The Development of a Multienzyme Isothermal Rapid Amplification Assay to Visually Detect Duck Hepatitis B Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Clinical Samples
2.2. Genomic DNA Extraction
2.3. Development of MIRA Assay for DHBV Detection
2.4. Specificity and Comparative Sensitivity Analysis
2.5. Detection Efficacy of Clinical Samples and Statistical Analysis
3. Results
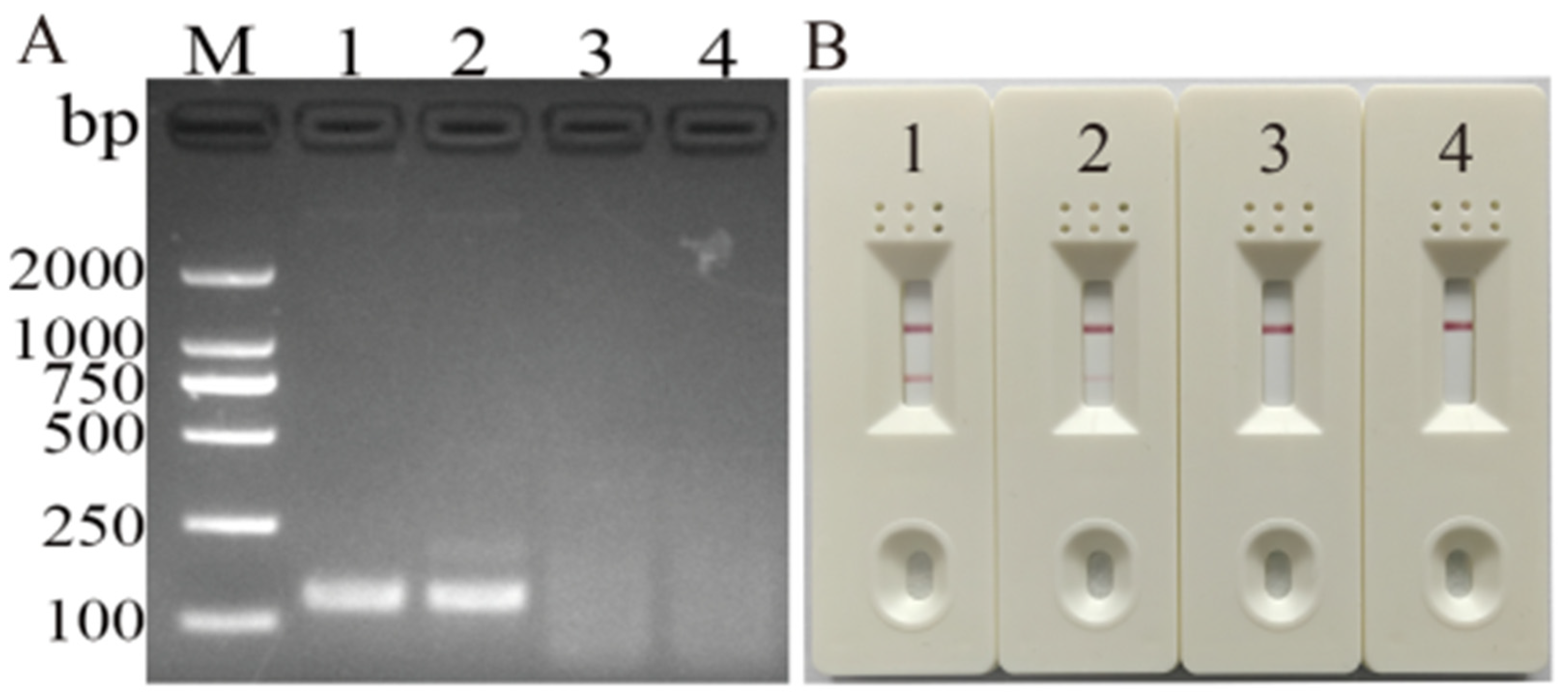
3.1. Screening for Primers and Probes
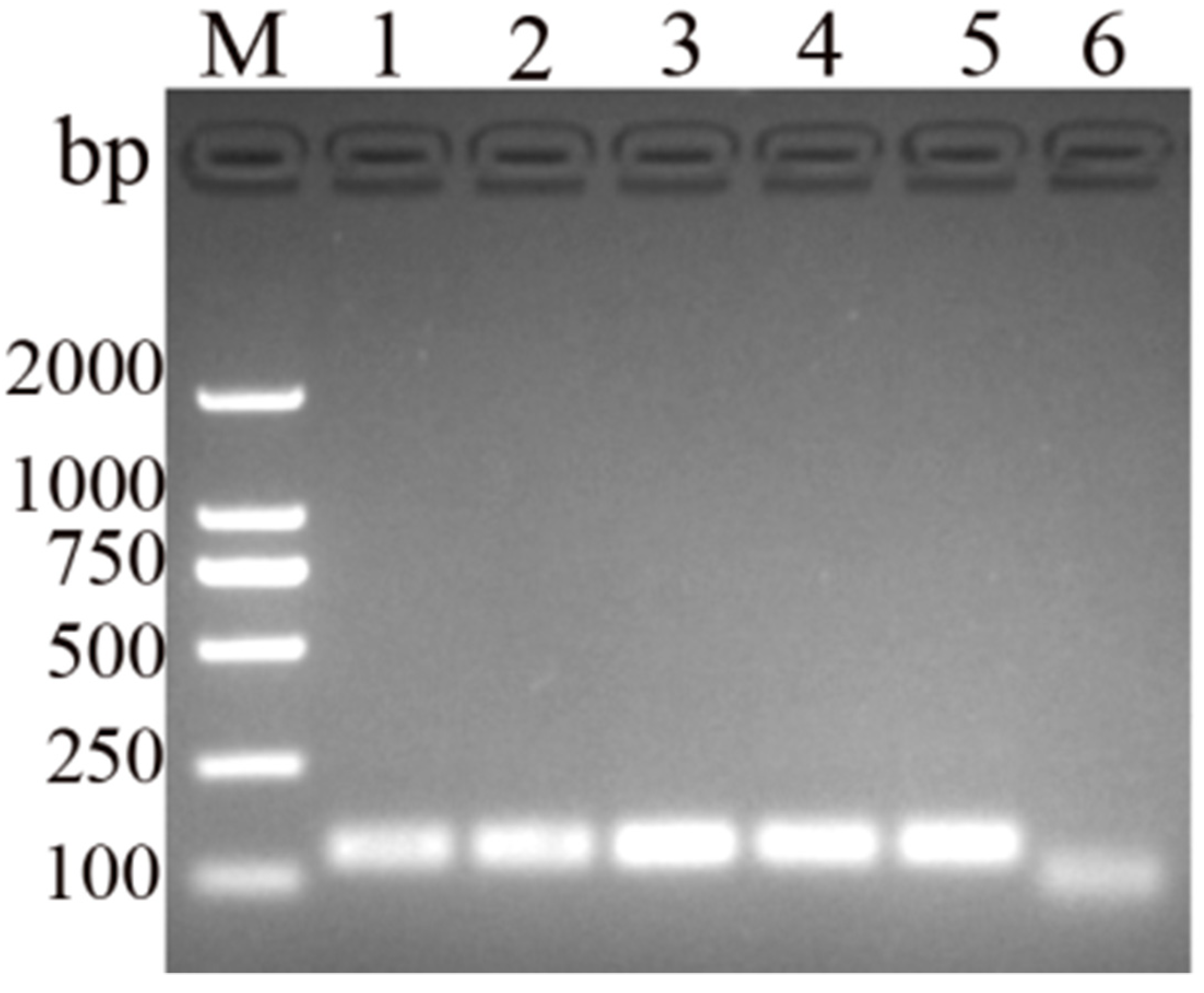
3.2. Optimization of MIRA Assay for DHBV Detection
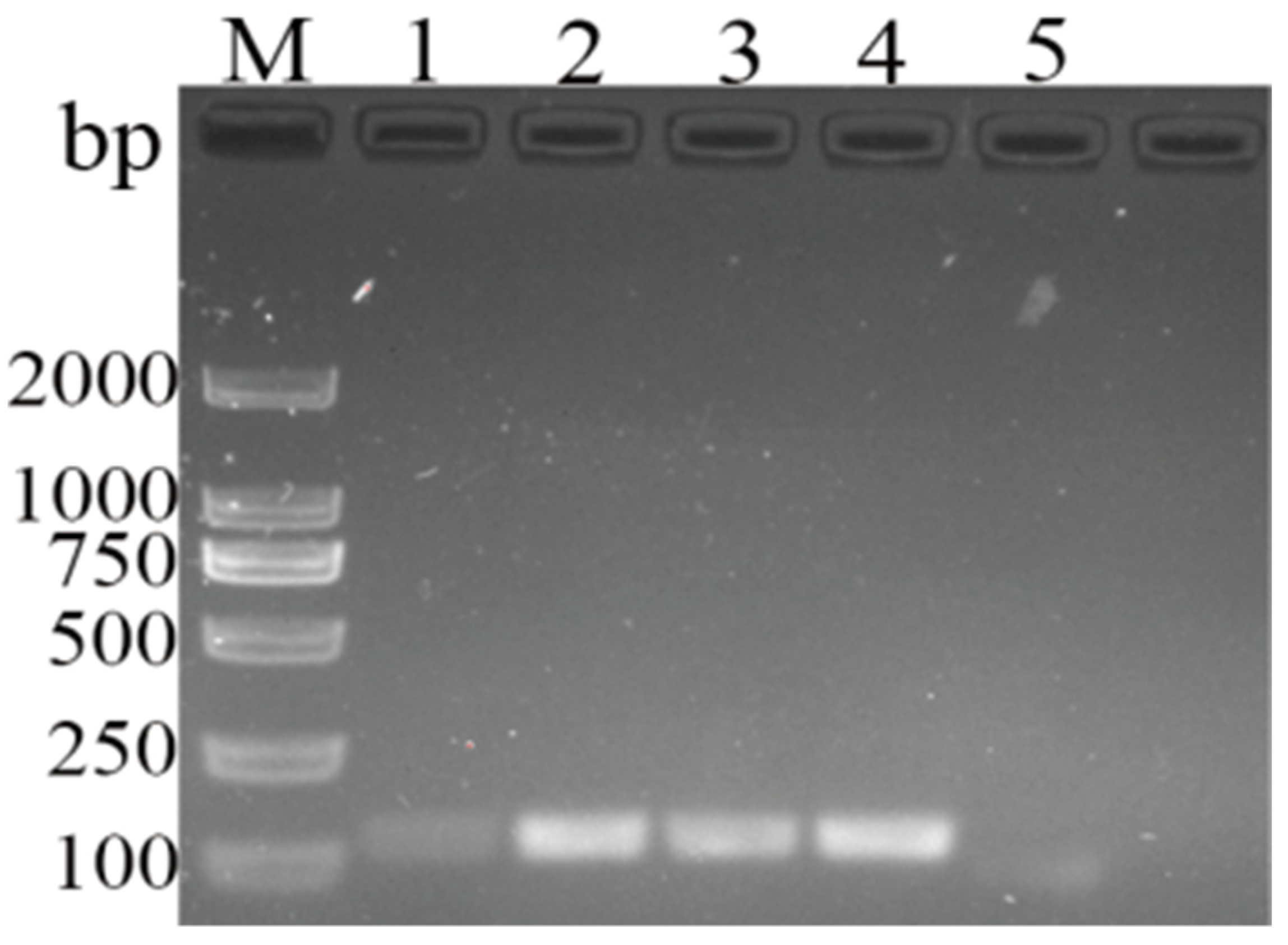
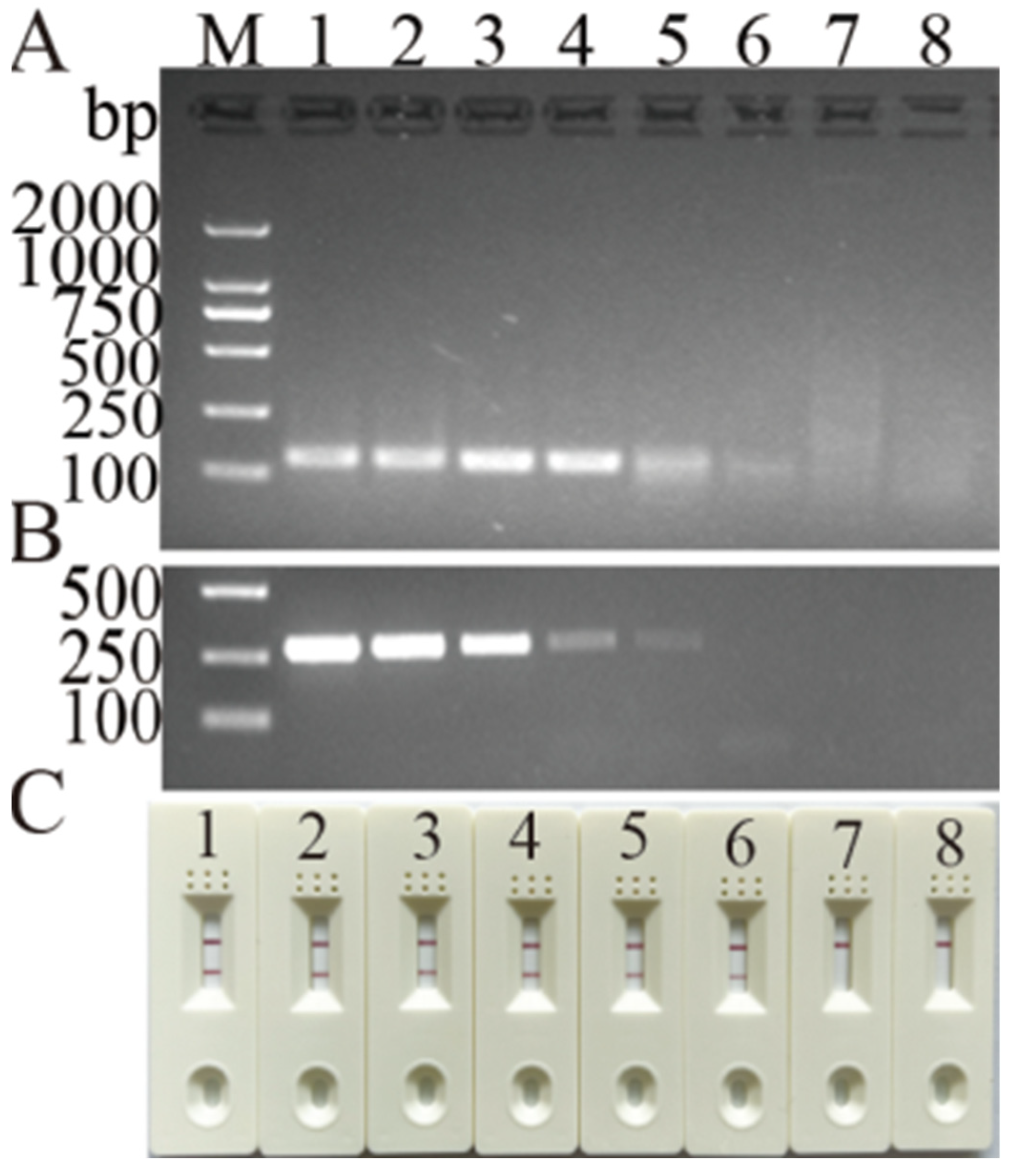
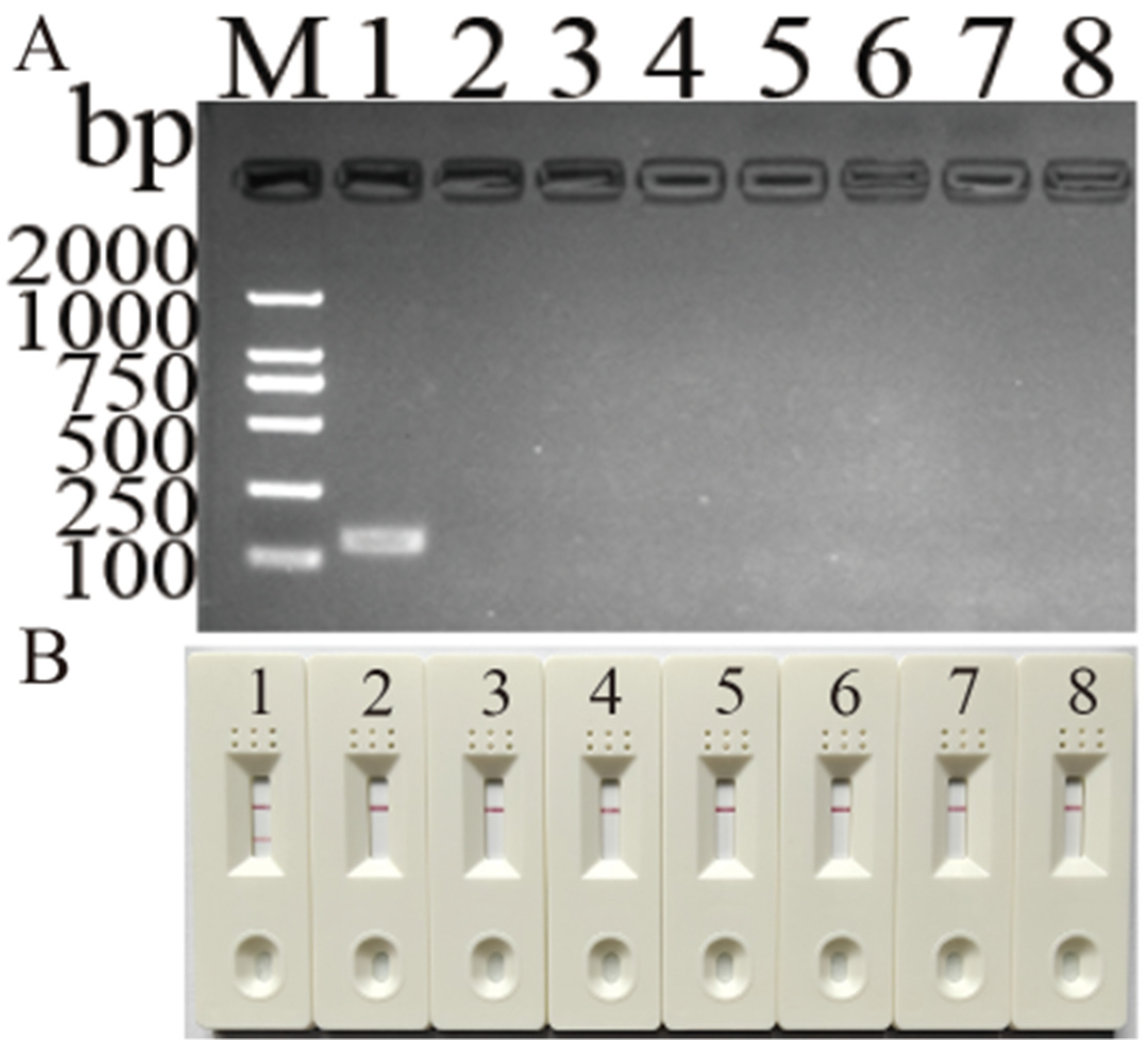
3.3. Specificity and Detection Limit of Newly Developed MIRA Assay for DHBV Detection
3.4. Clinical Sample Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, W.S.; Seal, G.; Summers, J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J. Virol. 1980, 36, 829–836. [Google Scholar] [CrossRef]
- Jilbert, A.R.; Kotlarski, I. Immune responses to duck hepatitis B virus infection. Dev. Comp. Immunol. 2000, 24, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Tavis, J.E. RNA elements directing translation of the duck hepatitis B Virus polymerase via ribosomal shunting. J. Virol. 2011, 85, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Robinson, W.S.; Marion, P.L. Duck hepatitis B virus replicates in the yolk sac of developing embryos. J. Virol. 1987, 61, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, J.; Qin, Y.; Liang, B.; Gu, Y.; Liang, L.; Liu, L.; Liu, Y.; Su, H. Glucose homeostasis is dysregulated in ducks infected with duck hepatitis B virus. Intervirology 2021, 64, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xu, S.; Li, W.; Xu, X.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Genome analysis and recombination characterization of duck hepatitis B virus isolated from ducks and geese in central China, 2017 to 2019. Poult. Sci. 2023, 102, 102641. [Google Scholar] [CrossRef] [PubMed]
- Lenhoff, R.J.; Luscombe, C.A.; Summers, J. Acute liver injury following infection with a cytopathic strain of duck hepatitis B virus. Hepatology 1999, 29, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Mu, X.; Xu, X.; Bi, C.; Ji, J.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China. Animals 2023, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jia, R.; Wang, M.; Huang, J.; Zhu, D.; Chen, S.; Yin, Z.; Wang, Y.; Chen, X.; Cheng, A. Cloning, expression and purification of duck hepatitis B virus (DHBV) core protein and its use in the development of an indirect ELISA for serologic detection of DHBV infection. Arch. Virol. 2014, 159, 897–904. [Google Scholar] [CrossRef]
- Sowmya, N.; Thakur, M.S.; Manonmani, H.K. Rapid and simple DNA extraction method for the detection of enterotoxigenic Staphylococcus aureus directly from food samples: Comparison of PCR and LAMP methods. J. Appl. Microbiol. 2012, 113, 106–113. [Google Scholar] [CrossRef]
- Bachman, J. Reverse-transcription PCR (RT-PCR). Meth. Enzymol. 2013, 530, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Giambrone, J.J.; Smith, B.F.; Huang, T. Detection of duck hepatitis B virus DNA on filter paper by PCR and SYBR green dye-based quantitative PCR. J. Clin. Microbiol. 2002, 40, 2584–2590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, J.; Xu, X.; Wu, Q.; Wang, X.; Li, W.; Yao, L.; Kan, Y.; Yuan, L.; Bi, Y.; Xie, Q. Simple and visible detection of duck hepatitis B virus in ducks and geese using loop-mediated isothermal amplification. Poult. Sci. 2020, 99, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Xie, Z.; Zhang, Y.; Xie, Z.; Xie, L.; Huang, J.; Zeng, T.; Wang, S.; Luo, S.; Li, M. A multiplex fluorescence-based loop-mediated isothermal amplification assay for identifying chicken parvovirus, chicken infectious anaemia virus, and fowl aviadenovirus serotype 4. Avian Pathol. 2023, 52, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, J.; Yang, D.; Zhang, Q.; Miao, D.; He, X.; Du, Y.; Zhang, W.; Ni, J.; Zhao, K. Visual and rapid detection of porcine epidemic diarrhea virus (PEDV) using reverse transcription loop-mediated isothermal amplification method. Animals 2022, 12, 2712. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, R.; Liu, S.; Wang, M.; Zhu, D.; Chen, S.; Liu, M.; Yin, Z.; Jing, B.; Cheng, A. Complete genome sequence of the novel duck hepatitis B virus strain SCP01 from Sichuan Cherry Valley duck. Springerplus 2016, 5, 1353. [Google Scholar] [CrossRef][Green Version]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent advances in recombinase polymerase amplification: Principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef]
- Chen, W.; Fan, J.; Li, Z.; Zhang, Y.; Qin, Y.; Wu, K.; Li, X.; Li, Y.; Fan, S.; Zhao, M. Development of recombinase-Aided amplification combined with disposable nucleic acid test strip for rapid detection of porcine circovirus Type 2. Front. Vet. Sci. 2021, 8, 676294. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2019, 144, 31–67. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Gao, L.; Yan, Z.; Zhao, Q.; Chen, F.; Xie, Q.; Zhang, X. Development and application of a reverse transcription recombinase-aided amplification assay for porcine epidemic diarrhea virus. Viruses 2022, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Heng, P.; Liu, J.; Song, Z.; Wu, C.; Yu, X.; He, Y. Rapid detection of Staphylococcus aureus using a novel multienzyme isothermal rapid amplification technique. Front. Microbiol. 2022, 13, 1027785. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xu, S.; Xu, X.; Ji, J.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Multienzyme isothermal rapid amplification and lateral flow dipstick combination assay for visible detection of chicken chaphamaparvovirus. Poult. Sci. 2023, 102, 103144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, J.; Sun, M.; Li, Z.; Wang, X.; He, Y.; Qi, J. Rapid and sensitive detection of Streptococcus iniae in Trachinotus ovatus using multienzyme isothermal rapid amplification. Int. J. Mol. Sci. 2023, 24, 7733. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Huang, A.L.; Qi, Z.Y.; Guo, S.H. Establishment and assessment of two methods for quantitative detection of serum duck hepatitis B virus DNA. World J. Gastroenterol. 2004, 10, 2666–2669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, L.; Wang, J.; Geng, Y.; Wang, J.; Li, R.; Shi, R.; Yuan, W. Equipment-free recombinase polymerase amplification assay using body heat for visual and rapid point-of-need detection of canine parvovirus 2. Mol. Cell. Probes. 2018, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Zhang, R.; Lin, M.; Shi, R.; Han, Q.; Wang, J.; Yuan, W. Visual and equipment-free reverse transcription recombinase polymerase amplification method for rapid detection of foot-and-mouth disease virus. BMC Vet. Res. 2018, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Li, R.; Shi, R.; Liu, L.; Yuan, W. Evaluation of an incubation instrument-free reverse transcription recombinase polymerase amplification assay for rapid and point-of-need detection of canine distemper virus. J. Virol. Methods 2018, 260, 56–61. [Google Scholar] [CrossRef]
- Ceruti, A.; Kobialka, R.M.; Ssekitoleko, J.; Okuni, J.B.; Blome, S.; Abd El Wahed, A.; Truyen, U. Rapid extraction and detection of African swine fever virus DNA using an isothermal recombinase polymerase amplification assay. Viruses 2021, 13, 1731. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence (5′–3′) | Localization |
|---|---|---|
| DHBV-1F | GM2GTCGGTCTCAGCCCTTTTCTCCTCCA | 1619–1644 1 |
| DHBV-1R | [5′-Biotin]-ACGAGCGTTTGGGTGGCAGAGGAGGAAG | 1717–1744 |
| DHBV-2F | AAATAYAATCCTGCKGACGGCCCATCCA | 2423–2450 |
| DHBV-2R | [5′-Biotin]-GGGGTGTAAYTYTTAAGTTCCACATAGC | 2521–2548 |
| Probe for DHBV detection | [5′-FAM]-TCTCTTCACTACTGCCCTCGGATCAGAAATC/IDSP/CTCGTCGCTTTAA-[3′ Spacer] | 1645–1689 |
| Samples | Detection Results | Statistics Analysis | ||
|---|---|---|---|---|
| Routine PCR Assay | MIRA Assay | Kappa (k) | p-Value of Kappa | |
| DHBV isolation | 34/34 | 34/34 | 1 | <0.001 |
| Serum from ducks | 54/136 | 59/136 | 0.924 | <0.001 |
| Serum from geese | 58/145 | 61/145 | 0.957 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Man, Y.; Xu, X.; Ji, J.; Wang, Y.; Yao, L.; Xie, Q.; Bi, Y. The Development of a Multienzyme Isothermal Rapid Amplification Assay to Visually Detect Duck Hepatitis B Virus. Vet. Sci. 2024, 11, 191. https://doi.org/10.3390/vetsci11050191
Xu S, Man Y, Xu X, Ji J, Wang Y, Yao L, Xie Q, Bi Y. The Development of a Multienzyme Isothermal Rapid Amplification Assay to Visually Detect Duck Hepatitis B Virus. Veterinary Sciences. 2024; 11(5):191. https://doi.org/10.3390/vetsci11050191
Chicago/Turabian StyleXu, Shuqi, Yuanzhuo Man, Xin Xu, Jun Ji, Yan Wang, Lunguang Yao, Qingmei Xie, and Yingzuo Bi. 2024. "The Development of a Multienzyme Isothermal Rapid Amplification Assay to Visually Detect Duck Hepatitis B Virus" Veterinary Sciences 11, no. 5: 191. https://doi.org/10.3390/vetsci11050191
APA StyleXu, S., Man, Y., Xu, X., Ji, J., Wang, Y., Yao, L., Xie, Q., & Bi, Y. (2024). The Development of a Multienzyme Isothermal Rapid Amplification Assay to Visually Detect Duck Hepatitis B Virus. Veterinary Sciences, 11(5), 191. https://doi.org/10.3390/vetsci11050191







