Effect of Peganum harmala Total Alkaloid Extract on Sexual Behavior and Sperm Parameters in Male Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Materials
2.3. Preparation of Plant Extract
2.4. Animal Preparation
2.5. Male Sexual Behavior Observation
2.6. Blood Sample and Gonadosomatic Index
2.7. Epididymal Sperm Count and Motility Analysis
2.8. Membrane Integrity and Antioxidant Status of Spermatozoa
2.9. Testosterone and Fructose Assay
2.10. Statistical Analysis
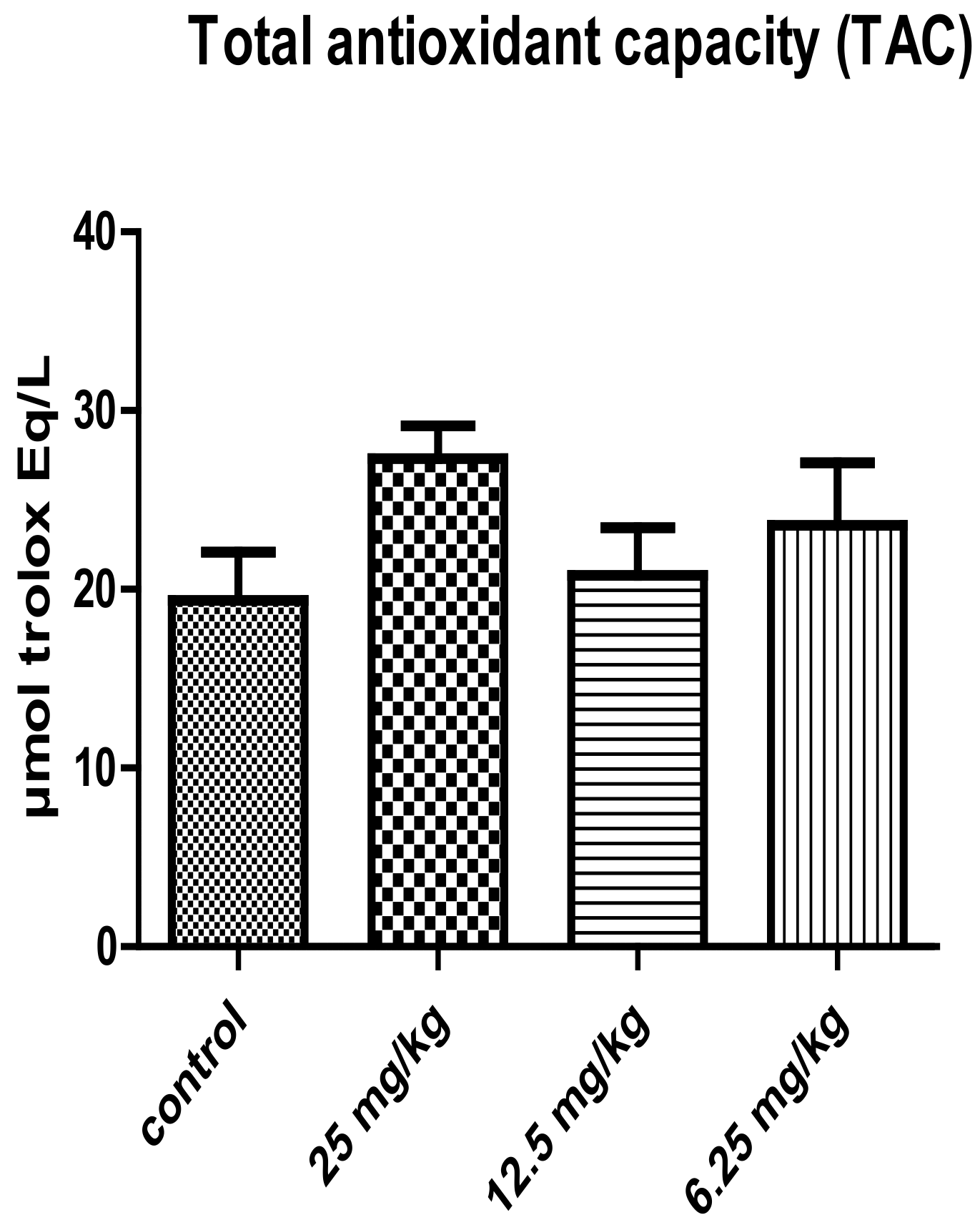
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musa, N.H.C.; Zain, H.H.M.; Muayad, T.M.; Ibrahim, H. Effect of lyophilized aqueous leaf extract of Aquilaria subintegra on aphrodisiac properties in mice. Asian Pac. J. Reprod. 2019, 8, 167. [Google Scholar]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Hassan, S.M.; Alkorashy, A.I.; Karar, M.M.; Ahmed, S.H.; Mohamed, S.O. Brain cortical and hippocampal dopamine: A new mechanistic approach for Eurycoma longifolia well-known aphrodisiac activity and its chemical characterization. Evid.-Based Complement. Altern. Med. 2019, 2019, e7543460. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Zubair, S.; Riaz, A.; Anwar, F.; Ahmad, B. Effect of venlafaxine, pramipexole, and valsartan on spermatogenesis in male rats. ACS Omega 2020, 5, 20481–20490. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebæk, N.E. Evidence for decreasing quality of semen during past 50 years. Br. Med. J. 1992, 305, 609–613. [Google Scholar] [CrossRef]
- Chaturapanich, G.; Chaiyakul, S.; Verawatnapakul, V.; Yimlamai, T.; Pholpramool, C. Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia 2012, 4, 323–328. [Google Scholar] [CrossRef]
- Hammad, A.; Muhammad, A. Evaluation of aphrodisiac activity of ethanol extract of Ganoderma lucidum in male Wistar rats. Clin. Phytosci. 2018, 4, 26. [Google Scholar]
- Ugwah-Oguejiofor, C.J.; Ogbulie, C.S.; Ugwah, M.O.; Umaru, M.L.; Adebisi, I.M.; Abubakar, K.; Alkali, Y. Effect of aqueous extract of the aerial parts of Carallumadalzielii N. E Brown on the testosterone levels of male Wistar rats. J. Pharm. Bioresour. 2018, 15, 175–179. [Google Scholar] [CrossRef]
- Gill, M.; Rai, A.; Kinra, M.; Sumalatha, S.; Rao, C.M.; Cheruku, S.P.; Devkar, R.; Kumar, N. Chemically characterised extract of Saracaasoca improves the sexual function in male Wistar rats. Andrologia 2018, 50, e13037. [Google Scholar] [CrossRef]
- Akintunde, W.O.; Abdulfatai, A.; Adeleke, M.T. Effect of Aqueous Extract of Carpolobia Lutea Stem on Some Reproduc-tive Parameters in Adult Male Wistar Rats. Pan Afr. J. Life Sci. 2020, 4, 25–29. [Google Scholar]
- Hosseini, A.; Shahrani, M.; Asgharian, S.; Anjomshoa, M.; Rostamzadeh, A.; Lorigooini, Z.; Asgharzadeh, N.; Azari, A. Ameliorative effect of Alliumatroviolaceum on sperm quality in cyclophosphamide-treated mice. Future J. Pharm. Sci. 2021, 7, 82. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Huang, X.; Zhang, Y.; Zhang, C. Main alkaloids of Peganum harmala L. and their different effects on dicot and monocot crops. Molecules 2013, 18, 2623–2634. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M.; Altun, M.L.; Kurucu, S. HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J. Pharm. Biomed. Anal. 2003, 31, 263–269. [Google Scholar] [CrossRef]
- Davoodi, H.; Ghaemi, E.; Mazandarani, M.; Shakeri, F.; Javid, S.N.; Klishadi, M. Anti-mycobacterial and anti-inflammatory activity of Peganum harmala. J. Chem. Pharm. Res. 2015, 7, 1611–1616. [Google Scholar]
- Diba, K.; Shoar, M.G.; Shabatkhori, M.; Khorshivand, Z. Anti fungal activity of alcoholic extract of Peganum harmala seeds. J. Med. Plant Res. 2011, 5, 5550–5554. [Google Scholar]
- Rharrabe, K.; Bakrim, A.; Ghailani, N.; Sayah, F. Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2007, 89, 137–145. [Google Scholar] [CrossRef]
- Apostolico, I.; Aliberti, L.; Caputo, L.; De Feo, V.; Fratianni, F.; Nazzaro, F.; Souza, L.F.; Khadhr, M. Chemical composition, antibacterial and phytotoxic activities of Peganum harmala seed essential oils from five different localities in Northern Africa. Molecules 2016, 21, 1235. [Google Scholar] [CrossRef]
- Komeili, G.; Hashemi, M.; Bameri-Niafar, M. Evaluation of antidiabetic and antihyperlipidemic effects of Peganum harmala seeds in diabetic rats. Cholesterol 2016, 2016, 7389864. [Google Scholar] [CrossRef]
- Jalali, A.; Dabaghian, F.; Zarshenas, M.M. Alkaloids of Peganum harmala: Anticancer biomarkers with promising outcomes. Curr. Pharm. Des. 2021, 27, 185–196. [Google Scholar] [CrossRef]
- Subhan, F.; Sultan, S.; Alam, W.; Tahir, F.; Dil, A.S. Aphrodisiac potential of Peganum harmala seeds. Hamdard Med. 1998, 41, 69–72. [Google Scholar]
- EL-Dwairi, Q.A.; Banihani, S.M. Histo-functional effects of Peganumharmala on male rat’s spermatogenesis and fertility. Neuroendocrinol. Lett. 2007, 28, 305–310. [Google Scholar] [PubMed]
- Hanon, H.A. Effects of aqueous and alcoholic extracts of Peganumharmala L. seeds on male fertility of white micetreated with olanzapin drug. J. Glob. Pharma Technol. 2009, 10, 426–436. [Google Scholar]
- ELghul, S.M.; Sasi, N.M.; Mohammed, E. Assessment the effect of aqueous extract of Peganum harmala seeds on fertility of male mice. Syr. J. Agric. Res. 2020, 7, 434–440. [Google Scholar]
- De Wit, M.; Hugo, A.; Shongwe, N. South African cactus pear seed oil: A comprehensive study on 42 spineless burbank Opuntia ficus-indica and Opuntia robusta cultivars. Eur. J. Lipid Sci. Technol. 2018, 120, 1700343. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Chuang, C.-H.; Chen, H.-C.; Wan, C.-J.; Chen, T.-L.; Lin, L.-Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT—Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Benbott, A.; Mahdia, D.; Zellaguia, A.; Moumena, Y.; Mosbaha, C. Effect of alkaloids extract of peganum harmala seeds on histo-function of rat’s testes. J. New Technol. Mater. 2018, 8, 70–76. [Google Scholar] [CrossRef]
- Revell, S.G.; Mrode, R.A. An osmotic resistance test for bovine semen. Anim. Reprod. Sci. 1994, 36, 7–86. [Google Scholar] [CrossRef]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef]
- Mann, T. Biochemistry of Semen and of the Male Reproductive Tract; Methuen & Go Ltd.: London, UK; John Wiley & Sons Inc.: New York, NY, USA, 1964. [Google Scholar]
- Malviya, N.; Jain, S.; Gupta, V.B.; Vyas, S. Recent studies on aphrodisiac herbs for the management of male sexual dysfunction-a review. Acta Pol. Pharm. 2011, 68, 3–8. [Google Scholar]
- JianFeng, C.; PengYing, Z.; ChengWei, X.; TaoTao, H.; YunGui, B.; KaoShan, C. Effect of aqueous extract of Arctium lappa L. (burdock) roots on the sexual behavior of male rats. BMC Complement. Altern. Med. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Nchegang, B.; Mezui, C.; Longo, F.; Nkwengoua, Z.E.; Amang, A.P.; Pan, P.V. Effects of the aqueous extract of Eremomastax speciosa (Acanthaceae) on sexual behavior in normal male rats. BioMed Res. Int. 2016, 2016, 9706429. [Google Scholar] [CrossRef]
- Vyas, N.Y.; Raval, M.A. Aphrodisiac and spermatogenic potential of alkaloidal fraction of Hygrophila spinosa T. Ander in rats. J. Ethnopharmacol. 2016, 194, 947–953. [Google Scholar] [CrossRef]
- Wankeu-Nya, M.; Watcho, P.; Nguelefack, T.B.; Carro-Juarez, M.; Tapondjou, L.; Kamanyi, A. Effects of Dracaena arborea (Dracaenaceae) on sexual dysfunction in 4 weeks hyperglycemic male rats. Asian Pac. J. Trop. Med. 2014, 7, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Obiandu, C.; Achinike, P.C. Evaluation of sexual behaviour in Momordica charantia treated male Wistar rats. Saudi J. Med. Pharm. Sci. 2020, 6, 183–185. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Sharma, V.; Dixit, V.K.; Thakur, M. A review on plants used for improvement of sexual performance and virility. BioMed Res. Int. 2014, 2014, 868062. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431. [Google Scholar] [CrossRef]
- Antoine, K.S.; Pierre, M.; Désiré, D.D.P.; Mesmer, F.N.N.; Pierre, W.; Théophile, D.; Pierre, K. Effect of aqueous extract of Allanblackia floribunda (Oliver) stem bark on sexual behaviour in adult male rats. World J. Pharm. Pharm. Sci. 2012, 1, 585–600. [Google Scholar]
- Yonezawa, T.; Hasegawa, S.; Asai, M.; Ninomiya, T.; Sasaki, T.; Cha, B.Y.; Teruya, T.; Ozawa, H.; Yagasaki, K.; Nagai, K.; et al. Harmine, a β-carboline alkaloid, inhibits osteoclast differentiation and bone resorption in vitro and in vivo. Eur. J. Pharmacol. 2011, 650, 511–518. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–666. [Google Scholar] [CrossRef]
- Karabaşoğlu, C.; Güneş, B.; Erbaş, O. Suicide-Related Genes. J. Exp. Basic Med. Sci. 2021, 2, 18–26. [Google Scholar]
- Kim, H.; Sablin, S.O.; Ramsay, R.R. Inhibition of monoamine oxidase A by β-carboline derivatives. Arch. Biochem. Biophys. 1997, 337, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhang, L.; Zhang, Y.; Sheng, Y.; Deng, G.; Li, S.; Cao, N.; Guan, H.; Cheng, X.; et al. Subchronic toxicity and concomitant toxicokinetics of long-term oral administration of total alkaloid extracts from seeds of Peganum harmala Linn: A 28-day study in rats. J. Ethnopharmacol. 2019, 238, 111866. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, M.R. The effects of aqueous extract of anacyclus pyrethrum on sperm count and reproductive organs in adult male rats. Zahedan J. Res. Med. Sci. 2015, 17, 42–46. [Google Scholar]
- Al-Mushhadani, M.T.; EArteen, H.; JJumaa, H. Effect of plant seeds Peganumharmala evaporation on fertility of white rats male treated with drug chlorpromazine. Rafidain J. Sci. 2014, 25, 1–12. [Google Scholar] [CrossRef]
- Derbak, H.; Moussaoui, M.; Benberkane, A.; Ayad, A. In-vitro effect of Peganum harmala total alkaloids on spermatozoa quality and oxidative stress of epididymal ram semen. Asian Pac. J. Reprod. 2021, 10, 232. [Google Scholar]
- Senhaji, S.; Lamchouri, F.; Boulfia, M.; Lachkar, N.; Bouabid, K.; Toufik, H. Mineral composition, content of phenolic compounds and in vitro antioxidant and antibacterial activities of aqueous and organic extracts of the seeds of Peganum harmala L. S. Afr. J. Bot. 2022, 147, 697–712. [Google Scholar] [CrossRef]
- Keshtmand, Z.; Oryan, S.; Ghanbari, A.; Khazaei, M. Protective effect of Tribulus terrestris hydroalcoholic extract against cisplatininduced cytotoxicity on sperm parameters in male mice. Int. J. Morphol. 2014, 32, 551–557. [Google Scholar] [CrossRef]
- Darszon, A.; López-Martínez, P.; Acevedo, J.J.; Hernández-Cruz, A.; Treviño, C.L. T-type Ca2+ channels in sperm function. Cell Calcium 2006, 40, 241–252. [Google Scholar] [CrossRef]
- Ghosh, A.; Jana, K.; Pakhira, B.P.; Tripathy, A.; Ghosh, D. Anti-fertility effect of aqueous-ethanolic (1:1) extract of the fruit of Terminalia chebula: Rising approach towards herbal contraception. Asian Pac. J. Reprod. 2021, 4, 201–207. [Google Scholar] [CrossRef]
- Tahtamouni, L.H.; Al-Khateeb, R.A.; Abdellatif, R.N.; Al-Mazaydeh, Z.A.; Yasin, S.R.; Al-Gharabli, S.; Elkarmi, A.Z. Anti-spermatogenic activities of Taraxacum officinale whole plant and leaves aqueous extracts. Vet. Res. Forum 2016, 7, 89. [Google Scholar] [PubMed]
- David, M.; Ain Qul Ahmad, M.; Zaman, W.; Jahan, S. A biochemical and histological approach to study antifertility effects of methanol leaf extract of Asplenium dalhousiae Hook. In adult male rats. Andrologia 2019, 51, e13262. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.; Magdi, Y.; Rashed, A.; Henkel, R.; Agarwal, A. Epididymal contribution to male infertility: An overlooked problem. Andrologia 2021, 53, e13721. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Wnuk, M.; Lewinska, A.; Oklejewicz, B.; Bartosz, G.; Tischner, M.; Bugno-Poniewierska, M. Redox status of equine seminal plasma reflects the pattern and magnitude of DNA damage in sperm cells. Theriogenology 2010, 74, 1677–1684. [Google Scholar] [CrossRef]
- Sarbishegi, M.; Gorgich, E.A.C.; Khajavi, O. Olive leaves extract improved sperm quality and antioxidant status in the testis of rat exposed to rotenone. Nephro-Urol. Mon. 2017, 9, e47127. [Google Scholar]
- Hosseinzadeh Colagar, A.; Karimi, F.; Jorsaraei, S.G. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran Red Crescent Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef]
- Fazeli, F.; Salimi, S. Correlation of seminal plasma total antioxidant capacity and malondialdehyde levels with sperm parameters in men with idiopathic infertility. Avicenna J. Med. Biochem 2016, 4, e29736. [Google Scholar] [CrossRef][Green Version]
- Schoenfeld, C.Y.; Amelar, R.D.; Dubin, L.; Numeroff, M. Prolactin, fructose, and zinc levels found in human seminal plasma. Fertil. Steril. 1979, 32, 206–208. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Dixit, V.K. Effects of Bryonia laciniosa seeds on sexual behaviour of male rats. Int. J. Impot. Res. 2010, 22, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T. Effect of a 60-Day Oral Gavage of a Crude Alkaloid Extract from Chromolaena odorata Leaves on Hormonal and Spermatogenic Indices of Male Rats. J. Androl. 2012, 33, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.M.; Aswar, U.M.; Mohan, V.; Bodhankar, S.L.; Zambare, G.N.; Thakurdesai, P.A. Study of furostenol glycoside fraction of Tribulus terresteris on male sexual function in rats. Pharm. Biol. 2008, 46, 191–198. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, Y.K. Aphrodisiac activity of Tribulus terrestris Linn. In experimental models in rats. J. Men’s Health 2011, 8, S75–S77. [Google Scholar]
- Alhowiriny, T.A.; Al-Rehaily, A.J.; El Tahir, K.E.; Al-Taweel, A.M.; Perveen, S. Molecular mechanisms that underlie the sexual stimulant actions of ginger (Zingiber officinale Rosocoe) and garden rocket (Eruca sativa L.). J. Med. Plants Res. 2013, 7, 2370–2379. [Google Scholar]
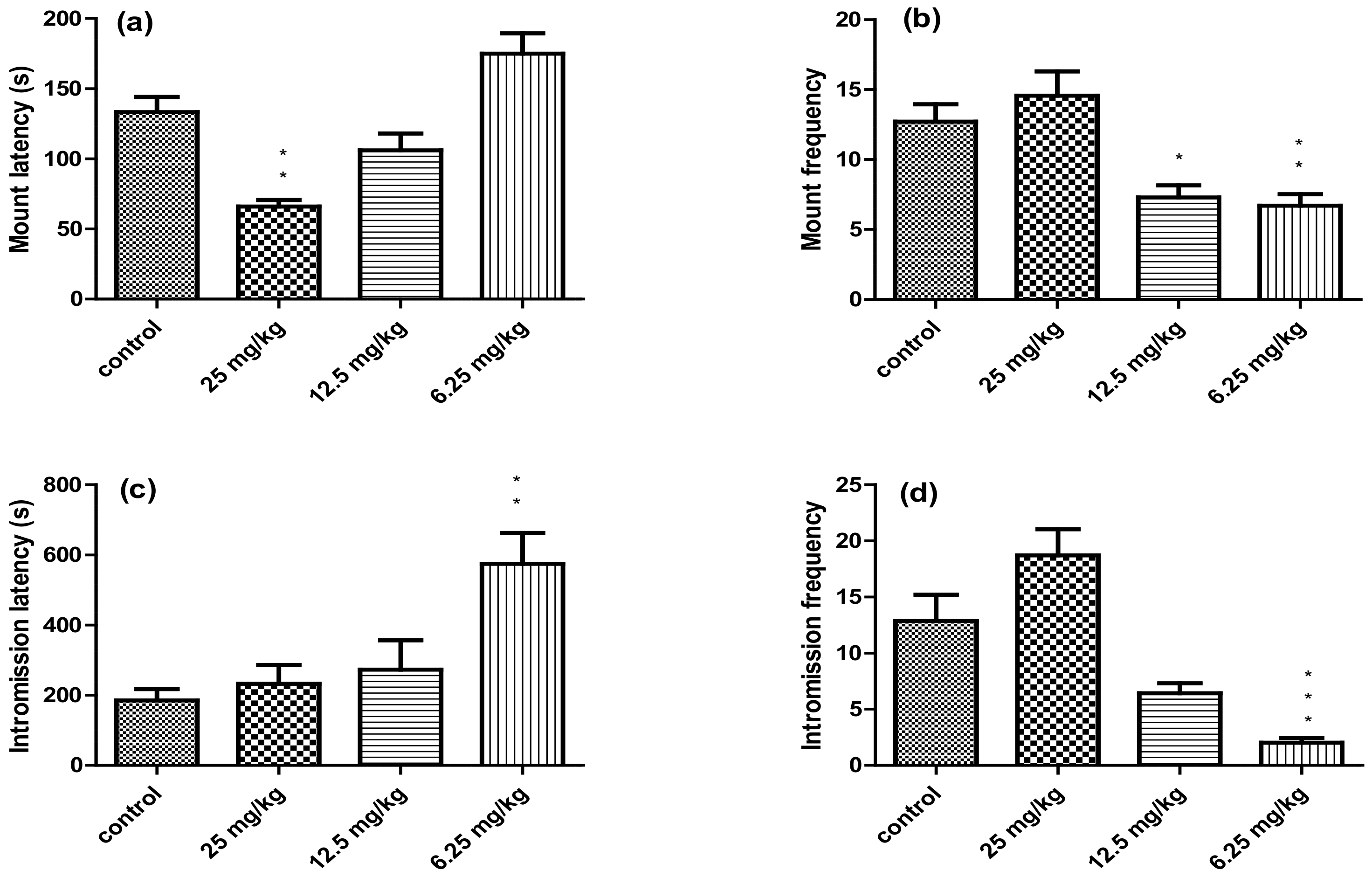
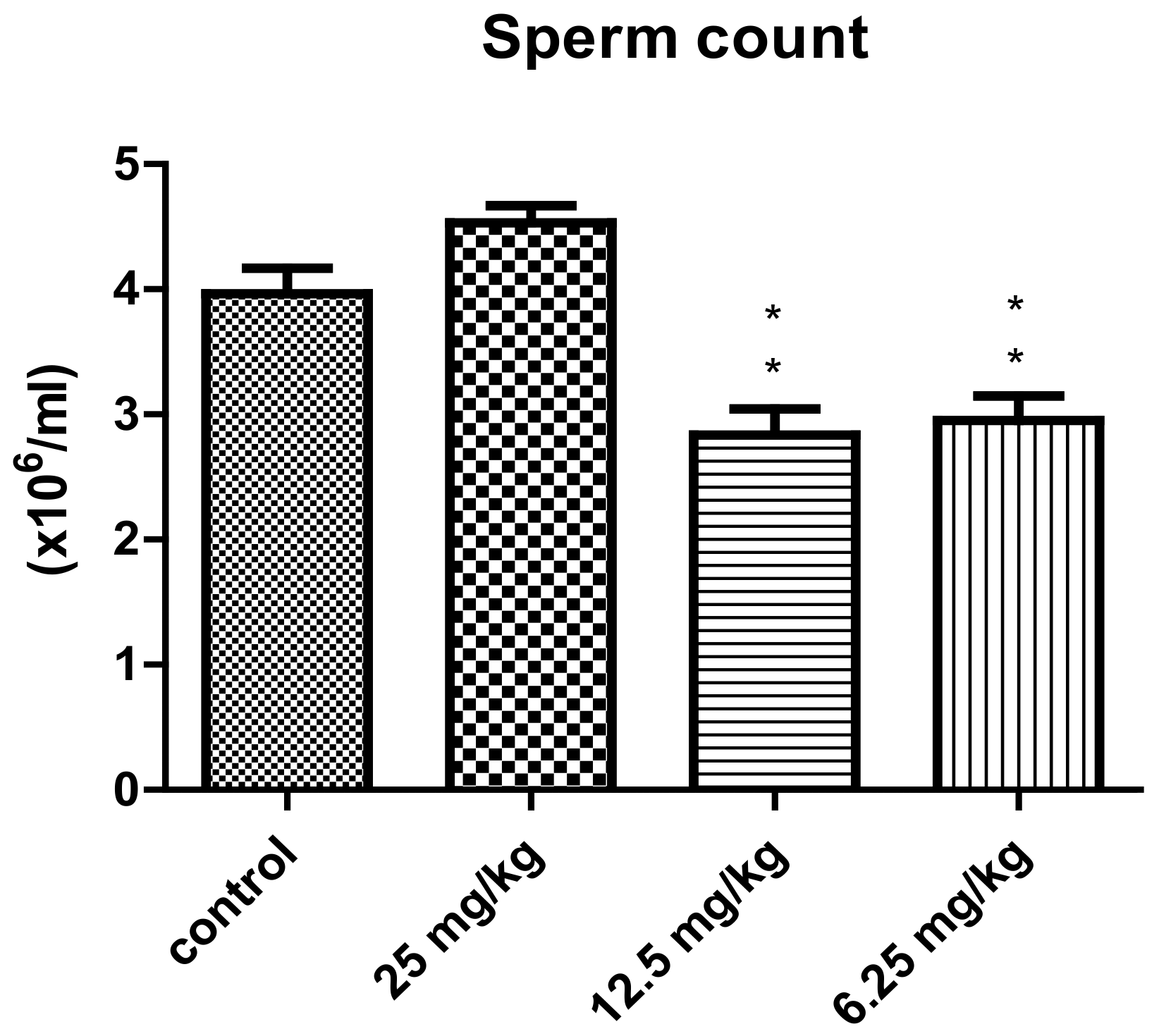
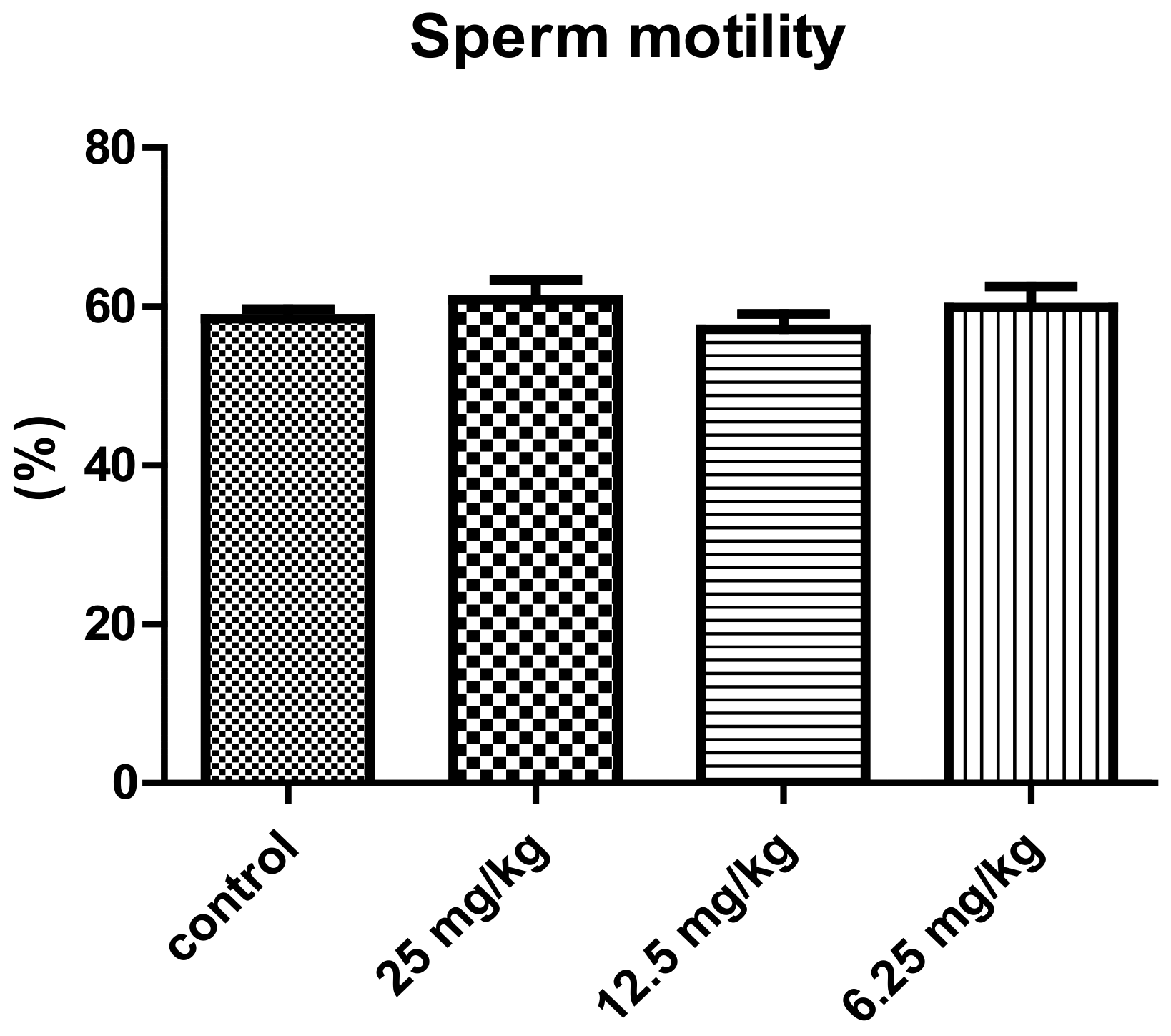
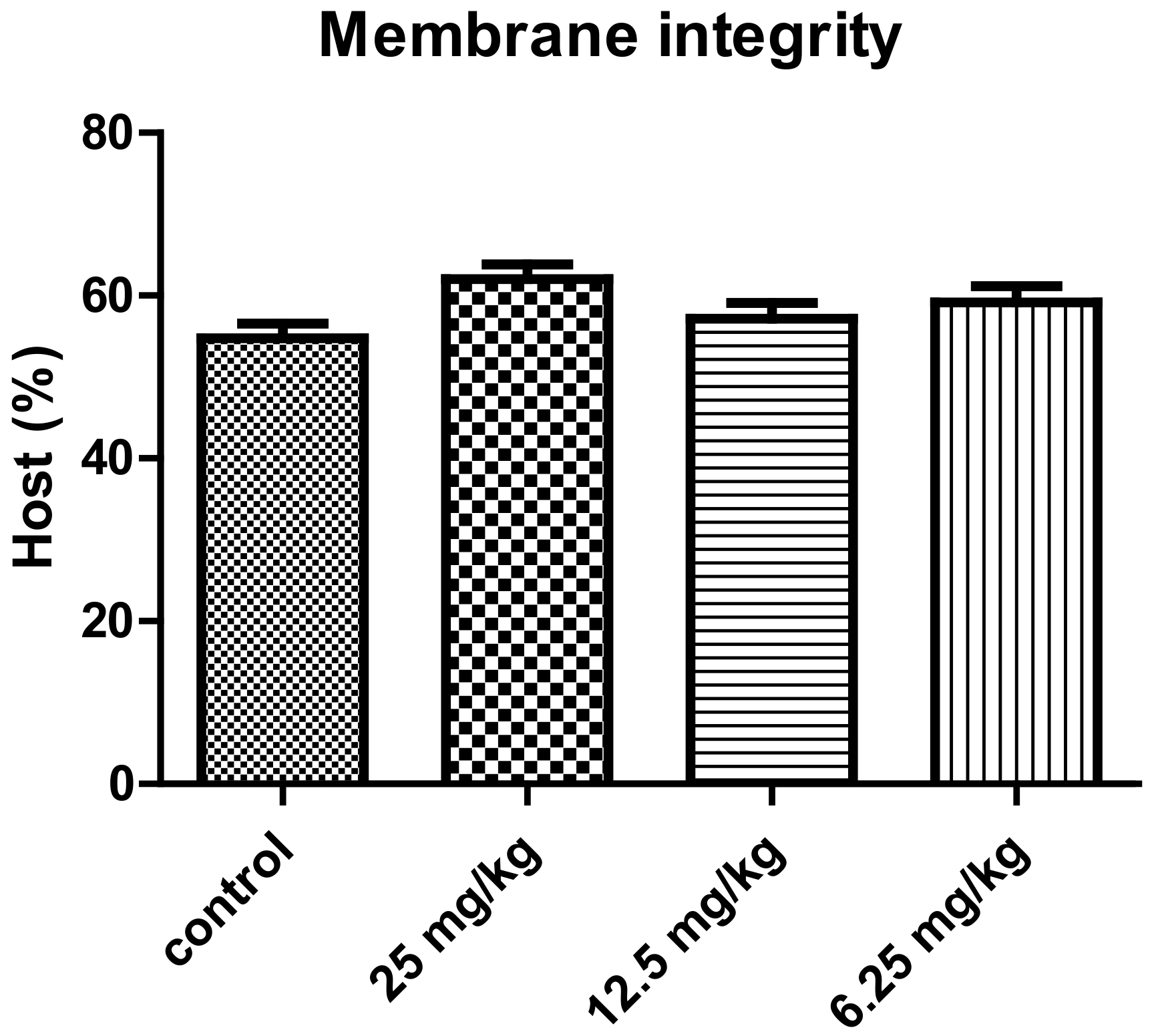
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | 25 mg/kg | 12.5 mg/kg | 6.25 mg/kg | |
| GSI | 0.140 ± 0.13 | 0.985 ± 0.06 *** | 1.11 ± 0.06 *** | 0.889 ± 0.04 *** |
| Epididymis weight (g) | 0.063 ± 0.001 | 0.067 ± 0.005 | 0.082 ± 0.004 * | 0.064 ± 0.004 |
| Seminal vesicle weight (g) | 0.181 ± 0.01 | 0.156± 0.01 | 0.183 ± 0.01 | 0.168 ± 0.01 |
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | 25 mg/kg | 12.5 mg/kg | 6.25 mg/kg | |
| Seminal fructose (mg/g) | 3.72 ± 0.38 | 4.22 ± 0.5 | 4.61 ± 0.68 | 4.62 ± 0.79 |
| Testosterone (ng/mL)) | 1.14 ± 0.39 | 2.08 ± 0.72 | 1.21 ± 0.55 | 1.37 ± 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derbak, H.; Imre, K.; Benabdelhak, A.C.; Moussaoui, M.; Kribeche, A.; Kebbi, R.; Ayad, A. Effect of Peganum harmala Total Alkaloid Extract on Sexual Behavior and Sperm Parameters in Male Mice. Vet. Sci. 2023, 10, 498. https://doi.org/10.3390/vetsci10080498
Derbak H, Imre K, Benabdelhak AC, Moussaoui M, Kribeche A, Kebbi R, Ayad A. Effect of Peganum harmala Total Alkaloid Extract on Sexual Behavior and Sperm Parameters in Male Mice. Veterinary Sciences. 2023; 10(8):498. https://doi.org/10.3390/vetsci10080498
Chicago/Turabian StyleDerbak, Hanane, Kálmán Imre, Amira Chahrazad Benabdelhak, Mohamed Moussaoui, Amina Kribeche, Rosa Kebbi, and Abdelhanine Ayad. 2023. "Effect of Peganum harmala Total Alkaloid Extract on Sexual Behavior and Sperm Parameters in Male Mice" Veterinary Sciences 10, no. 8: 498. https://doi.org/10.3390/vetsci10080498
APA StyleDerbak, H., Imre, K., Benabdelhak, A. C., Moussaoui, M., Kribeche, A., Kebbi, R., & Ayad, A. (2023). Effect of Peganum harmala Total Alkaloid Extract on Sexual Behavior and Sperm Parameters in Male Mice. Veterinary Sciences, 10(8), 498. https://doi.org/10.3390/vetsci10080498







