Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of the Bacterial Strains
2.2. Antimicrobial Susceptibility Testing
3. Results
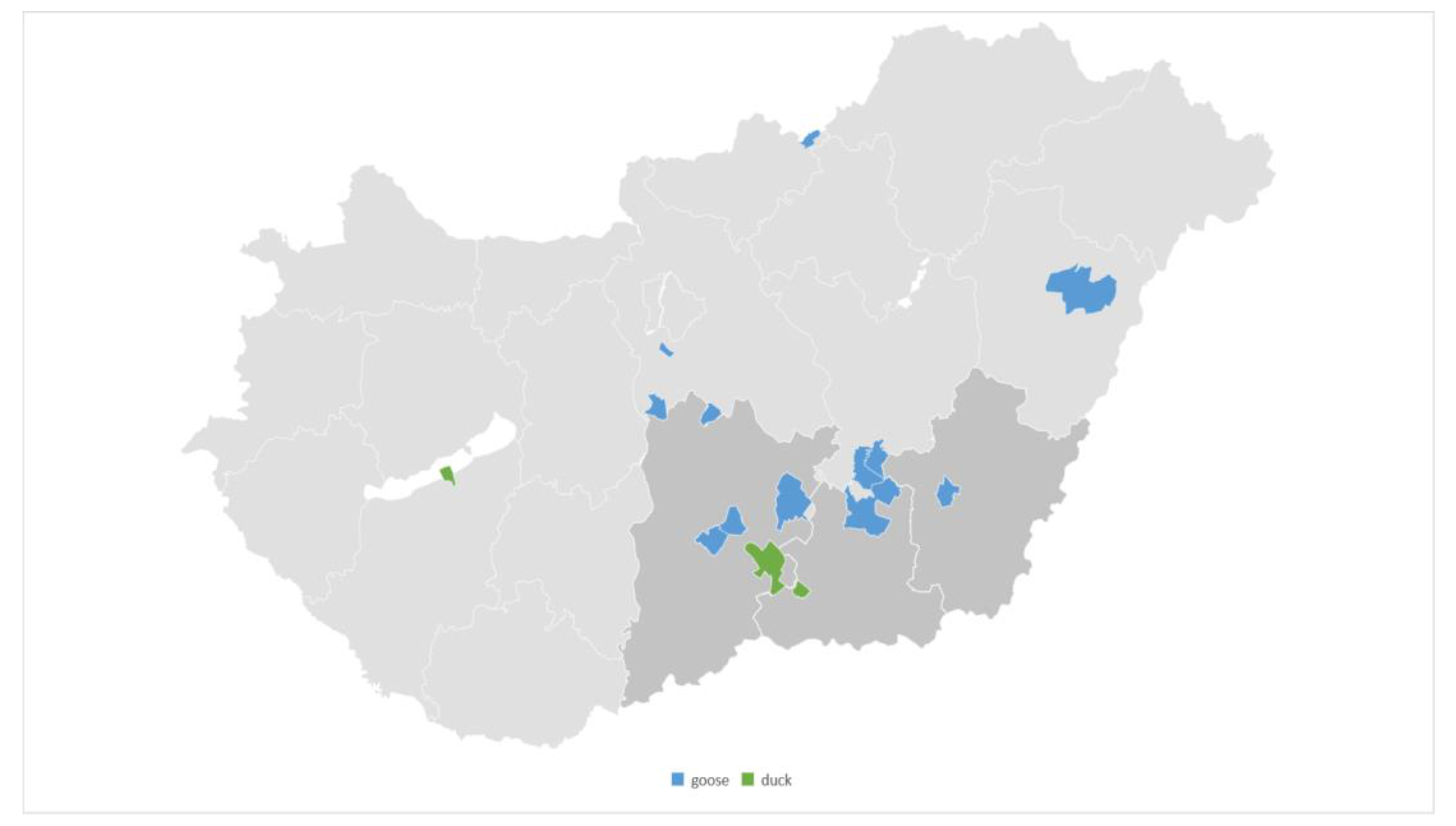
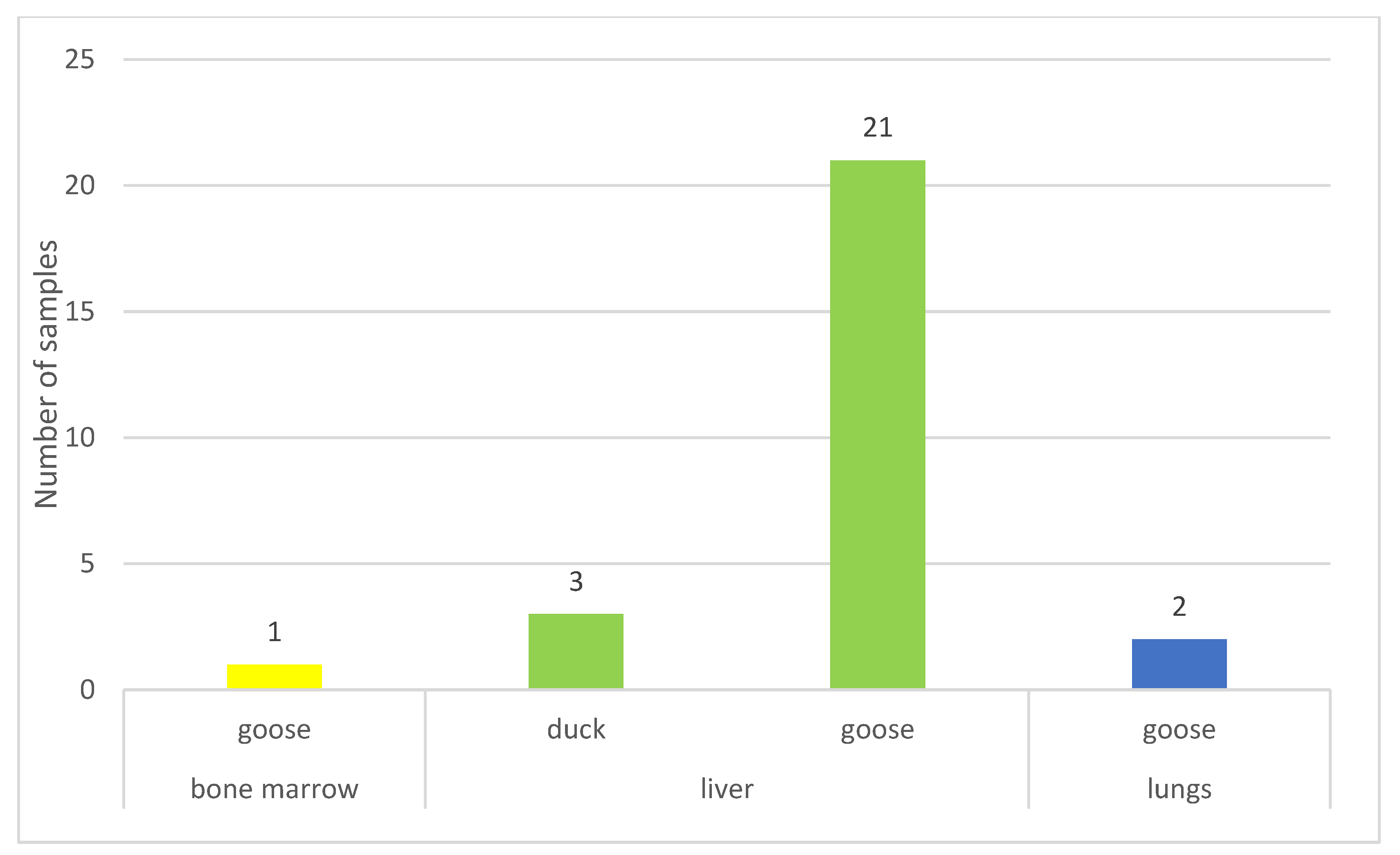
3.1. Origin of the Isolates
3.2. Antimicrobial Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sy, C.L.; Chen, P.-Y.; Cheng, C.-W.; Huang, L.-J.; Wang, C.-H.; Chang, T.-H.; Chang, Y.-C.; Chang, C.-J.; Hii, I.-M.; Hsu, Y.-L.; et al. Recommendations and Guidelines for the Treatment of Infections Due to Multidrug Resistant Organisms. J. Microbiol. Immunol. Infect. 2022, 55, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.; Gupta, S.; Seth, S.; James, S.; Fatima, F.; Chaurasia, P.; Ramachandran, S. Knowledgebase of Potential Multifaceted Solutions to Antimicrobial Resistance. Comput. Biol. Chem. 2022, 101, 107772. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.-F.; et al. Global Antimicrobial Resistance: A System-Wide Comprehensive Investigation Using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ikhimiukor, O.O.; Odih, E.E.; Donado-Godoy, P.; Okeke, I.N. A Bottom-up View of Antimicrobial Resistance Transmission in Developing Countries. Nat. Microbiol. 2022, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e01991-21. [Google Scholar] [CrossRef]
- Tejpar, S.; Rogers Van Katwyk, S.; Wilson, L.; Hoffman, S.J. Taking Stock of Global Commitments on Antimicrobial Resistance. BMJ Glob. Health 2022, 7, e008159. [Google Scholar] [CrossRef] [PubMed]
- Központi Statisztikai Hivatal. Magyar Statisztikai Évkönyv, 2022; XXIV; Központi Statisztikai Hivatal (KSH): Budapest, Hungary, 2023; ISBN 9771215786003. [Google Scholar]
- Singh, R.; Remington, B.; Blackall, P.; Turni, C. Epidemiology of Fowl Cholera in Free Range Broilers. Avian Dis. 2014, 58, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Miller, E.; Aguayo, J.M.; Figueroa, C.F.; Nezworski, J.; Studniski, M.; Wileman, B.; Johnson, T. Genomic Diversity and Molecular Epidemiology of Pasteurella multocida. PLoS ONE 2021, 16, e0249138. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.S.; Mowafy, R.E.; Fahmy, H.A.; Matter, A.A.; Samir, M. Pathognomonic Features of Pasteurella multocida Isolates among Various Avian Species in Sharkia Governorate, Egypt. World J. Microbiol. Biotechnol. 2023, 39, 335. [Google Scholar] [CrossRef]
- Chrzastek, K.; Kuczkowski, M.; Wieliczko, A.K.; Bednarek, K.J.; Wieliczko, A. Molecular Epidemiologic Investigation of Polish Avian Pasteurella multocida Strains Isolated from Fowl Cholera Outbreaks Showing Restricted Geographical and Host-Specific Distribution. Avian Dis. 2012, 56, 529–536. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Muhairwa, A.P.; Christensen, J.P.; Bisgaard, M. Investigations on the Carrier Rate of Pasteurella multocida in Healthy Commercial Poultry Flocks and Flocks Affected by Fowl Cholera. Avian Pathol. 2000, 29, 133–142. [Google Scholar] [CrossRef]
- Botzler, R.G. Epizootiology of Avian Cholera in Wildfowl. J. Wildl. Dis. 1991, 27, 367–395. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.E. Transmission of Pasteurella multocida Infection from the Brown Rat (Rattus norvegicus) to Domestic Poultry. Vet. Rec. 1983, 113, 133–134. [Google Scholar] [CrossRef]
- Simensen, E.; Olson, L.D. Aerosol Transmission of Pasteurella multocida in Turkeys. Avian Dis. 1980, 24, 1007–1010. [Google Scholar] [CrossRef]
- Megahed, M.M.M.; El-Nagar, A.M.A.; El-Demerdash, A.S.; Ayoub, M.A.; Tolba, H.M.N. Evaluation and Development of Diagnostic Tools for Rapid Detection of Riemerella anatipestifer and Pasteurella multocida in Ducks. J. Adv. Vet. Anim. Res. 2023, 10, 211–221. [Google Scholar] [CrossRef] [PubMed]
- San Millan, A.; Escudero, J.A.; Gutierrez, B.; Hidalgo, L.; Garcia, N.; Llagostera, M.; Dominguez, L.; Gonzalez-Zorn, B. Multiresistance in Pasteurella multocida Is Mediated by Coexistence of Small Plasmids. Antimicrob. Agents Chemother. 2009, 53, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yuan, D.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; Zhang, S.; et al. Emergence of a Multidrug-Resistant Hypervirulent Pasteurella multocida ST342 Strain with a floR-Carrying Plasmid. J. Glob. Antimicrob. Resist. 2020, 20, 348–350. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-Tof Mass Spectrometry for Microorganism Identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobicall, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI standards M07. [Google Scholar]
- Clinical and Laboratory Standards Institute CLSI. Methods For Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated From Animals, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; Volume CLSI standards VET06. [Google Scholar]
- Wilkie, I.W.; Harper, M.; Boyce, J.D.; Adler, B. Pasteurella multocida: Diseases and Pathogenesis. Curr. Top. Microbiol. Immunol. 2012, 361, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pintér, K.; Ádám, K.; Tibor, M. Antibiotic Susceptibility of Pasteurella multocida Strains, Genetic Background of Antimicrobial Resistance Literature Review. Hung. Vet. J. 2023, 147, 239–256. [Google Scholar] [CrossRef]
- Sellyei, B.; Varga, Z.; Szentesi-Samu, K.; Kaszanyitzky, É.; Magyar, T. Antimicrobial Susceptibility of Pasteurella multocida Isolated from Swine and Poultry. Acta Vet. Hung. 2009, 57, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Sellyei, B.; Thuma, Á.; Volokhov, D.; Varga, Z. Comparative Analysis of Pasteurella multocida Isolates from Acute and Chronic Fowl Cholera Cases in Hungary during the Period 2005 through 2010. Avian Dis. 2017, 61, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kang, M.S.; Jeong, O.M.; Lee, H.J.; Lee, J.Y.; Kwon, Y.K.; Park, J.W.; Kim, J.H. Investigation of Genetic Diversity of Pasteurella multocida Isolated from Diseased Poultry in Korea. Braz. J. Poult. Sci. 2021, 23. [Google Scholar] [CrossRef]
- Lees, P.; Concordet, D.; Aliabadi, F.S.; Toutain, P.-L. Drug Selection and Optimization of Dosage Schedules to Minimize Antimicrobial Resistance. In Antimicrobial Resistance in Bacteria of Animal Origin; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 49–71. ISBN 978-1-68367-185-5. [Google Scholar]
- Toutain, P.L.; Del Castillo, J.R.E.; Bousquet-Mélou, A. The Pharmacokinetic-Pharmacodynamic Approach to a Rational Dosage Regimen for Antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef] [PubMed]
- McKellar, Q.A.; Sanchez Bruni, S.F.; Jones, D.G. Pharmacokinetic/Pharmacodynamic Relationships of Antimicrobial Drugs Used in Veterinary Medicine. J. Vet. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Sárközy, G. Quinolones: A Class of Antimicrobial Agents. Vet. Med. 2001, 46, 257–274. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Hung. Vet. J. 2024, 146, 181–191. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Hung. Vet. J. 2022, 144, 285–298. [Google Scholar]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Hung. Vet. J. 2023, 145, 461–475. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Hung. Vet. J. 2023, 145, 497–510. [Google Scholar]
- Eid, S.; Marouf, S.; Hefny, H.Y.; Al-Atfeehy, N.M. Pasteurellaceae Members with Similar Morphological Patterns Associated with Respiratory Manifestations in Ducks. Vet. World 2019, 12, 2061–2069. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Islam, M.; Hossain, M.M.; Hassan, M.K.; Kabir, M.H.B.; Sabrin, M.S.; Khan, M.S.R. Isolation, Characterization and Antibiogram Study of Pasteurella multocida Isolated from Ducks of Kishoreganj District, Bangladesh. Int. J. Anim. Resour. 2016, 1, 69–76. [Google Scholar]
- Furian, T.Q.; Borges, K.A.; Laviniki, V.; da Silveira Rocha, S.L.; de Almeida, C.N.; do Nascimento, V.P.; Salle, C.T.P.; de Souza Moraes, H.L. Virulence Genes and Antimicrobial Resistance of Pasteurella multocida Isolated from Poultry and Swine. Braz. J. Microbiol. 2016, 47, 210–216. [Google Scholar] [CrossRef]
- Shivachandra, S.B.; Kumar, A.A.; Biswas, A.; Ramakrishnan, M.A.; Singh, V.P.; Srivastava, S.K. Antibiotic Sensitivity Patterns among Indian Strains of Avian Pasteurella multocida. Trop. Anim. Health Prod. 2004, 36, 743–750. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Hu, Z.; Zhang, Y.; Chang, Y.-F.; Zhou, Q.; Yan, Z.; Zhang, X.; Chen, L.; Li, W.; et al. Characterization of Pasteurella multocida Isolated from Ducks in China from 2017 to 2019. Microb. Pathog. 2021, 160, 105196. [Google Scholar] [CrossRef]
- Tan, M.-F.; Li, H.-Q.; Yang, Q.; Zhang, F.-F.; Tan, J.; Zeng, Y.-B.; Wei, Q.-P.; Huang, J.-N.; Wu, C.-C.; Li, N.; et al. Prevalence and Antimicrobial Resistance Profile of Bacterial Pathogens Isolated from Poultry in Jiangxi Province, China from 2020 to 2022. Poult. Sci. 2023, 102, 102830. [Google Scholar] [CrossRef]
- Gálfi, P.; Csikó, G.; Jerzsele, Á. Állatorvosi Gyógyszertan III; Második, Javított Kiadás; Robbie-Vet Kft.: Budapest, Hungary, 2015. [Google Scholar]
- Hasan, J.; Hug, M. Pasteurella multocida. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Somogyi, Z.; Mag, P.; Simon, R.; Kerek, Á.; Makrai, L.; Biksi, I.; Jerzsele, Á. Susceptibility of Actinobacillus pleuropneumoniae, Pasteurella multocida and Streptococcus suis Isolated from Pigs in Hungary between 2018 and 2021. Antibiotics 2023, 12, 1298. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What Do I Need to Know about Aminoglycoside Antibiotics? Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 89–93. [Google Scholar] [CrossRef]
- Gray, P.; Jenner, R.; Norris, J.; Page, S.; Browning, G. Antimicrobial Prescribing Guidelines for Poultry. Aust. Vet. J. 2021, 99, 181–235. [Google Scholar] [CrossRef] [PubMed]
- Bialvaei, A.Z.; Samadi Kafil, H. Colistin, Mechanisms and Prevalence of Resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef] [PubMed]
| ECOFF (µg/mL) 4 | MIC90 (µg/mL) 3 | MIC50 (µg/mL) 2 | 0.007 | 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | Breakpoint 1 (µg/mL) | Antibiotic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 0.06 | 0.06 | 24 | 3 | ≥1 | Penicillin | ||||||||||
| 88.9 | 11.1 | |||||||||||||||
| 0.25 | 0.125 | 0.125 | 2 | 25 | ≥1 | Amoxicillin–clavulanic acid 5 | ||||||||||
| 7.4 | 92.6 | |||||||||||||||
| 0.06 | 0.015 | 0.015 | 25 | 2 | ≥8 | Ceftiofur | ||||||||||
| 92.6 | 7.4 | |||||||||||||||
| 64 | 8 | 8 | 2 | 24 | 1 | ≥128 | Spectinomycin | |||||||||
| 7.4 | 88.9 | 3.7 | ||||||||||||||
| 1 | 0.25 | 0.25 | 27 | ≥8 | Florfenicol | |||||||||||
| 100 | ||||||||||||||||
| 1 | 0.5 | 0.5 | 1 | 26 | ≥32 | Chloramphenicol | ||||||||||
| 3.7 | 96.3 | |||||||||||||||
| 32 | 8 | 4 | 1 | 14 | 10 | 2 | ≥32 | Tilmicosin | ||||||||
| 3.7 | 51.9 | 37 | 7.4 | |||||||||||||
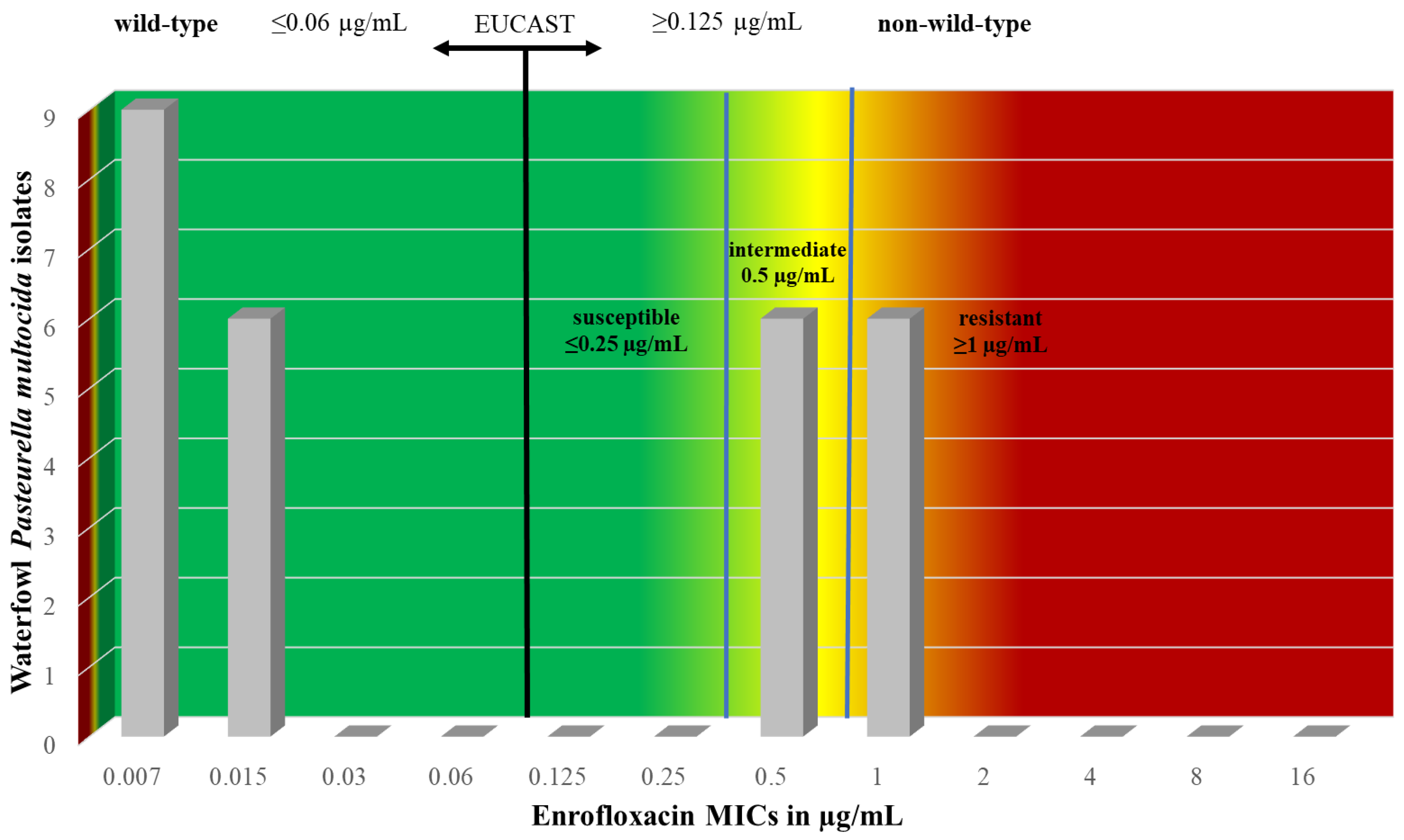
| 0.06 | 1 | 0.015 | 9 | 6 | 6 | 6 | ≥1 | Enrofloxacin | ||||||||
| 33.3 | 22.2 | 22.2 | 22.2 |
| ECOFF (µg/mL) 3 | MIC90 (µg/mL) 2 | MIC50 (µg/mL) 1 | 0.007 | 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | Antibiotic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64 | 32 | 16 | 3 | 16 | 5 | 2 | 1 | Tiamulin | ||||||||||
| 11.1 | 59.3 | 18.5 | 7.4 | 3.7 | ||||||||||||||
| 0.125 | 8 | 1 | 1 | 2 | 5 | 10 | 1 | 5 | 3 | Potent sulphonamide 4 | ||||||||
| 3.7 | 7.4 | 18.5 | 37.0 | 3.7 | 18.5 | 11.1 | ||||||||||||
| 0.5 | 0.125 | 0.125 | 27 | Amoxicillin | ||||||||||||||
| 100.0 | ||||||||||||||||||
| 1 | 0.125 | 0.03 | 10 | 8 | 5 | 1 | 1 | 2 | Doxycycline | |||||||||
| 37.0 | 29.6 | 18.5 | 3.7 | 3.7 | 7.4 | |||||||||||||
| 8 | 4 | 2 | 17 | 9 | 1 | Cephalexin | ||||||||||||
| 63.0 | 33.3 | 3.7 | ||||||||||||||||
| 8 | 2 | 2 | 25 | 2 | Gentamicin | |||||||||||||
| 92.6 | 7.4 | |||||||||||||||||
| 0.125 | 0.03 | 0.03 | 27 | Cefquinome | ||||||||||||||
| 100.0 |
| MIC90 (µg/mL) 2 | MIC50 (µg/mL) 1 | 0.007 | 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | Antibiotic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.015 | 27 | Cefotaxime | ||||||||||||||
| 100 | |||||||||||||||||
| 0.25 | 0.25 | 3 | 7 | 16 | 1 | Imipenem | |||||||||||
| 11.1 | 25.9 | 59.3 | 3.7 | ||||||||||||||
| 32 | 32 | 3 | 24 | Tilozin | |||||||||||||
| 11.1 | 88.9 | ||||||||||||||||
| 0.015 | 0.015 | 1 | 25 | 1 | Ceftriaxone | ||||||||||||
| 3.7 | 92.6 | 3.7 | |||||||||||||||
| 32 | 32 | 1 | 26 | Lincomycin | |||||||||||||
| 3.7 | 96.3 | ||||||||||||||||
| 4 | 2 | 2 | 3 | 7 | 7 | 6 | 1 | 1 | Colistin | ||||||||
| 7.4 | 11.1 | 25.9 | 25.9 | 22.2 | 3.7 | 3.7 | |||||||||||
| 2 | 0.06 | 3 | 7 | 4 | 1 | 6 | 6 | Marbofloxacin | |||||||||
| 11.1 | 25.9 | 14.8 | 3.7 | 22.2 | 22.2 | ||||||||||||
| 128 | 64 | 1 | 6 | 15 | 5 | Clindamycin | |||||||||||
| 3.7 | 22.2 | 55.6 | 18.5 | ||||||||||||||
| 0.5 | 0.015 | 5 | 8 | 1 | 2 | 11 | Levofloxacin | ||||||||||
| 7.4 | 29.6 | 3.7 | 7.4 | 40.7 | |||||||||||||
| 0.5 | 0.06 | 2 | 8 | 1 | 1 | 10 | 4 | Gatifloxacin | |||||||||
| 7.4 | 29.6 | 3.7 | 3.7 | 37 | 14.8 | ||||||||||||
| 32 | 32 | 2 | 25 | Lincomycin–spectinomycin 3 | |||||||||||||
| 7.4 | 92.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023. Vet. Sci. 2024, 11, 194. https://doi.org/10.3390/vetsci11050194
Kerek Á, Szabó Á, Jerzsele Á. Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023. Veterinary Sciences. 2024; 11(5):194. https://doi.org/10.3390/vetsci11050194
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, and Ákos Jerzsele. 2024. "Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023" Veterinary Sciences 11, no. 5: 194. https://doi.org/10.3390/vetsci11050194
APA StyleKerek, Á., Szabó, Á., & Jerzsele, Á. (2024). Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023. Veterinary Sciences, 11(5), 194. https://doi.org/10.3390/vetsci11050194







