Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Examination
3. Complementary Diagnostic Exams
4. Treatment Options
4.1. Conservative Therapies
4.1.1. Physiotherapeutic Modalities
Physical and Mechanical Agents
- Manual Therapy
- Thermal therapy
- Cold therapy
- Heat Therapy
- Kynesiotape and Bandage
- Therapeutic Exercise
- Water Exercise—Hydrotherapy
Electrotherapy
- Therapeutic Ultrasound
- Laser
- Extracorporeal Shock Wave
- Electromagnetic Field
- Electrostimulation
- Transcutaneous Electrical Nerve Stimulation (TENS)
- Neural Electrical Muscle Stimulation (NEMS)
- Vibration Plates
4.1.2. Pharmacologic Conservative Therapies
Anti-Inflammatories
Hyaluronic Acid (HyA)
PSGAGs
PPS
Polyacrylamide Hydrogel
Bisphosphonates: Tiludronate and Clodronate
4.2. Surgical Techniques
Tendon Splitting
4.3. Regenerative Therapies
4.3.1. Hemoderivatives
PRP
ACS
APS
α2M
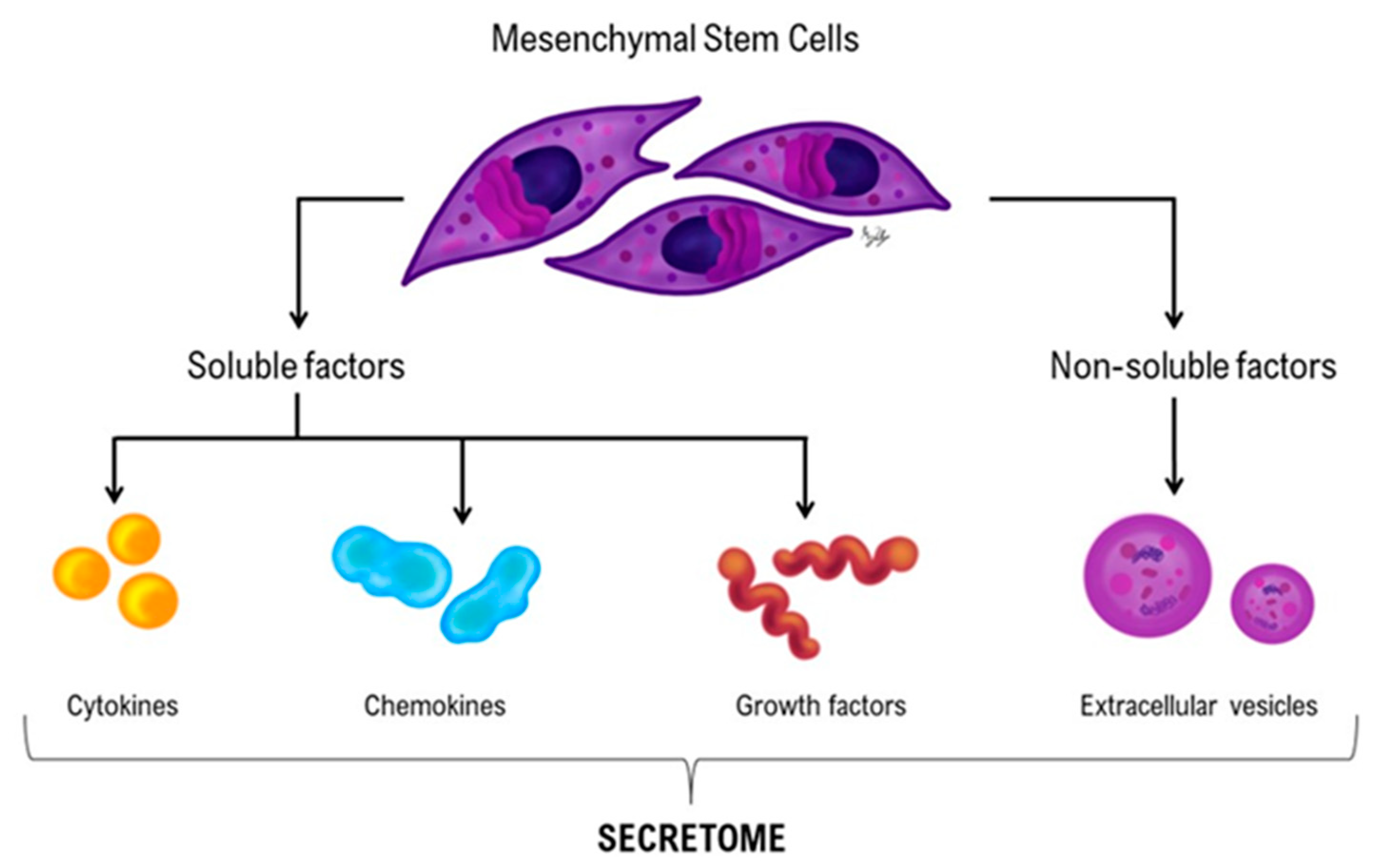
4.3.2. Mesenchymal Stromal/Stem Cell-Based Therapies
Mesenchymal Stromal/Stem Cell Therapies
Autologous Chondrocyte Implantation (ACI)
Mesenchymal Stromal/Stem Cell-Free Therapies
5. Prognosis
6. Discussion
7. Conclusions, Challenges, and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18F-NaF | Fluorine-18-sodium fluoride |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ACI | Autologous chondrocyte implantation |
| ACS | Autologous conditioned serum |
| AFS-MSC | Amniotic fluid mesenchymal stromal/stem cell |
| AI | Anti-inflammatory |
| APS | Autologous protein serum |
| AT-MSC | Adipose tissue mesenchymal stromal/stem cell |
| BM-MSC | Bone marrow mesenchymal stromal/stem cell |
| CD | Cluster differentiation |
| CD | Complementary diagnostic exams |
| cm | Centimeter |
| CM | Conditioned medium |
| cm2 | Square centimeter |
| COX | Cycloxigenase |
| CSA | Cross sectional area |
| CT | Computed tomography |
| CTA | Computed tomography arthrography |
| DDFT | Deep digital flexor tendon |
| DNA | Desoxyribonucleic acid |
| DP-MSC | Dental pulp mesenchymal stromal/stem cell |
| ECM | Extracellular matrix |
| ESC | Embryonic stem cell |
| ESWT | Extracorporeal shock wave therapy |
| EV | Extracellular vesicles |
| FEI | Federation Equestre Internationale |
| FGF | Fibroblast growth factor |
| FL | Forelimbs |
| GAG | Glycosaminoglycans |
| GF | Growth factor |
| h | Hour |
| HILT | High intensity laser therapy |
| HL | Hindlimbs |
| HLA | Human leucocyte antigen |
| HSC | Hematopoietic stem cell |
| HyA | Hyaluronic acid |
| Hz | Hertz |
| IA | Intra-articular |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| IM | Intramuscular |
| IPSC | Induced pluripotent stem cell |
| IRAP | Interleukin-1 receptor antagonist protein |
| IRU | Radioisotope uptake |
| IV | Endovenous |
| Kg | Kilogram |
| KT | Kynesiotaping |
| LLLT | Low level laser therapy |
| mg | Milligram |
| MHz | Megahertz |
| min | Minute |
| mL | Milliliter |
| MRI | Magnetic resonance image |
| MSC | Mesenchymal stem/stromal cells |
| MSC-CM | Mesenchymal stem/stromal cells conditioned medium |
| MT-MSC | Muscle tissue mesenchymal stromal/stem cell |
| mW | Milliwatts |
| NEMS | Neural electrical muscle stimulation |
| nm | Nanometers |
| NSAID | Non-steroidal anti-inflammatory |
| OA | Osteoarthritis |
| °C | Degree Celsius |
| PDEGF | Platelet derived epidermal growth factor |
| PDGF | Platelet derived growth factor |
| PEMF | Pulsed electromagnetic frequency |
| PET | Positron emission tomography |
| PGE2 | Prostaglandin E2 |
| PHyd | Polyacrylamide hydrogel |
| Po -MSC | Periosteum mesenchymal stromal/stem cell |
| PO | Per os |
| PPS | Pentosan polysulfate |
| PRP | Platelet-rich plasma |
| PSAG | Polysulfated glycosaminoglycan |
| RNA | Ribonucleic acid |
| ROM | Range of motion |
| SAIDs | Steroidal anti-inflammatories |
| SF | Synovial fluid |
| SL | Suspensory ligament |
| SM-MSC | Synovial membrane mesenchymal stromal/stem cell |
| TENS | Transcutaneous electrical nerve stimulation |
| TGF | Transforming growth factor |
| U/S | Ultrasound |
| UC-MSC | Umbilical cord derived Whartons jelly mesenchymal stromal/stem cell |
| VEGF | Vascular endothelial growth factor |
| W | Watts |
| WT | Water treadmill |
| X-ray | Radiograph |
| α2M | Alfa-2 macroglobulin |
References
- Smith, R.K.W.; McIlwraith, C.W. “One Health” in tendinopathy research: Current concepts. J. Orthop. Res. 2021, 39, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.W.; Bolwell, C.F.; Gee, E.K.; Rosanowski, S.M. Equine musculoskeletal development and performance: Impact of the production system and early training. Anim. Prod. Sci. 2020, 60, 2069–2079. [Google Scholar] [CrossRef]
- Smith, R.; Webbon, P. Harnessing the stem cell for the treatment of tendon injuries: Heralding a new dawn? Br. J. Sports Med. 2005, 39, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Shokry, M.; Mostafo, A.; Tohamy, A.; El-Sharkawi, M. Autologous mesenchymal stem cells for treatment of acute superficial digital flexor tendonitis in athletic horses-A clinical study of 1 5 cases. Pferdeheilkunde 2020, 36, 43–48. [Google Scholar] [CrossRef]
- Salz, R.O.; Elliott, C.R.; Zuffa, T.; Bennet, E.D.; Ahern, B.J. Treatment of racehorse superficial digital flexor tendonitis–a comparison of stem cell treatments to controlled exercise rehabilitation in 213 cases. Equine Vet. J. 2023, 55, 979–987. [Google Scholar] [CrossRef]
- Lim, W.B.; Al-Dadah, O. Conservative treatment of knee osteoarthritis: A review of the literature. World J. Orthop. 2022, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.S.; Muran, A.; Zaslav, K.; Grande, D. Orthobiologics: An Updated Definition. Open J. Regen. Med. 2023, 12, 36–48. [Google Scholar] [CrossRef]
- Redden, R.F. Clinical and radiographic examination of the equine foot. Aaep Proc. 2003, 49, 169–185. [Google Scholar]
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J.; Van Erck-Westergen, E. Equine Sports Medicine and Surgery: Equine Sports Medicine and Surgery-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Hinchcliff, K.W. Clinical Epidemiology and Evidence-Based Medicine. Equine Intern. Med. -E-Book 2017, 1, 218. [Google Scholar]
- Schumacher, J.; Taylor, D.R.; Schramme, M.C.; Schumacher, J. Localization of pain in the equine foot emphasizing the physical examination and analgesic techniques. Aaep Proc. 2012, 58, 156. [Google Scholar]
- Mahmoud, E.; Hassaneen, A.S.A.; Noby, M.A.; Mawas, A.; Abdel-Hady, A.-N.A. Equine osteoarthritis: An overview of different treatment strategies. SVU-Int. J. Vet. Sci. 2021, 4, 85–96. [Google Scholar] [CrossRef]
- Park, R.D.; Nelson, T.R.; Hoopes, P.J. Magnetic resonance imaging of the normal equine digit and metacarpophalangeal joint. Vet. Radiol. 1987, 28, 105–116. [Google Scholar] [CrossRef]
- Kraft, S.L.; Gavin, P. Physical principles and technical considerations for equine computed tomography and magnetic resonance imaging. Vet. Clin. North Am. Equine Pract. 2001, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Fraschetto, C.; Dancot, M.; Vandersmissen, M.; Denoix, J.-M.; Coudry, V. Conservative management of equine tarsal collateral ligament injuries may allow return to normal performance. J. Am. Vet. Med. Assoc. 2023, 261, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Coudry, V.; Denoix, J.M. Ultrasonographic examination of the palmar aspect of the pastern of the horse: Digital flexor tendons and digital sheath. Equine Vet. Educ. 2013, 25, 196–203. [Google Scholar] [CrossRef]
- Denoix, J.-M. Diagnostic techniques for identification and documentation of tendon and ligament injuries. Vet. Clin. North Am. Equine Pract. 1994, 10, 365–407. [Google Scholar] [CrossRef] [PubMed]
- Werpy, N.M.; Denoix, J.-M. Imaging of the equine proximal suspensory ligament. Vet. Clin. Equine Pract. 2012, 28, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.S. Ultrasonography of the hock. In Atlas of Equine Ultrasonography; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 173–188. [Google Scholar]
- Dik, K.J. Ultrasonography of the equine tarsus. Vet. Radiol. Ultrasound 1993, 34, 36–43. [Google Scholar] [CrossRef]
- Denoix, J.-M. Ultrasonographic examination of joints, a revolution in equine locomotor pathology. Bull. De L’académie Vétérinaire De Fr. 2009, 162, 313–325. [Google Scholar] [CrossRef][Green Version]
- De Bastiani, G.; de La Côrte, F.D.; Brass, K.E.; Kommers, G.D.; Denoix, J.M. Association of ultrasound and anatomopathologic findings of equine metacarpophalangeal lesions. J. Equine Vet. Sci. 2014, 34, 1218–1225. [Google Scholar] [CrossRef]
- Brommer, H.; Rijkenhuizen, A.; Brama, P.; Barneveld, A.; Van Weeren, P. Accuracy of diagnostic arthroscopy for the assessment of cartilage damage in the equine metacarpophalangeal joint. Equine Vet. J. 2004, 36, 331–335. [Google Scholar] [CrossRef]
- Merkely, G.; Ackermann, J.; Lattermann, C. Articular cartilage defects: Incidence, diagnosis, and natural history. Oper. Tech. Sports Med. 2018, 26, 156–161. [Google Scholar] [CrossRef]
- Ehrle, A.; Lilge, S.; Clegg, P.D.; Maddox, T.W. Equine flexor tendon imaging part 2: Current status and future directions in advanced diagnostic imaging, with focus on the deep digital flexor tendon. Vet. J. 2021, 278, 105763. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.; Brown, C.; McNally, E.; Price, A.; Tracey, I.; Jezzard, P.; Carr, A.; Glyn-Jones, S. Non-invasive imaging of cartilage in early osteoarthritis. Bone Jt. J. 2013, 95, 738–746. [Google Scholar] [CrossRef]
- Doll, C.U.; Bohner, M.; Berner, D.; Buettner, K.; Horstmeier, C.; Winter, K.; Burk, J. Approaches to standardising the magnetic resonance image analysis of equine tendon lesions. Vet. Rec. Open 2023, 10, e257. [Google Scholar] [CrossRef]
- Barrett, M.F.; Goorchenko, G.E.; Frisbie, D.D. Comparison of Ultrasound and Magnetic Resonance Imaging for Identifying Soft Tissue Abnormalities in the Palmar Aspect of the Equine Digit. Animals 2023, 13, 2328. [Google Scholar] [CrossRef] [PubMed]
- Mair, T.; Kinns, J.; Jones, R.; Bolas, N. Magnetic resonance imaging of the distal limb of the standing horse. Equine Vet. Educ. 2005, 17, 74–78. [Google Scholar] [CrossRef]
- Vanderperren, K.; Raes, E.; Hoegaerts, M.; Saunders, J.H. Diagnostic imaging of the equine tarsal region using radiography and ultrasonography. Part 1: The soft tissues. Vet. J. 2009, 179, 179–187. [Google Scholar] [CrossRef]
- Biggi, M. Equine scintigraphy: Basic principles and interpretation. UK-Vet Equine 2020, 4, 84–86. [Google Scholar] [CrossRef]
- Dyson, S. Musculoskeletal scintigraphy of the equine athlete. In Proceedings of the Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 4–14. [Google Scholar]
- Spriet, M. Positron emission tomography: A horse in the musculoskeletal imaging race. Am. J. Vet. Res. 2022, 83, 7. [Google Scholar] [CrossRef]
- Spriet, M.; Espinosa, P.; Kyme, A.Z.; Stepanov, P.; Zavarzin, V.; Schaeffer, S.; Katzman, S.A.; Galuppo, L.D.; Beylin, D. Positron emission tomography of the equine distal limb: Exploratory study. Vet. Radiol. Ultrasound 2016, 57, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.-M. Physical Therapy and Massage for the Horse: Biomechanics-Excercise-Treatment; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Yang, S.-M.; Chen, W.-S. Conservative treatment of tendon injuries. Am. J. Phys. Med. Rehabil. 2020, 99, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Frick, A. Stretching exercises for horses: Are they effective? J. Equine Vet. Sci. 2010, 30, 50–59. [Google Scholar] [CrossRef]
- Kaneps, A.J. Practical rehabilitation and physical therapy for the general equine practitioner. Vet. Clin. Equine Pract. 2016, 32, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, A.; Orsini, J. A comparison of seven methods for continuous therapeutic cooling of the equine digit. Equine Vet. J. 2016, 48, 120–124. [Google Scholar] [CrossRef]
- Marlin, D. Evaluation of the cooling efficacy of different equine leg cooling methods. Comp. Exerc. Physiol. 2019, 15, 113–122. [Google Scholar] [CrossRef]
- Montgomery, L.; Elliott, S.B.; Adair, H.S. Muscle and tendon heating rates with therapeutic ultrasound in horses. Vet. Surg. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Ericson, C.; Stenfeldt, P.; Hardeman, A.; Jacobson, I. The effect of kinesiotape on flexion-extension of the thoracolumbar back in horses at trot. Animals 2020, 10, 301. [Google Scholar] [CrossRef]
- Garcia Piqueres, M.; Forés Jackson, P. Evaluation of kinesio taping applied to the equine thoracolumbar spine: Clinical response and mechanical nociceptive threshold. J. Vet. Med. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Mattos, L.H.L.; Yamada, A.L.M.; dos Santos, V.H.; Hussni, C.A.; Rodrigues, C.A.; Watanabe, M.J.; Alves, A.L.G. Treatment With Therapeutic Bandages to Control Equine Postarthroscopic Tibio-Patellofemoral Swelling. J. Equine Vet. Sci. 2017, 54, 87–92. [Google Scholar] [CrossRef]
- Davidson, E.J. Controlled exercise in equine rehabilitation. Vet. Clin. Equine Pract. 2016, 32, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.L. Rehabilitation of tendon and ligament injuries. AAEP Proc. 1997, 43, 306–309. [Google Scholar]
- Kannus, P.; Jozsa, L.; Natri, A.; Järvinen, M. Effects of training, immobilization and remobilization on tendons. Scand. J. Med. Sci. Sports 1997, 7, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. JBJS 2005, 87, 187–202. [Google Scholar]
- Muñoz, A.; Saitua, A.; Becero, M.; Riber, C.; Satué, K.; de Medina, A.S.; Argüelles, D.; Castejón-Riber, C. The use of the water treadmill for the rehabilitation of musculoskeletal injuries in the sport horse. J. Vet. Res. 2019, 63, 439. [Google Scholar] [CrossRef] [PubMed]
- Nankervis, K.; Tranquille, C.; McCrae, P.; York, J.; Lashley, M.; Baumann, M.; King, M.; Sykes, E.; Lambourn, J.; Miskimmin, K.-A.; et al. Consensus for the General Use of Equine Water Treadmills for Healthy Horses. Animals 2021, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- King, M.R. Principles and application of hydrotherapy for equine athletes. Vet. Clin. Equine Pract. 2016, 32, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Tsutani, K.; Okuizumi, H.; Mutoh, Y.; Ohta, M.; Handa, S.; Okada, S.; Kitayuguchi, J.; Kamada, M.; Shiozawa, N. Effectiveness of aquatic exercise and balneotherapy: A summary of systematic reviews based on randomized controlled trials of water immersion therapies. J. Epidemiol. 2010, 20, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Angulo, L.L. Effect of of Water Level on Kinematics of Healthy Horses Walked on an Aquatic Treadmill Compared to Conventional Rehabilitation Techniques. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2012. [Google Scholar]
- Nankervis, K.J.; Launder, E.J.; Murray, R.C. The use of treadmills within the rehabilitation of horses. J. Equine Vet. Sci. 2017, 53, 108–115. [Google Scholar] [CrossRef]
- Tranquille, C.A.; Tacey, J.B.; Walker, V.A.; Nankervis, K.J.; Murray, R.C. International survey of equine water treadmills—Why, when, and how? J. Equine Vet. Sci. 2018, 69, 34–42. [Google Scholar] [CrossRef]
- Kaneps, A.J.; Beverly, M. Therapeutic Ultrasound. Available online: http://kanepsequine.com/services (accessed on 22 April 2024).
- Porter, M. Equine rehabilitation therapy for joint disease. Vet. Clin. Equine Pract. 2005, 21, 599–607. [Google Scholar] [CrossRef]
- Riegel, R.J.; Godbold, J.C., Jr. Fundamental information. In Laser Therapy in Veterinary Medicine: Photobiomodulation; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 7–18. [Google Scholar]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Kaub, L.; Schmitz, C. More than ninety percent of the light energy emitted by near-infrared laser therapy devices used to treat musculoskeletal disorders is absorbed within the first ten millimeters of biological tissue. Biomedicines 2022, 10, 3204. [Google Scholar] [CrossRef] [PubMed]
- Jadah, N.A.; Shamkhi, I.A. A comparative study of low-level laser efficacy on autologous activity of PRP injected in knee arthritis, in vivo study. Lasers Med. Sci. 2021, 36, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Liu, X.; Jiang, L.; Chen, Y.; Lu, J.; Zhu, B.; Liu, X.; Li, Y.; Wang, D.; Li, S. The functions and mechanisms of low-level laser therapy in tendon repair. Front. Physiol. 2022, 13, 808374. [Google Scholar] [CrossRef]
- Wu, M.; Luan, L.; Pranata, A.; Witchalls, J.; Adams, R.; Bousie, J.; Han, J. Is high intensity laser therapy more effective than other physical therapy modalities for treating knee osteoarthritis? A systematic review and network meta-analysis. Front. Med. 2022, 9, 956188. [Google Scholar] [CrossRef]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef]
- Calatrava, I.R.; Valenzuela, J.S.; Gómez-Villamandos, R.; Redondo, J.; Gómez-Villamandos, J.; Jurado, I.A. Histological and clinical responses of articular cartilage to low-level laser therapy: Experimental study. Lasers Med. Sci. 1997, 12, 117–121. [Google Scholar] [CrossRef]
- Jankaew, A.; You, Y.-L.; Yang, T.-H.; Chang, Y.-W.; Lin, C.-F. The effects of low-level laser therapy on muscle strength and functional outcomes in individuals with knee osteoarthritis: A double-blinded randomized controlled trial. Sci. Rep. 2023, 13, 165. [Google Scholar] [CrossRef]
- Zielińska, P.; Soroko, M.; Godlewska, M.; Śniegucka, K.; Dudek, K.; Howell, K. Photothermal Effects of High-Intensity Laser Therapy on the Superficial Digital Flexor Tendon Area in Clinically Healthy Racehorses. Animals 2022, 12, 1253. [Google Scholar] [CrossRef]
- Zielińska, P.; Nicpoń, J.; Kiełbowicz, Z.; Soroko, M.; Dudek, K.; Zaborski, D. Effects of high intensity laser therapy in the treatment of tendon and ligament injuries in performance horses. Animals 2020, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Wyszyńska, J.; Bal-Bocheńska, M. Efficacy of high-intensity laser therapy in treating knee osteoarthritis: A first systematic review. Photomed. Laser Surg. 2018, 36, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Ilieva, E.M. Effectiveness of high intensity laser therapy for reduction of pain in knee osteoarthritis. Pain Res. Manag. 2016, 2016, 9163618. [Google Scholar] [CrossRef] [PubMed]
- Štiglić-Rogoznica, N.; Stamenković, D.; Frlan-Vrgoč, L.; Avancini-Dobrović, V.; Schnurrer-Luke Vrbanić, T. Analgesic effect of high intensity laser therapy in knee osteoarthritis. Coll. Antropol. 2011, 35, 183–185. [Google Scholar] [PubMed]
- Riegel, R.J. Fundamentals of Equine Laser Therapy. In Laser Therapy in Veterinary Medicine: Photobiomodulation; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 335–343. [Google Scholar]
- Auersperg, V.; Trieb, K. Extracorporeal shock wave therapy: An update. EFORT Open Rev. 2020, 5, 584–592. [Google Scholar] [CrossRef]
- Wang, C.-J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Schroeder, A.N.; Tenforde, A.S.; Jelsing, E.J. Extracorporeal shockwave therapy in the management of sports medicine injuries. Curr. Sports Med. Rep. 2021, 20, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Stania, M.; Juras, G.; Chmielewska, D.; Polak, A.; Kucio, C.; Krol, P. Extracorporeal shock wave therapy for Achilles tendinopathy. BioMed Res. Int. 2019, 2019, 3086910. [Google Scholar] [CrossRef]
- Ho, K.-D.; Yang, C.-L.; Lo, H.-Y.; Yeh, H.-J. Extracorporeal shockwave therapy with a modified technique on tendon and ligament for knee osteoarthritis: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2022, 101, 11–17. [Google Scholar] [CrossRef]
- Yocom, A.; Bass, L. Review of the application and efficacy of extracorporeal shockwave therapy in equine tendon and ligament injuries. Equine Vet. Educ. 2019, 31, 271–277. [Google Scholar] [CrossRef]
- Schmitz, C. Improving extracorporeal shock wave therapy with 904 or 905 nm pulsed, high power laser pretreatment. Preprints 2021, 1, 138. [Google Scholar]
- Schlachter, C.; Lewis, C. Electrophysical therapies for the equine athlete. Vet. Clin. Equine Pract. 2016, 32, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Schils, S.J. Review of electrotherapy devices for use in veterinary medicine. In Proceedings of the 55th Annual Convention of the American Association of Equine Practitioners, Las Vegas, NV, USA, 5–9 December 2009; pp. 68–73. [Google Scholar]
- Hyytiäinen, H.K.; Boström, A.; Asplund, K.; Bergh, A. A Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Electrotherapy. Animals 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.G.; Rodríguez-Hurtado, I.; Álvarez, C.T.; Ortiz, G. Effectiveness of neuromuscular electrical stimulation and dynamic mobilization exercises on equine multifidus muscle cross-sectional area. J. Equine Vet. Sci. 2022, 113, 103934. [Google Scholar] [CrossRef] [PubMed]
- Buchner, H.H.F.; Zimmer, L.; Haase, L.; Perrier, J.; Peham, C. Effects of whole body vibration on the horse: Actual vibration, muscle activity, and warm-up effect. J. Equine Vet. Sci. 2017, 51, 54–60. [Google Scholar] [CrossRef]
- Nowlin, C.; Nielsen, B.; Mills, J.; Robison, C.; Schott, H.; Peters, D. Acute and prolonged effects of vibrating platform treatment on horses: A pilot study. J. Equine Vet. Sci. 2018, 62, 116–122. [Google Scholar] [CrossRef]
- Sugg, S.J. Effects of Whole Body Vibration on Lameness, Stride Length, Cortisol, and Other Parameters in Healthy Horses; Middle Tennessee State University: Nashville, TN, USA, 2018. [Google Scholar]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Åstrom, M. Histopathology of common tendinopathies: Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Sass, F.A.; Fuchs, M.; Pumberger, M.; Geissler, S.; Duda, G.N.; Perka, C.; Schmidt-Bleek, K. Immunology guides skeletal muscle regeneration. Int. J. Mol. Sci. 2018, 19, 835. [Google Scholar] [CrossRef] [PubMed]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet 2010, 376, 1751–1767. [Google Scholar] [CrossRef]
- Lin, M.-T.; Chiang, C.-F.; Wu, C.-H.; Huang, Y.-T.; Tu, Y.-K.; Wang, T.-G. Comparative effectiveness of injection therapies in rotator cuff tendinopathy: A systematic review, pairwise and network meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2019, 100, 336–349.e15. [Google Scholar] [CrossRef]
- Everhart, J.S.; Cole, D.; Sojka, J.H.; Higgins, J.D.; Magnussen, R.A.; Schmitt, L.C.; Flanigan, D.C. Treatment options for patellar tendinopathy: A systematic review. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 861–872. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.R.; Hari, R.; Jüni, P. Intra-articular corticosteroids for osteoarthritis of the knee. JAMA 2016, 316, 2671–2672. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Welch, V.; Gee, T.L.; Bourne, R.; Wells, G.A. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021, 133, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Arvind, V.; Huang, A.H. Reparative and maladaptive inflammation in tendon healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.H.; LaValley, M.; McAlindon, T.; Felson, D.T. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: A meta-analysis. JAMA 2003, 290, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-B.; Seo, I.-W.; Shin, Y.-S. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: A network meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.-F.; Samson, A.; Crielaard, J.-M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2015, 5, 264. [Google Scholar] [CrossRef]
- Strauss, E.J.; Hart, J.A.; Miller, M.D.; Altman, R.D.; Rosen, J.E. Hyaluronic acid viscosupplementation and osteoarthritis: Current uses and future directions. Am. J. Sports Med. 2009, 37, 1636–1644. [Google Scholar] [CrossRef]
- da Silva Xavier, A.A.; da Rosa, P.P.; de Brum Mackmill, L.; Roll, V.F.B. An assessment of the effectiveness of hyaluronic acid and polyacrylamide hydrogel in horses with osteoarthritis: Systematic review and network meta-analysis. Res. Vet. Sci. 2021, 134, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Raman, R.; Richette, P.; Bard, H.; Jerosch, J.; Conrozier, T.; Chevalier, X.; Migliore, A. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Proc. Semin. Arthritis Rheum. 2015, 45, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyère, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res. 2017, 69, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Rydell, N.W.; Butler, J.; Balazs, E.A. Hyaluronic acid in synovial fluid: VI. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of arthritis in track horses. Acta Vet. Scand. 1970, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; You, D.; Zhao, S.; Zhu, Z.; Xu, M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Medicine 2020, 99, e19388. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, H.; Liang, G.; Zeng, L.-f.; Yang, W.; Liu, J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; He, Z.; Shu, L.; Li, X.; Ma, M.; Ye, C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, P.; Gao, C.; Fu, L.; Liao, Z.; Tian, G.; Yin, H.; Li, M.; Sui, X.; Yuan, Z.; et al. Recent advances in tendon tissue engineering strategy. Front. Bioeng. Biotechnol. 2023, 11, 1115312. [Google Scholar] [CrossRef]
- Agostini, F.; de Sire, A.; Paoloni, M.; Finamore, N.; Ammendolia, A.; Mangone, M.; Bernetti, A. Effects of hyaluronic acid injections on pain and functioning in patients affected by tendinopathies: A narrative review. J. Back Musculoskelet. Rehabil. 2022, 35, 949–961. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Locke, R.C.; Witherel, C.E.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, J.A. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 2021, 14, 014106. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: Repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.; Gao, R.; Yuan, J.; Zhang, J.; Li, H.; Xie, Z.; Wang, Y. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021, 128, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, X.; Xu, H.; Xing, Y.; Wu, T.; Sun, X.; Kuang, M.; Ma, X.; Liu, W.; Yang, Q. Wharton’s jelly MSC-derived extracellular vehicles—Loaded hyaluronic acid-alginate adhesives for treatment of osteoarthritis. J. Mater. Sci. Technol. 2023, 142, 240–252. [Google Scholar] [CrossRef]
- Koch, D.W.; Goodrich, L.R.; Smanik, L.E.; Steward, S.K.; Ernst, N.S.; Trumble, T.N.; Parks, A.H.; Haussler, K.K.; King, M.; Ellis, K. Principles of Therapy for Lameness: SYSTEMIC/PARENTERAL. In Adams and Stashak’s Lameness in Horses; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 875–947. [Google Scholar]
- O’Shea, C. Arthritis prevention in sport horses. In Proceedings of the 68th Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, USA, 18–22 November 2022. [Google Scholar]
- Zanotto, G.M.; Frisbie, D.D. Current joint therapy usage in equine practice: Changes in the last 10 years. Equine Vet. J. 2022, 54, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Melrose, J. Pentosan Polysulfate affords pleotropic protection to multiple cells and tissues. Pharmaceuticals 2023, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Shekhter, A.; Zar, V.; Voloshin, V.; Lopatin, V. Tissue and cell reaction of the synovial media to intraarticular injection of polymer viscoprosthesis “Noltrex” in experimental conditions. Alm. Clin. Med. 2013, 28, 20–24. [Google Scholar] [CrossRef][Green Version]
- Christensen, L.; Camitz, L.; Illigen, K.; Hansen, M.; Sarvaa, R.; Conaghan, P. Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthr. Cartil. 2016, 24, 1999–2002. [Google Scholar] [CrossRef]
- Tnibar, A.; Persson, A.; Jensen, H. Mechanisms of action of an intraarticular 2.5% polyacrylamide hydrogel (Arthramid Vet) in a goat model of osteoarthritis: Preliminary observations. SM J. Biomed. Eng. 2017, 3, 1022. [Google Scholar]
- Moreau, M.; Rialland, P.; Pelletier, J.-P.; Martel-Pelletier, J.; Lajeunesse, D.; Boileau, C.; Caron, J.; Frank, D.; Lussier, B.; del Castillo, J.R. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res. Ther. 2011, 13, 1–13. [Google Scholar] [CrossRef]
- Fan, T.M.; de Lorimier, L.P.; Charney, S.C.; Hintermeister, J.G. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J. Vet. Intern. Med. 2005, 19, 74–80. [Google Scholar] [PubMed]
- Tischmacher, A.; Wilford, S.; Allen, K.; Mitchell, R.D.; Parkin, T.; Denoix, J.-M. Retrospective Analysis of the Use of Tiludronate in Equine Practice: Safety on 1804 Horses, Efficacy on 343 Horses. J. Equine Vet. Sci. 2022, 115, 104007. [Google Scholar] [CrossRef]
- Vergara-Hernandez, F.B.; Nielsen, B.D.; Colbath, A.C. Is the Use of Bisphosphonates Putting Horses at Risk? An Osteoclast Perspective. Animals 2022, 12, 1722. [Google Scholar] [CrossRef]
- Bertuglia, A.; Basano, I.; Pagliara, E.; Bottegaro, N.B.; Spinella, G.; Bullone, M. Effect of intravenous tiludronate disodium administration on the radiographic progression of osteoarthritis of the fetlock joint in Standardbred racehorses. J. Am. Vet. Med. Assoc. 2021, 259, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Yocom, A.; Contino, E.; Kawcak, C. Review of the Mechanism of Action and Use of Bisphosphonates in Horses. J. Equine Vet. Sci. 2023, 127, 104503. [Google Scholar] [CrossRef]
- Suva, L.J.; Cooper, A.; Watts, A.E.; Ebetino, F.H.; Price, J.; Gaddy, D. Bisphosphonates in veterinary medicine: The new horizon for use. Bone 2021, 142, 115711. [Google Scholar] [CrossRef] [PubMed]
- Story, M.R.; Haussler, K.K.; Nout-Lomas, Y.S.; Aboellail, T.A.; Kawcak, C.E.; Barrett, M.F.; Frisbie, D.D.; McIlwraith, C.W. Equine cervical pain and dysfunction: Pathology, diagnosis and treatment. Animals 2021, 11, 422. [Google Scholar] [CrossRef]
- Mitchell, A.; Watts, A.E.; Ebetino, F.H.; Suva, L.J. Bisphosphonate use in the horse: What is good and what is not? BMC Vet. Res. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Knych, H.K.; Finno, C.J.; Katzman, S.; Ryan, D.; McKemie, D.S.; Kass, P.H.; Arthur, R.M. Clodronate detection and effects on markers of bone resorption are prolonged following a single administration to horses. Equine Vet. J. 2023, 55, 696–706. [Google Scholar] [CrossRef]
- Mama, K.R.; Hector, R.C. Therapeutic developments in equine pain management. Vet. J. 2019, 247, 50–56. [Google Scholar] [CrossRef]
- Krueger, C.R.; Mitchell, C.F.; Leise, B.S.; Knych, H.K. Pharmacokinetics and pharmacodynamics of clodronate disodium evaluated in plasma, synovial fluid and urine. Equine Vet. J. 2020, 52, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, H.A.; Mitchell, C.F.; Gillett, A.N.; McNulty, M.A. Tiludronate and clodronate do not affect bone structure or remodeling kinetics over a 60 day randomized trial. BMC Vet. Res. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Denoix, J.M.; Thibaud, D.; Riccio, B. Tiludronate as a new therapeutic agent in the treatment of navicular disease: A double-blind placebo-controlled clinical trial. Equine Vet. J. 2003, 35, 407–413. [Google Scholar] [CrossRef]
- Argüelles, D.; Saitua, A.; de Medina, A.S.; Muñoz, J.A.; Muñoz, A. Clinical efficacy of clodronic acid in horses diagnosed with navicular syndrome: A field study using objective and subjective lameness evaluation. Res. Vet. Sci. 2019, 125, 298–304. [Google Scholar] [CrossRef]
- Osborn, M.L.; Cornille, J.L.; Blas-Machado, U.; Uhl, E.W. The equine navicular apparatus as a premier enthesis organ: Functional implications. Vet. Surg. 2021, 50, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.; Thibaud, D.; Smith, R. Tiludronate infusion in the treatment of bone spavin: A double blind placebo-controlled trial. Equine Vet. J. 2010, 42, 381–387. [Google Scholar] [CrossRef]
- Dubuc, J. Distal tarsal joints osteoarthritis: Evidence behind bisphosphonates and NSAIDs to improve lameness. Vet. Evid. 2020, 5. [Google Scholar] [CrossRef]
- Soto, S.A.; Barbará, A.C. Bisphosphonates: Pharmacology and clinical approach to their use in equine osteoarticular diseases. J. Equine Vet. Sci. 2014, 34, 727–737. [Google Scholar] [CrossRef]
- Delguste, C.; Amory, H.; Doucet, M.; Piccot-Crezollet, C.; Thibaud, D.; Garnero, P.; Detilleux, J.; Lepage, O. Pharmacological effects of tiludronate in horses after long-term immobilization. Bone 2007, 41, 414–421. [Google Scholar] [CrossRef]
- Kamm, L.; McIlwraith, W.; Kawcak, C. A review of the efficacy of tiludronate in the horse. J. Equine Vet. Sci. 2008, 28, 209–214. [Google Scholar] [CrossRef]
- Ortved, K.F. Regenerative medicine and rehabilitation for tendinous and ligamentous injuries in sport horses. Vet. Clin. Equine Pract. 2018, 34, 359–373. [Google Scholar] [CrossRef]
- Fortier, L.A.; Smith, R.K. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. North Am. Equine Pract. 2008, 24, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Godek, P. Regenerative Medicine and Cell Biology. Regen. Med. 2022, 1, 1. [Google Scholar]
- Fukuda, K.; Kuwano, A.; Kuroda, T.; Tamura, N.; Mita, H.; Okada, Y.; Kasashima, Y. Optimal double-spin method for maximizing the concentration of platelets in equine platelet-rich plasma. J. Equine Sci. 2020, 31, 105–111. [Google Scholar] [CrossRef]
- Garbin, L.C.; Olver, C.S. Platelet-rich products and their application to osteoarthritis. J. Equine Vet. Sci. 2020, 86, 102820. [Google Scholar] [CrossRef] [PubMed]
- Garbin, L.C.; Contino, E.K.; Olver, C.S.; Frisbie, D.D. A safety evaluation of allogeneic freeze-dried platelet-rich plasma or conditioned serum compared to autologous frozen products equivalents in equine healthy joints. BMC Vet. Res. 2022, 18, 141. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Marques-Smith, P.; Kallerud, A.S.; Johansen, G.M.; Boysen, P.; Jacobsen, A.M.; Reitan, K.M.; Henriksen, M.M.; Löfgren, M.; Fjordbakk, C.T. Is clinical effect of autologous conditioned serum in spontaneously occurring equine articular lameness related to ACS cytokine profile? BMC Vet. Res. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Geburek, F.; Lietzau, M.; Beineke, A.; Rohn, K.; Stadler, P.M. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res. Ther. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Genç, E.; Yüksel, S.; Çağlar, A.; Beytemur, O.; Güleç, M.A. Comparison on effects of platelet-rich plasma versus autologous conditioned serum on Achilles tendon healing in a rat model. Acta Orthop. Traumatol. Turc. 2020, 54, 438. [Google Scholar] [CrossRef]
- Fjordbakk, C.; Johansen, G.; Løvås, A.; Oppegård, K.; Storset, A. Surgical stress influences cytokine content in autologous conditioned serum. Equine Vet. J. 2015, 47, 212–217. [Google Scholar] [CrossRef]
- Stashak, T.S. Adams’ Lameness in Horses; Verlag, M., Schaper, H., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Clegg, P. Differential diagnosis of a swollen hock in the horse. InPractice 2003, 25, 328–341. [Google Scholar] [CrossRef]
- Kümmerle, J.M.; Kummer, M.R. Arthroscopically accessible anatomy of the tarsal collateral ligaments in the horse. Vet. Surg. 2013, 42, 267–274. [Google Scholar] [CrossRef]
- Patel, S.; Jindal, K.; Dhillon, M.S. The future of injectable orthobiologic substances for knee osteoarthritis: Options beyond platelet-rich plasma. J. Musculoskelet. Surg. Res. 2020, 4, 173. [Google Scholar]
- Kaneps, A.J. A one-health perspective: Use of hemoderivative regenerative therapies in canine and equine patients. J. Am. Vet. Med. Assoc. 2023, 261, 301–308. [Google Scholar] [CrossRef]
- Linardi, R.L.; Dodson, M.E.; Moss, K.L.; King, W.J.; Ortved, K.F. The effect of autologous protein solution on the inflammatory cascade in stimulated equine chondrocytes. Front. Vet. Sci. 2019, 6, 64. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; Morris, M.J. A comparative review of autologous conditioned serum and autologous protein solution for treatment of osteoarthritis in horses. Front. Vet. Sci. 2021, 8, 602978. [Google Scholar] [CrossRef]
- Soontararak, S.; Ardaum, P.; Senarat, N.; Yangtara, S.; Lekcharoensuk, C.; Putchong, I.; Kashemsant, N.; Vijarnsorn, M.; Chow, L.; Dow, S. In vitro anti-inflammatory and regenerative effects of autologous conditioned serum from dogs with osteoarthritis. Animals 2022, 12, 2717. [Google Scholar] [CrossRef]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative strategies in tendon tissue engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef]
- Gaesser, A.M.; Underwood, C.; Linardi, R.L.; Even, K.M.; Reef, V.B.; Shetye, S.S.; Mauck, R.L.; King, W.J.; Engiles, J.B.; Ortved, K.F. Evaluation of autologous protein solution injection for treatment of superficial digital flexor tendonitis in an equine model. Front. Vet. Sci. 2021, 8, 697551. [Google Scholar] [CrossRef]
- Geburek, F.; Gaus, M.; van Schie, H.T.; Rohn, K.; Stadler, P.M. Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies–a randomized prospective controlled clinical trial. BMC Vet. Res. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Peng, C.; Yang, L.; Labens, R.; Gao, Y.; Zhu, Y.; Li, J. A systematic review and meta-analysis of the efficacy of platelet-rich plasma products for treatment of equine joint disease. Equine Vet. J. 2024. [Google Scholar] [CrossRef]
- Abu-Seida, A.M. Regenerative therapy for equine osteoarthritis: A concise review. Asian J. Anim. Vet. Adv 2015, 10, 500–508. [Google Scholar] [CrossRef][Green Version]
- da Fontoura Pereira, R.C.; De La Côrte, F.D.; Brass, K.E.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.L.; Cezar, A.S.; Inkelmann, M.A. Evaluation of three methods of platelet-rich plasma for treatment of equine distal limb skin wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef]
- Gottschall, J.; Johnston, V.L.; Rzad, L.; Anderson, A.J.; Aster, R. Importance of white blood cells in platelet storage. Vox Sang. 1984, 47, 101–107. [Google Scholar] [CrossRef]
- Giraldo, C.E.; López, C.; Álvarez, M.E.; Samudio, I.J.; Prades, M.; Carmona, J.U. Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2013, 9, 1–10. [Google Scholar] [CrossRef]
- McCarrel, T.M. Equine Platelet-Rich Plasma. Vet. Clin. Equine Pract. 2023, 39, 429–442. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; Lopez, C.; Carmona, J.U. A critical overview of the use of platelet-rich plasma in equine medicine over the last decade. Front. Vet. Sci. 2021, 8, 641818. [Google Scholar] [CrossRef]
- Bosch, G.; van Schie, H.T.; de Groot, M.W.; Cadby, J.A.; van de Lest, C.H.; Barneveld, A.; van Weeren, P.R. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J. Orthop. Res. 2010, 28, 211–217. [Google Scholar] [CrossRef]
- Bosch, G.; Moleman, M.; Barneveld, A.; van Weeren, P.R.; Van Schie, H. The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand. J. Med. Sci. Sports 2011, 21, 554–561. [Google Scholar] [CrossRef]
- Santangelo, K.; Nuovo, G.; Bertone, A. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr. Cartil. 2012, 20, 1610–1618. [Google Scholar] [CrossRef]
- Hopper, S.A. Regenerative Medicine–Under-standing IRAP, PRP and stem cell Therapies. Rood Riddle 2015. [Google Scholar]
- Crawford, D.C.; Miller, L.E.; Block, J.E. Conservative management of symptomatic knee osteoarthritis: A flawed strategy? Orthop. Rev. 2013, 5, e2. [Google Scholar]
- Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis 2011, 2011, 454873. [Google Scholar]
- Genç, E.; Beytemur, O.; Yuksel, S.; Eren, Y.; Çağlar, A.; Küçükyıldırım, B.O.; Güleç, M.A. Investigation of the biomechanical and histopathological effects of autologous conditioned serum on healing of Achilles tendon. Acta Orthop. Traumatol. Turc. 2018, 52, 226–231. [Google Scholar] [CrossRef]
- Bertone, A.L.; Ishihara, A.; Zekas, L.J.; Wellman, M.L.; Lewis, K.B.; Schwarze, R.A.; Barnaba, A.R.; Schmall, M.L.; Kanter, P.M.; Genovese, R.L. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am. J. Vet. Res. 2014, 75, 141–151. [Google Scholar] [CrossRef]
- Ortved, K.F. Equine Autologous Conditioned Serum and Autologous Protein Solution. Vet. Clin. N. Am. Equine Pract. 2023, 39, 443–451. [Google Scholar] [CrossRef]
- Knott, L.E.; Fonseca-Martinez, B.A.; O’Connor, A.M.; Goodrich, L.R.; McIlwraith, C.W.; Colbath, A.C. Current use of biologic therapies for musculoskeletal disease: A survey of board-certified equine specialists. Vet. Surg. 2022, 51, 557–567. [Google Scholar] [CrossRef]
- Velloso Alvarez, A.; Boone, L.H.; Braim, A.P.; Taintor, J.S.; Caldwell, F.; Wright, J.C.; Wooldridge, A.A. A survey of clinical usage of non-steroidal intra-articular therapeutics by equine practitioners. Front. Vet. Sci. 2020, 7, 579967. [Google Scholar] [CrossRef]
- Wang, S.; Wei, X.; Zhou, J.; Zhang, J.; Li, K.; Chen, Q.; Terek, R.; Fleming, B.C.; Goldring, M.B.; Ehrlich, M.G. Identification of α2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol. 2014, 66, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, B.; Wei, L.; Wang, S. Alpha-2-macroglobulin, a native and powerful proteinase inhibitor, prevents cartilage degeneration disease by inhibiting majority of catabolic enzymes and cytokines. Curr. Mol. Biol. Rep. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Xiang, C.; Wei, X.; Sun, X.; Li, R.; Li, P.; Sun, J.; Wei, D.; Chen, Y.; Zhang, Y. Early supplemental α2-macroglobulin attenuates cartilage and bone damage by inhibiting inflammation in collagen II-induced arthritis model. Int. J. Rheum. Dis. 2019, 22, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Szponder, T.; Latalski, M.; Danielewicz, A.; Krać, K.; Kozera, A.; Drzewiecka, B.; Nguyen Ngoc, D.; Dobko, D.; Wessely-Szponder, J. Osteoarthritis: Pathogenesis, animal models, and new regenerative therapies. J. Clin. Med. 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Velloso Alvarez, A.; Boone, L.H.; Pondugula, S.R.; Caldwell, F.; Wooldridge, A.A. Effects of autologous conditioned serum, autologous protein solution, and triamcinolone on inflammatory and catabolic gene expression in equine cartilage and synovial explants treated with IL-1β in co-culture. Front. Vet. Sci. 2020, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Tokawa, P.K.A.; Brossi, P.M.; Baccarin, R.Y.A. Autologous conditioned serum in equine and human orthopedic therapy: A systematic review. Res. Vet. Sci. 2022, 146, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Hraha, T.; Doremus, K.; McIlwraith, C.; Frisbie, D. Autologous conditioned serum: The comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet. J. 2011, 43, 516–521. [Google Scholar] [CrossRef]
- Lasarzik, J.; Bondzio, A.; Rettig, M.; Estrada, R.; Klaus, C.; Ehrle, A.; Einspanier, R.; Lischer, C.J. Evaluation of two protocols using autologous conditioned serum for intra-articular therapy of equine osteoarthritis—A pilot study monitoring cytokines and cartilage-specific biomarkers. J. Equine Vet. Sci. 2018, 60, 35–42.e32. [Google Scholar] [CrossRef]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem cells in veterinary medicine—Current state and treatment options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Medical therapies with adult stem/progenitor cells (MSCs): A backward journey from dramatic results in vivo to the cellular and molecular explanations. J. Cell. Biochem. 2012, 113, 1460–1469. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Kim, H.-S.; Shin, T.-H.; Kang, I.; Lee, J.Y.; Kim, J.-J.; Kang, H.K.; Seo, Y.; Lee, S.; Yu, K.-R. PGE2 maintains self-renewal of human adult stem cells via EP2-mediated autocrine signaling and its production is regulated by cell-to-cell contact. Sci. Rep. 2016, 6, 26298. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Leal Reis, I.; Lopes, B.; Sousa, P.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Pedrosa, S.S.; Rêma, A.; Oliveira, C.; Porto, B. Allogenic Synovia-Derived Mesenchymal Stem Cells for Treatment of Equine Tendinopathies and Desmopathies—Proof of Concept. Animals 2023, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Colbath, A.C.; Dow, S.W.; Phillips, J.N.; McIlwraith, C.W.; Goodrich, L.R. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties in vitro. Stem Cells Dev. 2017, 26, 503–511. [Google Scholar] [CrossRef]
- Leal Reis, I.; Lopes, B.; Sousa, P.; Sousa, A.C.; Branquinho, M.V.; Caseiro, A.R.; Rêma, A.; Briote, I.; Mendonça, C.M.; Santos, J.M.; et al. Treatment of Equine Tarsus Long Medial Collateral Ligament Desmitis with Allogenic Synovial Membrane Mesenchymal Stem/Stromal Cells Enhanced by Umbilical Cord Mesenchymal Stem/Stromal Cell-Derived Conditioned Medium: Proof of Concept. Animals 2024, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Reis, I.; Lopes, B.; Sousa, P.; Sousa, A.; Caseiro, A.R.; Briote, I.; Rocha, A.M.; Pereira, J.; Mendonça, C.; Santos, J. Equine Metacarpophalangeal Joint Partial and Full Thickness Defects Treated with Allogenic Equine Synovial Membrane Mesenchymal Stem/Stromal Cell and Umbilical Cord Mesenchymal Stem/Stromal Cell Conditioned Medium: The Proof-of-Concept. Preprints 2024, 1, 1915. [Google Scholar]
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: A meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Im, G.-I.; Shin, Y.-W.; Lee, K.-B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr. Cartil. 2005, 13, 845–853. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Xie, H.-Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.-C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: Current status and future perspectives. Stem Cell Rev. Rep. 2017, 13, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Terada, N.; Hamazaki, T.; Oka, M.; Hoki, M.; Mastalerz, D.M.; Nakano, Y.; Meyer, E.M.; Morel, L.; Petersen, B.E.; Scott, E.W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002, 416, 542–545. [Google Scholar] [CrossRef]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: A phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- To, K.; Zhang, B.; Romain, K.; Mak, C.; Khan, W. Synovium-derived mesenchymal stem cell transplantation in cartilage regeneration: A PRISMA review of in vivo studies. Front. Bioeng. Biotechnol. 2019, 7, 314. [Google Scholar] [CrossRef]
- Zupan, J.; Drobnič, M.; Stražar, K. Synovium-derived mesenchymal stem/stromal cells and their promise for cartilage regeneration. Adv. Exp. Med. Biol. 2020, 1212, 87–106. [Google Scholar]
- Kondo, S.; Muneta, T.; Nakagawa, Y.; Koga, H.; Watanabe, T.; Tsuji, K.; Sotome, S.; Okawa, A.; Kiuchi, S.; Ono, H. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J. Orthop. Res. 2017, 35, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.; Kwon, H.; Kim, S.; Cho, D.; Kang, S.; You, J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch Jr, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise review: Wharton’s jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef]
- Anzalone, R.; Iacono, M.L.; Corrao, S.; Magno, F.; Loria, T.; Cappello, F.; Zummo, G.; Farina, F.; La Rocca, G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010, 19, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J. Cell. Physiol. 2020, 235, 9230–9240. [Google Scholar] [CrossRef] [PubMed]
- Thitiset, T.; Damrongsakkul, S.; Yodmuang, S.; Leeanansaksiri, W.; Apinun, J.; Honsawek, S. A novel gelatin/chitooligosaccharide/demineralized bone matrix composite scaffold and periosteum-derived mesenchymal stem cells for bone tissue engineering. Biomater. Res. 2021, 25, 19. [Google Scholar] [CrossRef]
- Moon, D.K.; Kim, B.G.; Lee, A.R.; In Choe, Y.; Khan, I.; Moon, K.M.; Jeon, R.-H.; Byun, J.-H.; Hwang, S.-C.; Woo, D.K. Resveratrol can enhance osteogenic differentiation and mitochondrial biogenesis from human periosteum-derived mesenchymal stem cells. J. Orthop. Surg. Res. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental pulp-derived mesenchymal stem cells for modeling genetic disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, A.R.; Santos Pedrosa, S.; Ivanova, G.; Vieira Branquinho, M.; Almeida, A.; Faria, F.; Amorim, I.; Pereira, T.; Maurício, A.C. Mesenchymal Stem/Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/Stromal Cells secretome. PLoS ONE 2019, 14, e0221378. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Presen, D.M.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019, 38, 101465. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Saether, E.E.; Aktas, E.; Vanderby, R. Mesenchymal stem cell therapy on tendon/ligament healing. J. Cytokine Biol. 2017, 2, 1–3. [Google Scholar] [CrossRef]
- Godwin, E.; Young, N.; Dudhia, J.; Beamish, I.; Smith, R. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Zhou, Z.; Taub, P.J.; Ramcharan, M.; Li, Y.; Akinbiyi, T.; Maharam, E.R.; Leong, D.J.; Laudier, D.M.; Ruike, T. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS ONE 2011, 6, e17531. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Yea, J.-H.; Kim, Y.; Jo, C.H. Comparison of mesenchymal stem cells from bone marrow, umbilical cord blood, and umbilical cord tissue in regeneration of a full-thickness tendon defect in vitro and in vivo. Biochem. Biophys. Rep. 2023, 34, 101486. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Gittel, C.; Heller, S.; Pfeiffer, B.; Paebst, F.; Ahrberg, A.B.; Brehm, W. Gene expression of tendon markers in mesenchymal stromal cells derived from different sources. BMC Res. Notes 2014, 7, 1–6. [Google Scholar] [CrossRef]
- Conze, P.; Van Schie, H.T.; Weeren, R.v.; Staszyk, C.; Conrad, S.; Skutella, T.; Hopster, K.; Rohn, K.; Stadler, P.; Geburek, F. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen. Med. 2014, 9, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The potential for synovium-derived stem cells in cartilage repair. Curr. Stem Cell Res. Ther. 2018, 13, 174–184. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium–and adipose synovium–derived cells compared with subcutaneous fat–derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Ju, Y.J.; Nagase, T.; Nimura, A.; Mochizuki, T.; Ichinose, S.; Von der Mark, K.; Sekiya, I. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 2007, 25, 689–696. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Archer, C.W.; Dowthwaite, G.P.; Francis-West, P. Development of synovial joints. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 144–155. [Google Scholar] [CrossRef]
- Koyama, E.; Shibukawa, Y.; Nagayama, M.; Sugito, H.; Young, B.; Yuasa, T.; Okabe, T.; Ochiai, T.; Kamiya, N.; Rountree, R.B. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008, 316, 62–73. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: A tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Vousooghi, N.; Rahimnia, R.; Razeghian, E.; Rajaeian, S.; Seyhoun, I.; Sharif, S.; Verdi, J. Recent advances in mesenchymal stem/stromal cells (MSCs)-based approaches for osteoarthritis (OA) therapy. Cell Biol. Int. 2023, 47, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Gregory, C.A.; Spees, J.L. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. Proc. Natl. Acad. Sci. USA 2003, 100, 11917–11923. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef] [PubMed]
- Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 2009, 266, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Grande, D.A.; Southerland, S.S.; Manji, R.; Pate, D.W.; Schwartz, R.E.; Lucas, P.A. Repair of articular cartilage defects using mesenchymal stem cells. Tissue Eng. 1995, 1, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.; Rao, J. Tomorrow’s skeleton staff: Mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004, 37, 97–110. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef]
- Granero-Molto, F.; Weis, J.A.; Longobardi, L.; Spagnoli, A. Role of mesenchymal stem cells in regenerative medicine: Application to bone and cartilage repair. Expert Opin. Biol. Ther. 2008, 8, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Stanford, W.L.; Kandel, R.A. Mesenchymal stem and progenitor cells for cartilage repair. Skelet. Radiol. 2007, 36, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent developed strategies for enhancing chondrogenic differentiation of MSC: Impact on MSC-based therapy for cartilage regeneration. Stem Cells Int. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sadlik, B.; Jaroslawski, G.; Puszkarz, M.; Blasiak, A.; Oldak, T.; Gladysz, D.; Whyte, G.P. Cartilage Repair in the Knee Using Umbilical Cord Wharton’s Jelly–Derived Mesenchymal Stem Cells Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthrosc. Tech. 2018, 7, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.P.; Ha, P.; Xiao, C.Y.; Kim, S.Y.; Jensen, A.R.; Easley, J.; Yao, Q.; Zhang, X. Updates on mesenchymal stem cell therapies for articular cartilage regeneration in large animal models. Front. Cell Dev. Biol. 2022, 10, 982199. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-N.; Zhu, S.-Y.; He, H.-C.; Yu, X.; Xu, Y.; He, C.-Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- De La Garza-Rodea, A.S.; Van Der Velde-Van Dijke, I.; Boersma, H.; Gonçalves, M.A.; Van Bekkum, D.W.; De Vries, A.A.; Knaän-Shanzer, S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 2012, 21, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Song, S.-Y.; Kim, E. Advanced Therapy medicinal products for autologous chondrocytes and comparison of regulatory systems in target countries. Regen. Ther. 2022, 20, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Welch, T.; Mandelbaum, B.; Tom, M. Autologous chondrocyte implantation: Past, present, and future. Sports Med. Arthrosc. Rev. 2016, 24, 85–91. [Google Scholar] [CrossRef]
- Xia, J.; Minamino, S.; Kuwabara, K.; Arai, S. Stem cell secretome as a new booster for regenerative medicine. Biosci. Trends 2019, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the stem cell secretome in regenerative engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Bouche Djatche, W.H.; Zhu, H.; Ma, W.; Li, Y.; Li, Z.; Zhao, H.; Liu, Z.; Qiao, H. Potential of mesenchymal stem cell-derived conditioned medium/secretome as a therapeutic option for ocular diseases. Regen. Med. 2023, 18, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Jafarinia, M.; Alsahebfosoul, F.; Salehi, H.; Eskandari, N.; Ganjalikhani-Hakemi, M. Mesenchymal stem cell-derived extracellular vesicles: A novel cell-free therapy. Immunol. Investig. 2020, 49, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Shin, S.; Do, M.; Oh, B.H.; Song, Y.; Bui, V.D.; Lee, E.S.; Jo, D.-G.; Cho, Y.W.; Kim, D.-H. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J. Control. Release 2021, 330, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef]
- Fan, W.-J.; Liu, D.; Pan, L.-Y.; Wang, W.-Y.; Ding, Y.-L.; Zhang, Y.-Y.; Ye, R.-X.; Zhou, Y.; An, S.-B.; Xiao, W.-F. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 2022, 10, 949690. [Google Scholar] [CrossRef]
- Fang, W.H.; Agrawal, D.K.; Thankam, F.G. “Smart exosomes”: A smart approach for tendon regeneration. Tissue Eng. Part B Rev. 2022, 28, 613–625. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef]
- Lyu, K.; Liu, T.; Chen, Y.; Lu, J.; Jiang, L.; Liu, X.; Liu, X.; Li, Y.; Li, S. A “cell-free treatment” for tendon injuries: Adipose stem cell-derived exosomes. Eur. J. Med. Res. 2022, 27, 75. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn III, R.F.; Otsuru, S.; Lovering, R.M. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes isolated from adipose-derived stem cells: A new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Piercy, R.J.; Rivero, J. Muscle disorders of equine athletes. In Equine Sports Medicine and Surgery E-Book: Basic and Clinical Sciences of the Equine Athlete; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; p. 109. [Google Scholar]
- Marshall-Gibson, M.E.; Durham, M.G.; Seabaugh, K.A.; Moorman, V.J.; Ferris, D.J. Survey of equine veterinarians regarding primary equine back pain in the United States. Front. Vet. Sci. 2023, 10, 1224605. [Google Scholar] [CrossRef] [PubMed]
- Willow, R.; Guzmán, K.E.; Panek, C.L.; Colbath, A.C. Stem cells and platelet-rich plasma for the treatment of naturally occurring equine tendon and ligament injuries: A systematic review and meta-analysis. J. Am. Vet. Med. Assoc. 2024, 1, 1–11. [Google Scholar]
- Rhatomy, S.; Prasetyo, T.E.; Setyawan, R.; Soekarno, N.R.; Romaniyanto, F.; Sedjati, A.P.; Sumarwoto, T.; Utomo, D.N.; Suroto, H.; Mahyudin, F. Prospect of stem cells conditioned medium (secretome) in ligament and tendon healing: A systematic review. Stem Cells Transl. Med. 2020, 9, 895–902. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal stem cells current clinical applications: A systematic review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Carter-Arnold, J.; Neilsen, N.; Amelse, L.; Odoi, A.; Dhar, M. In vitro analysis of equine, bone marrow-derived mesenchymal stem cells demonstrates differences within age-and gender-matched horses. Equine Vet. J. 2014, 46, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Colbath, A.C.; Dow, S.W.; McIlwraith, C.W.; Goodrich, L.R. Mesenchymal stem cells for treatment of musculoskeletal disease in horses: Relative merits of allogeneic versus autologous stem cells. Equine Vet. J. 2020, 52, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Pérez Fraile, A.; González-Cubero, E.; Martínez-Flórez, S.; Olivera, E.R.; Villar-Suárez, V. Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review. Vet. Sci. 2023, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Peter, V.G.; Coleridge, M.O.; Bathe, A.P. Immediate pre-operative computed tomography for surgical planning of equine fracture repair: A retrospective review of 55 cases. PLoS ONE 2022, 17, e0278748. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, C.; Eggleston, R.; Peroni, J.; Parks, A. Desmitis of the medial tarsal collateral ligament in 7 horses. Equine Vet. Educ. 2012, 24, 72–80. [Google Scholar] [CrossRef]
- Santos, V.H. Synovial-Derived Mesenchymal Stem Cells Encapsulated in Alginate Beads Provide Better Outcomes for Equine Tarsus Chondral Lesions. J. Orthop. Sports Med. 2023, 5, 265–279. [Google Scholar] [CrossRef]
- Rose, P.L.; Moore, I. Imaging diagnosis--avulsion of the medial collateral ligament of the tarsus in a horse. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2003, 44, 657–659. [Google Scholar] [CrossRef]
- Mitchell, R.; DaSilva, D.; Rosenbaum, C.; Blikslager, A.; Edwards III, R. Ultrasound findings in tendons and ligaments of lame sport horses competing or training in South Florida venues during the winter seasons of 2007 through 2016. Equine Vet. Educ. 2021, 33, 306–309. [Google Scholar] [CrossRef]
- Ireland, J.; Clegg, P.; McGowan, C.; McKane, S.; Chandler, K.; Pinchbeck, G. Comparison of owner-reported health problems with veterinary assessment of geriatric horses in the United Kingdom. Equine Vet. J. 2012, 44, 94–100. [Google Scholar] [CrossRef]
- Jammes, M.; Contentin, R.; Audigié, F.; Cassé, F.; Galéra, P. Effect of pro-inflammatory cytokine priming and storage temperature of the mesenchymal stromal cell (MSC) secretome on equine articular chondrocytes. Front. Bioeng. Biotechnol. 2023, 11, 1204737. [Google Scholar] [CrossRef]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Mariën, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking progenitor cells for hippiatric regenerative medicine: Optimized establishment of safe and consistent cell sources for standardized veterinary therapeutic protocols. Am. J. Biomed. Sci. 2020, 8, 252–271. [Google Scholar]
| Musculoskeletal Injuries Therapeutical Options | ||||
|---|---|---|---|---|
| Conservative Treatments | Surgical Techniques | Regenerative Treatments | ||
| Physiotherapy | Pharmacological | Hemoderivatives | Stem-Cell Based Therapies | |
|
|
|
|
|
|
|
|
| |
|
|
|
| |
|
|
| ||
|
| |||
|
| |||
| ||||
| ||||
| ||||
| ||||
| ||||
| Weeks after Injury | Exercise | Confinment |
|---|---|---|
| 0–4 | Hand walk, 5–10 min, Twice daily. | Stall rest |
| 5–8 | Hand walk, 10–15 min, Three times daily. | Stall rest or small paddock |
| 9–12 | Increase time walk 5 min/day Three times daily | Stall rest or small paddock |
| 13–16 | If sound and continued improvement in lesion parameters: ride at the walk 20–25 min daily, hand walk 30 min daily. | Stall rest or small paddock |
| 17–20 | Ride at the walk 30 min, add 3–5 min trot. On week 18, add 3–5 min additional trot per week. | Stall rest or small paddock |
| 21–recovery | Ride at the walk 30 min, ride at the trot 15 min per session, add 3 min canter. On week 22–24, add 3–5 min canter per session | Small paddock |
| Weeks after Injury | Exercise | Confinment |
|---|---|---|
| 0–4 | - | Stall rest |
| 5–6 | Hand walk, 15 min/day. | Stall rest or small paddock |
| 7–8 | Hand walk, 30 min/day. | Stall rest or small paddock |
| 9–16 | Exercise in small paddock 6 × 6 m. | Stall rest or small paddock |
| 16–recovery | Gradually increase exercise until full work. | Stall rest or small paddock |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, I.L.; Lopes, B.; Sousa, P.; Sousa, A.C.; Caseiro, A.R.; Mendonça, C.M.; Santos, J.M.; Atayde, L.M.; Alvites, R.D.; Maurício, A.C. Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review. Vet. Sci. 2024, 11, 190. https://doi.org/10.3390/vetsci11050190
Reis IL, Lopes B, Sousa P, Sousa AC, Caseiro AR, Mendonça CM, Santos JM, Atayde LM, Alvites RD, Maurício AC. Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review. Veterinary Sciences. 2024; 11(5):190. https://doi.org/10.3390/vetsci11050190
Chicago/Turabian StyleReis, Inês L., Bruna Lopes, Patrícia Sousa, Ana C. Sousa, Ana R. Caseiro, Carla M. Mendonça, Jorge M. Santos, Luís M. Atayde, Rui D. Alvites, and Ana C. Maurício. 2024. "Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review" Veterinary Sciences 11, no. 5: 190. https://doi.org/10.3390/vetsci11050190
APA StyleReis, I. L., Lopes, B., Sousa, P., Sousa, A. C., Caseiro, A. R., Mendonça, C. M., Santos, J. M., Atayde, L. M., Alvites, R. D., & Maurício, A. C. (2024). Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review. Veterinary Sciences, 11(5), 190. https://doi.org/10.3390/vetsci11050190







