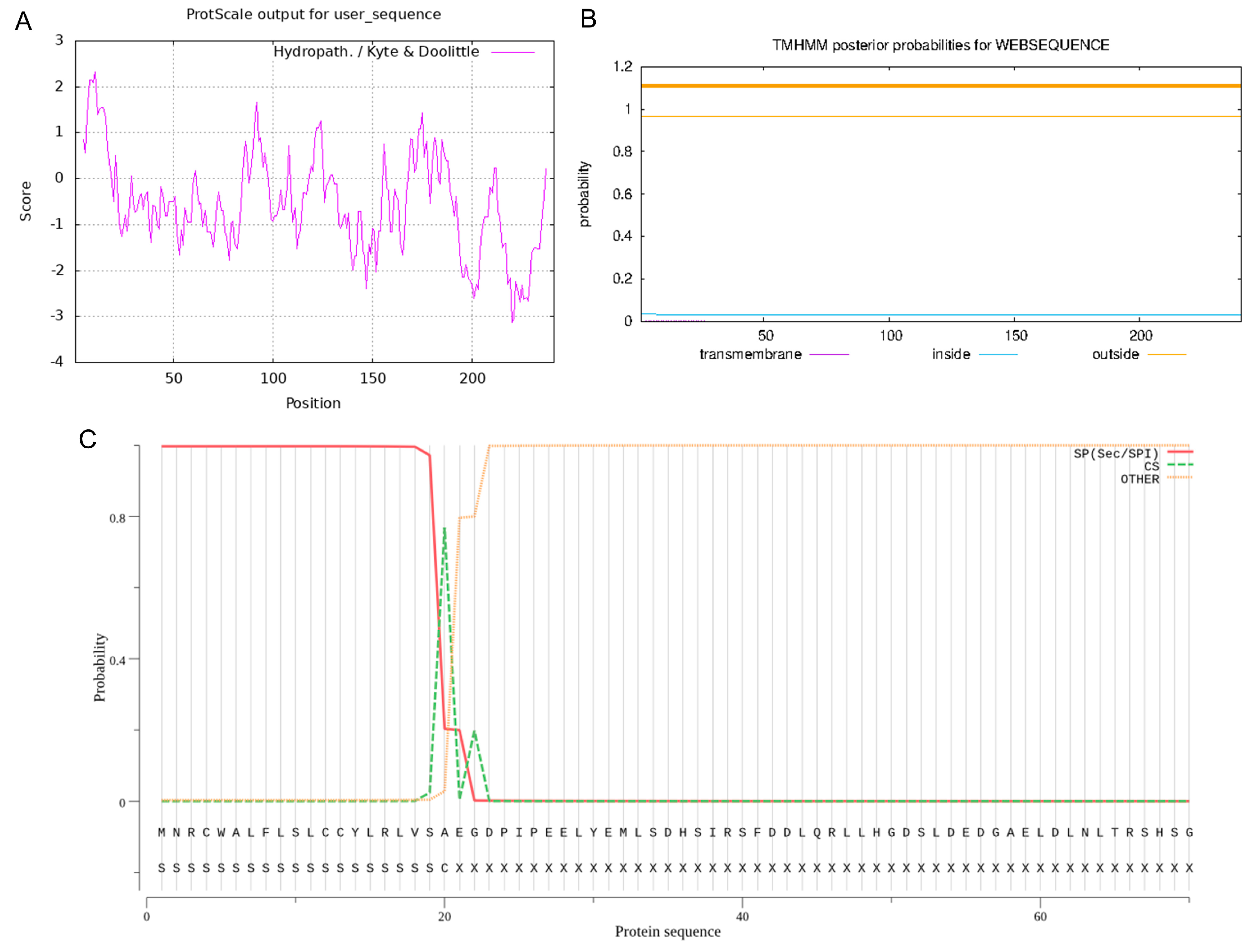
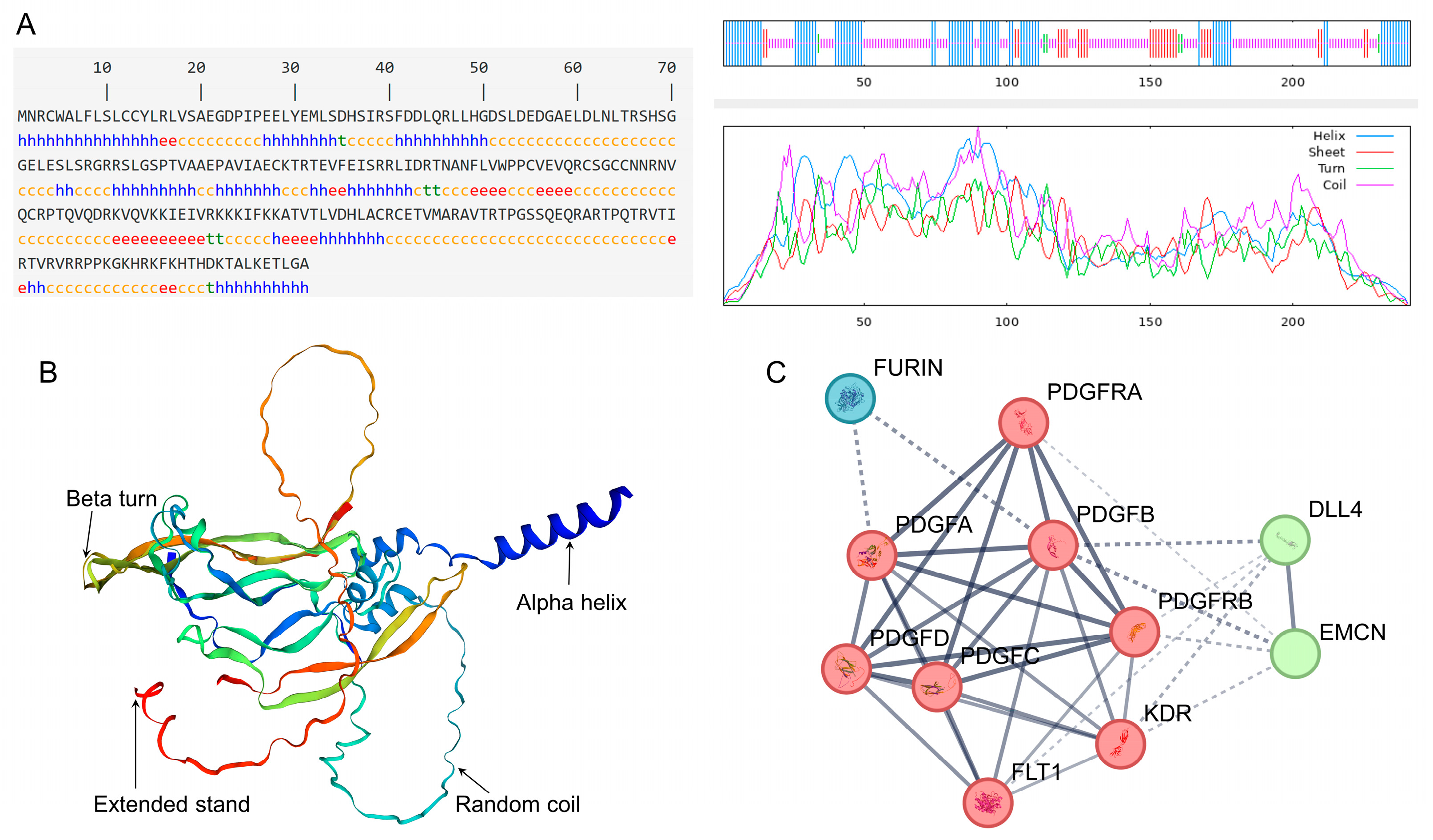
3.3. Spatial Structure Analysis of PDGFB Protein in Tibetan Sheep
3.4. Analysis of Interacting Proteins with Tibetan Sheep PDGFB Protein
3.5. PDGFB mRNA Expression in Testis and Epididymis Tissues
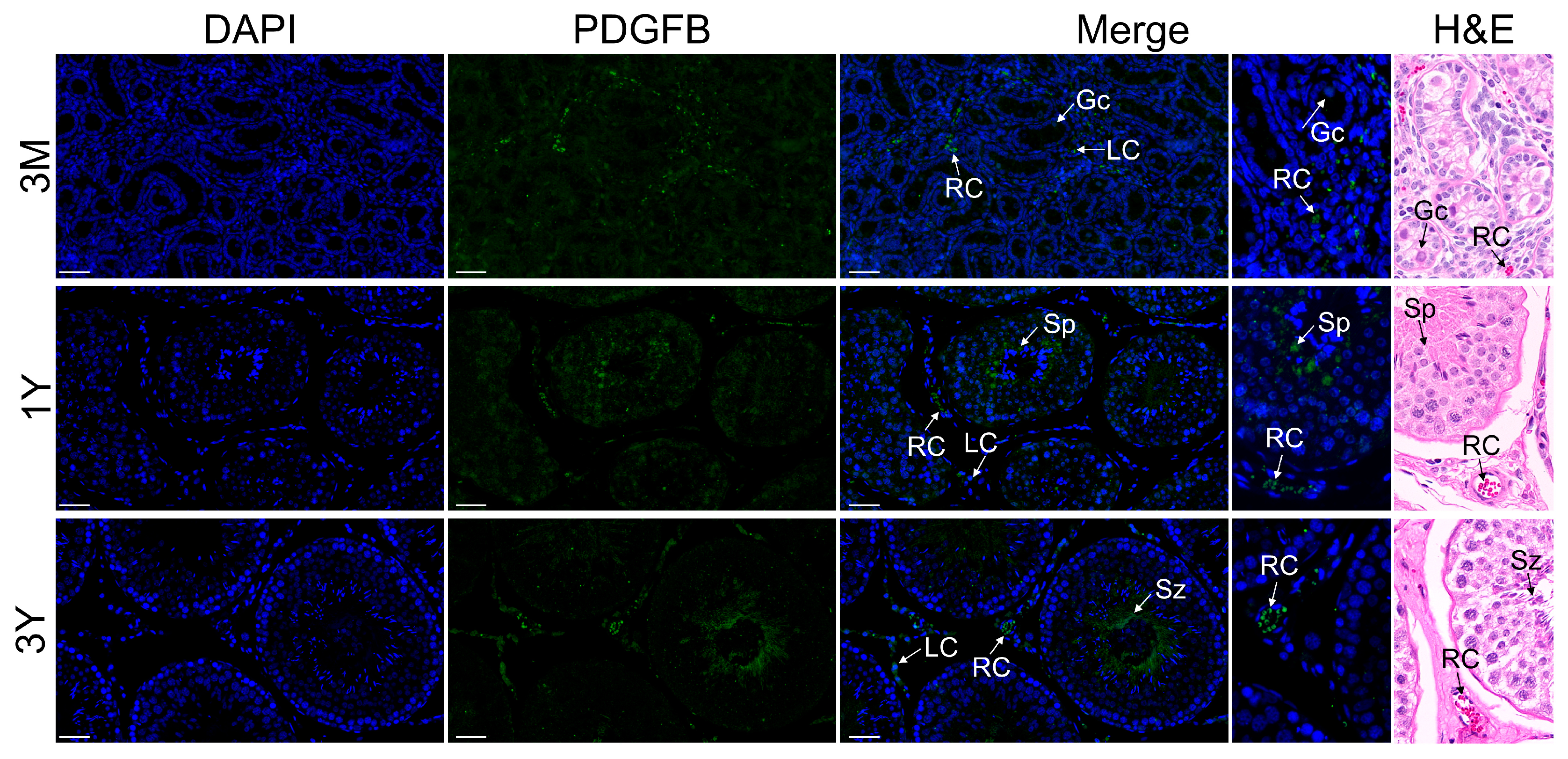
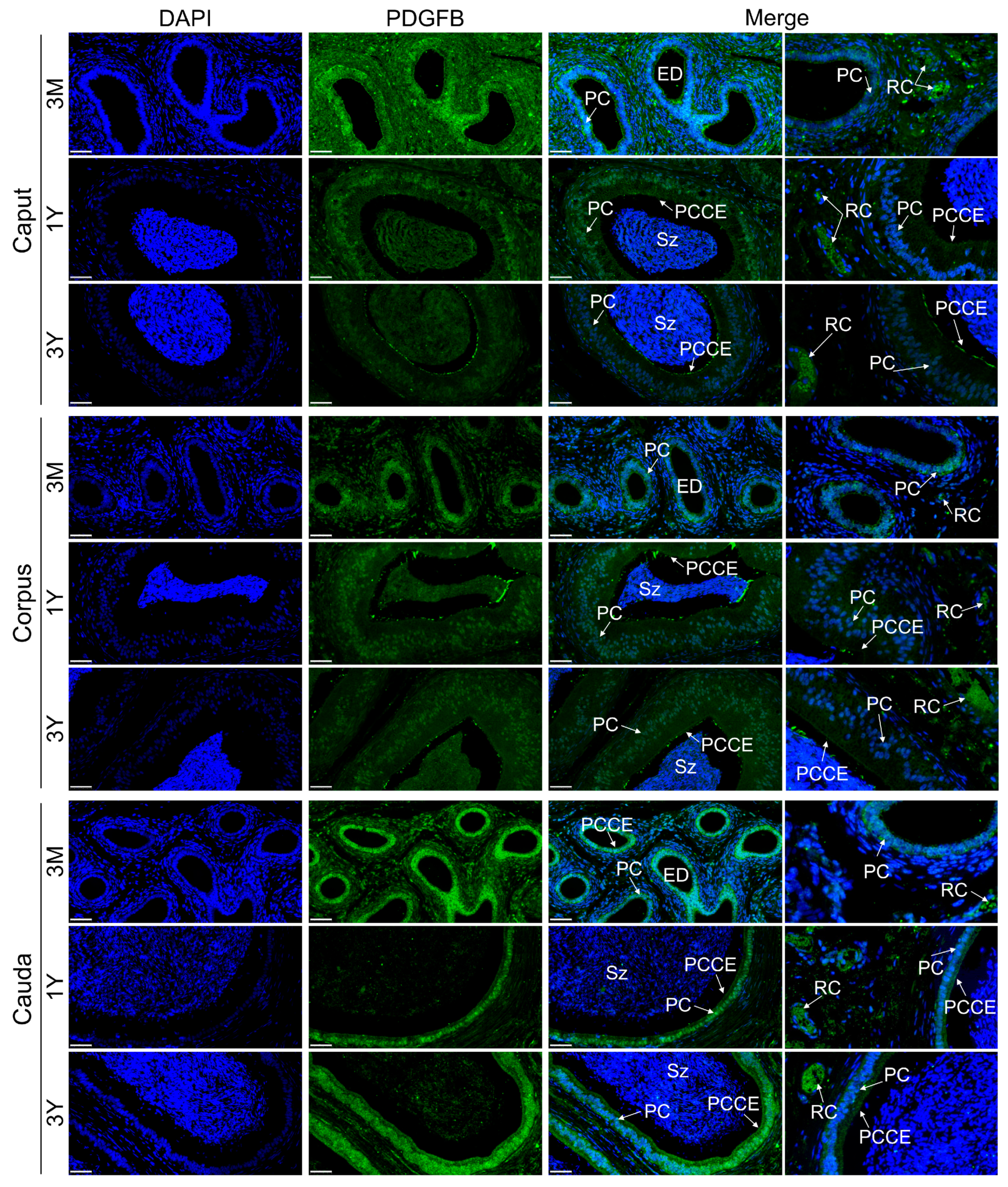
3.6. Localization of PDGFB Protein in Testis and Epididymis Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.; Sun, X.; Zhao, S.; Hu, M.; Li, D.; Qi, S.; Jiao, X.; Sun, Y.; Wang, C.; Zhu, X.; et al. Dietary alfalfa powder supplementation improves growth and development, body health, and meat quality of Tibetan sheep. Food Chem. 2022, 396, 133709. [Google Scholar] [CrossRef]
- Han, B.; Tian, D.; Li, X.; Liu, S.; Tian, F.; Liu, D.; Wang, S.; Zhao, K. Multiomics analyses provide new insight into genetic variation of reproductive adaptability in Tibetan sheep. Mol. Biol. Evol. 2024, 41, msae058. [Google Scholar] [CrossRef]
- Kumar, L.; Solanki, S.; Jain, A.; Botts, M.; Gupta, R.; Rajput, S.; Roti Roti, E. MAPKs signaling is obligatory for male reproductive function in a development-specific manner. Front. Reprod. Health 2024, 6, 1330161. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef]
- Lee, V.; Hinton, B.; Hirashima, T. Collective cell dynamics and luminal fluid flow in the epididymis: A mechanobiological perspective. Andrology 2023, 12, 939–948. [Google Scholar] [CrossRef]
- Mariani, S.; Basciani, S.; Arizzi, M.; Spera, G.; Gnessi, L. PDGF and the testis. Trends Endocrin. Met. 2002, 13, 11–17. [Google Scholar] [CrossRef]
- Gnessi, L.; Emidi, A.; Jannini, E.A.; Carosa, E.; Maroder, M.; Arizzi, M.; Ulisse, S.; Spera, G. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J. Cell Biol. 1995, 131, 1105–1121. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Xu, X.; Huang, B.; Sun, H.; Li, L.; Zhang, M.; Liu, J. A PDGFB mutation causes paroxysmal nonkinesigenic dyskinesia with brain calcification. Mov. Disord. 2017, 32, 1104–1106. [Google Scholar] [CrossRef]
- Ricci, G.; Catizone, A.; Galdieri, M. Embryonic mouse testis development: Role of platelet derived growth factor (PDGF-BB). J. Cell. Physiol. 2004, 200, 458–467. [Google Scholar] [CrossRef]
- Fang, Q. Effects of PDGF-BB and EGF on the Proliferation of Goat Spermatogonial Stem Cells In Vitro. Master’s Thesis, Northwest Agriculture and Forestry University, Yangling, China, 2018. [Google Scholar]
- Basciani, S.; Mariani, S.; Arizzi, M.; Brama, M.; Ricci, A.; Betsholtz, C.; Bondjers, C.; Ricci, G.; Catizone, A.; Galdieri, M.; et al. Expression of platelet-derived growth factor (PDGF) in the epididymis and analysis of the epididymal development in PDGF-A, PDGF-B, and PDGF receptor beta deficient mice. Biol. Reprod. 2004, 70, 168–177. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, T.; An, X.; Yin, D.; Chen, N.; Ma, Y. Regulation of GDF9 and CDKN1B expression in Tibetan sheep testes during different stages of maturity. Gene Expr. Patterns. 2022, 43, 119218. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Huang, F.; Dong, L.; Liu, L.; Jia, X.; Chi, J.; Ma, Y.; Deng, M.; Chen, Y.; et al. Hydrolyzed bound phenolics from rice bran alleviate hyperlipidemia and improve gut microbiota dysbiosis in high-fat-diet fed mice. Nutrients 2022, 14, 1277. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; Wang, X.; Yin, E.; Chen, N.; Kang, L.; Zhao, X.; Ma, Y. Cloning, Molecular Characterization and Expression Patterns of DMRTC2 Implicated in Germ Cell Development of Male Tibetan Sheep. Int. J. Mol. Sci. 2020, 21, 2448. [Google Scholar] [CrossRef]
- Betsholtz, C. Biology of platelet-derived growth factors in development. Birth Defects Res. C Embryo Today 2004, 69, 272–285. [Google Scholar] [CrossRef]
- Rhen, T.; Jangula, A.; Schroeder, A.; Woodward-Bosh, R. The platelet-derived growth factor signaling system in snapping turtle embryos, Chelydra serpentina: Potential role in temperature-dependent sex determination and testis development. Gen. Comp. Endocr. 2009, 161, 335–343. [Google Scholar] [CrossRef]
- Schmahl, J.; Rizzolo, K.; Soriano, P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008, 22, 3255–3267. [Google Scholar] [CrossRef]
- Odeh, H.; Kleinguetl, C.; Ge, R.; Zirkin, B.; Chen, H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol. Reprod. 2014, 90, 123. [Google Scholar] [CrossRef]
- Shen, H. Expression and Analysis of PDGF and CDYL Genes in Testis of the Chinese Red Steppe Cattle at Different Months. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2023. [Google Scholar]
- Bresnick, E.H.; Hewitt, K.J.; Mehta, C.; Keles, S.; Paulson, R.F.; Johnson, K.D. Mechanisms of erythrocyte development and regeneration: Implications for regenerative medicine and beyond. Development 2018, 145, dev151423. [Google Scholar] [CrossRef]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte metabolism. Acta. Physiol. 2024, 240, e14081. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Das, R.; Patil, P.K. Visualization of poikilocytosis as an emerging erythrocytic biomarker for fish health assessment. Anim. Res. One Health. 2024, 2, 136–157. [Google Scholar] [CrossRef]
- Palomino-Schätzlein, M.; Lamas-Domingo, R.; Ciudin, A.; Gutiérrez-Carcedo, P.; Marés, R.; Aparicio-Gómez, C.; Hernández, C.; Simó, R.; Herance, J.R. A translational in vivo and in vitro metabolomic study reveals altered metabolic pathways in red blood cells of type 2 diabetes. J. Clin. Med. 2020, 9, 1619. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Tomsig, J.; Turner, T. Growth factors and the epididymis. J. Androl. 2006, 27, 348–357. [Google Scholar] [CrossRef]
- Breton, S.; Ruan, Y.; Park, Y.; Kim, B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J. Androl. 2016, 18, 3–9. [Google Scholar] [CrossRef]
- Zhang, M.; Sheng, X.; Zhang, H.; Wang, Q.; Xu, M.; Weng, Q.; Watanabe, G.; Taya, K. Seasonal changes in morphology and immunoreactivity of PDGF-A and its receptor PDGFR-α in the epididymis of wild ground squirrels (Citellus dauricus Brandt). J. Reprod. Dev. 2012, 58, 353–359. [Google Scholar] [CrossRef]
- Okamura, N.; Tamba, M.; Uchiyama, Y.; Sugita, Y.; Dacheux, F.; Syntin, P.; Dacheux, J. Direct evidence for the elevated synthesis and secretion of procathepsin L in the distal caput epididymis of boar. Biochim. Biophys. Acta 1995, 1245, 221–226. [Google Scholar] [CrossRef]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial dynamics in the epididymis: Role in the maturation, protection, and storage of spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef]



| Gene Name | Primer Sequences (5′~3′) | Product Length | Usage |
|---|---|---|---|
| PDGFB | F: TGAGATCGTGCGGAAGAAGAAG R: GAATGGTCACCCGAGTTTGG | 161 bp | qRT-PCR |
| F: ATGAATCGCTGCTGGGCG R: CTAGGCTCCAAGGGTCTCCT | 726 bp | RT-PCR | |
| β-actin | F: CTTCCAGCCTTCCTTCCTGG R: GCCAGGGCAGTGATCTCTTT | 180 bp | qRT-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Pu, L.; Tian, C.; Qi, X.; Song, J.; Liao, Y.; Mo, B.; Li, T. Exploring the Molecular Characteristics and Role of PDGFB in Testis and Epididymis Development of Tibetan Sheep. Vet. Sci. 2024, 11, 266. https://doi.org/10.3390/vetsci11060266
Chen H, Pu L, Tian C, Qi X, Song J, Liao Y, Mo B, Li T. Exploring the Molecular Characteristics and Role of PDGFB in Testis and Epididymis Development of Tibetan Sheep. Veterinary Sciences. 2024; 11(6):266. https://doi.org/10.3390/vetsci11060266
Chicago/Turabian StyleChen, Haolin, Ling Pu, Chengcheng Tian, Xingcai Qi, Juanjuan Song, Yan Liao, Bentian Mo, and Taotao Li. 2024. "Exploring the Molecular Characteristics and Role of PDGFB in Testis and Epididymis Development of Tibetan Sheep" Veterinary Sciences 11, no. 6: 266. https://doi.org/10.3390/vetsci11060266
APA StyleChen, H., Pu, L., Tian, C., Qi, X., Song, J., Liao, Y., Mo, B., & Li, T. (2024). Exploring the Molecular Characteristics and Role of PDGFB in Testis and Epididymis Development of Tibetan Sheep. Veterinary Sciences, 11(6), 266. https://doi.org/10.3390/vetsci11060266




