Retrospective Study of Clinicopathological Changes and Prediction Model for Canine Vascular Neoplasms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor and Control Dogs
2.2. Clinicopathological Data
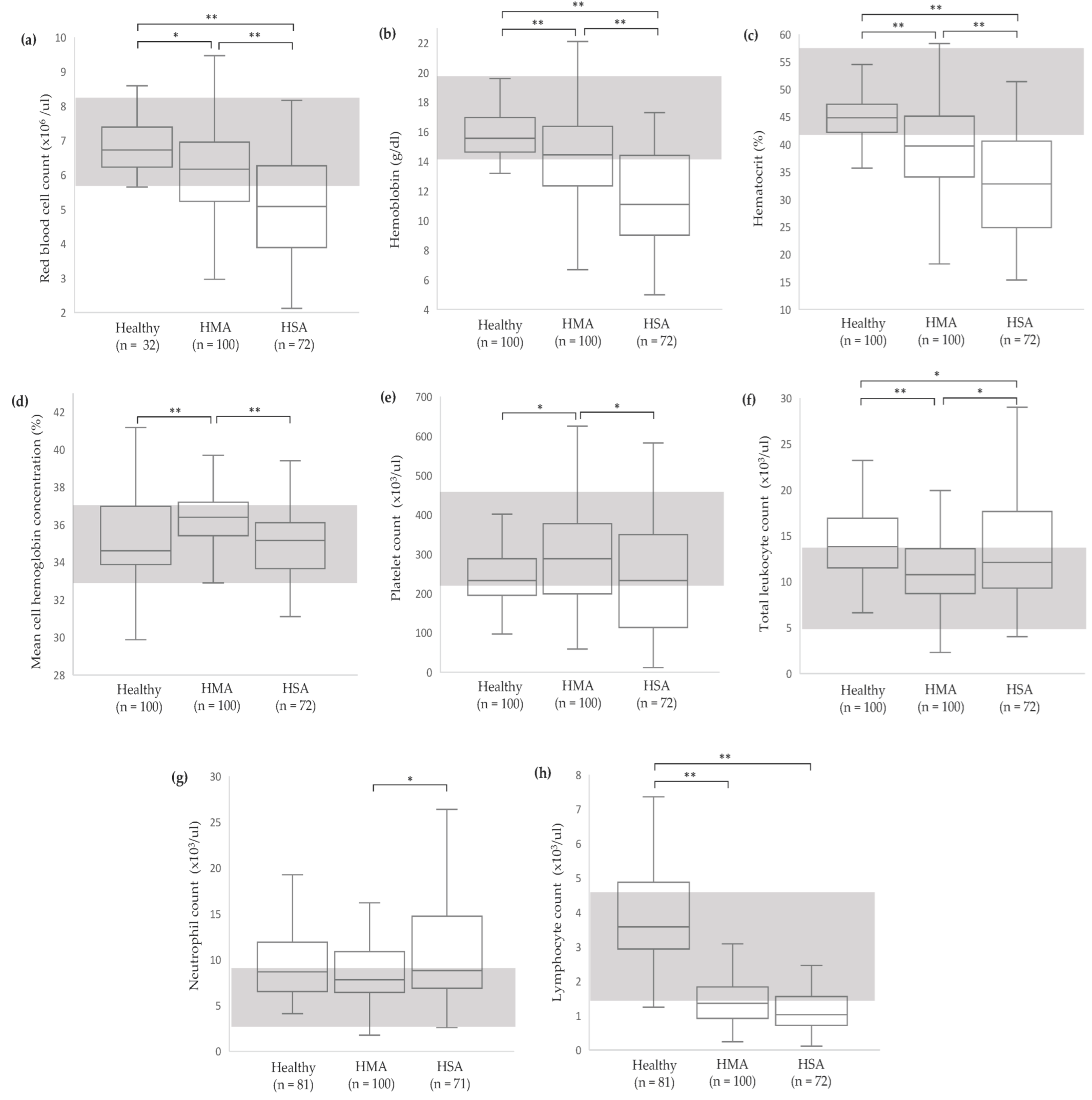
2.2.1. Complete Blood Count (CBC)
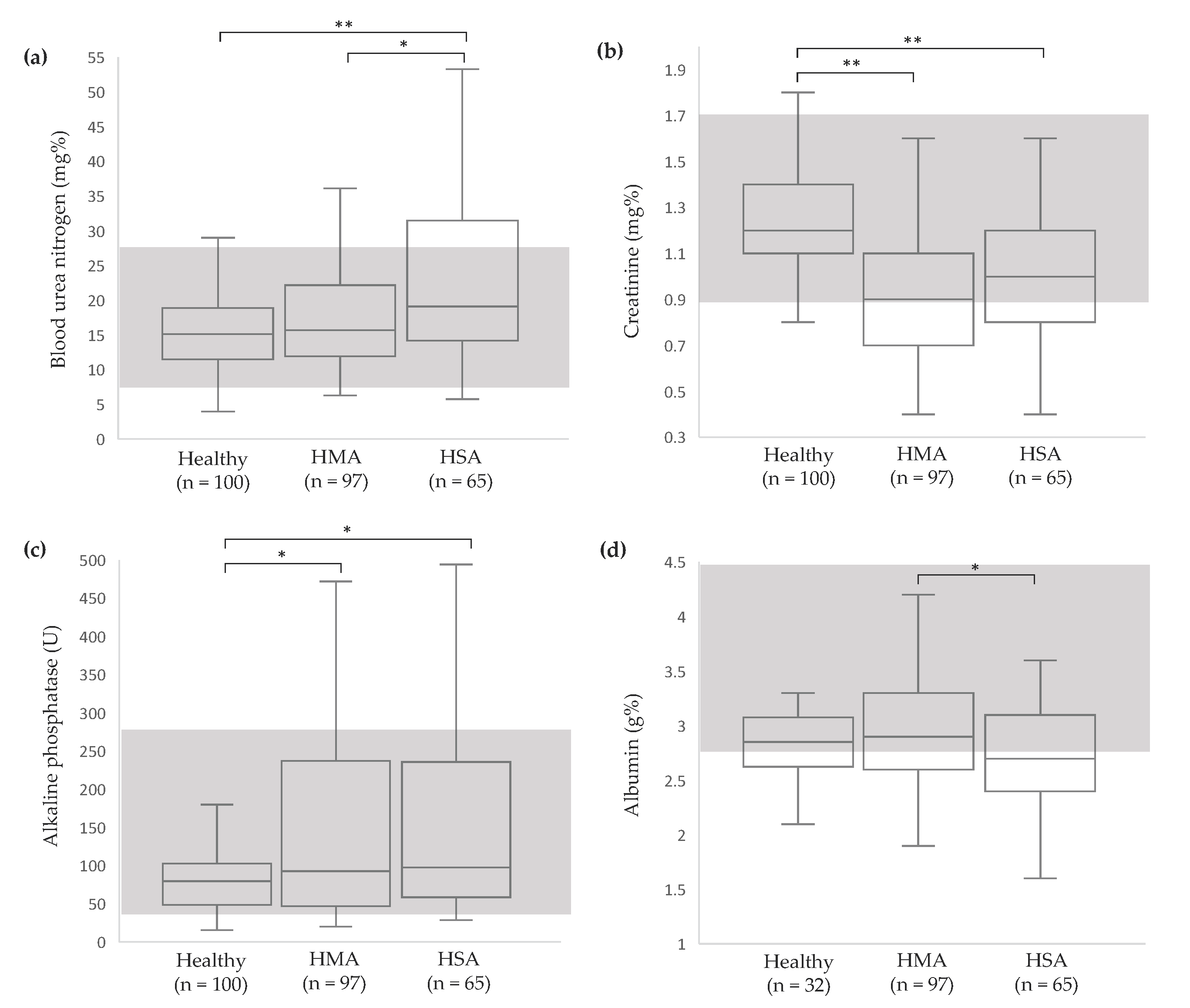
2.2.2. Serum Chemistry
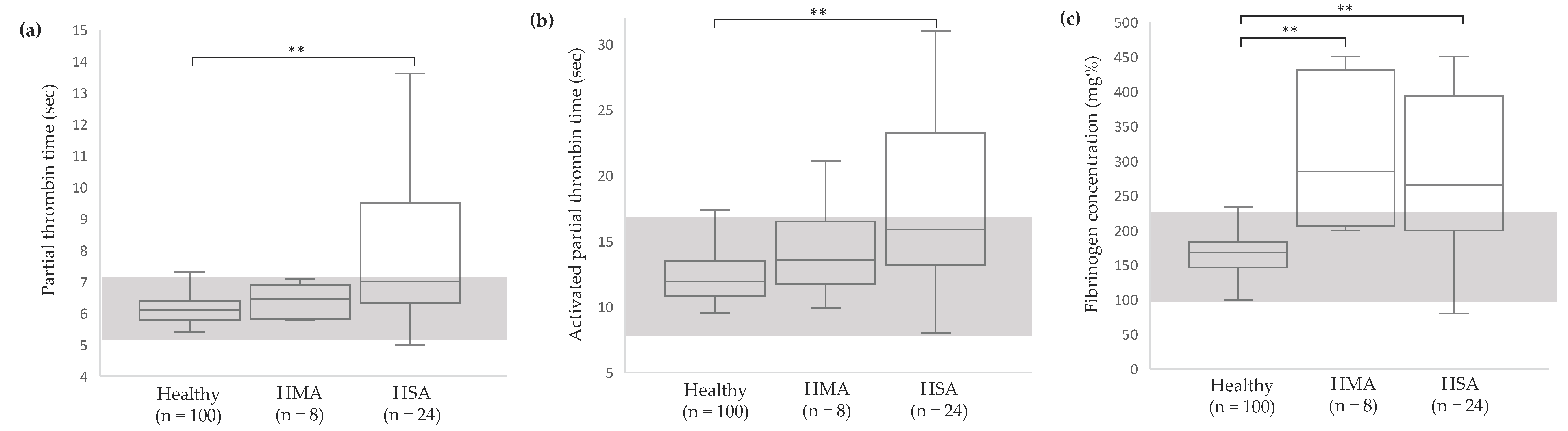
2.2.3. Hemostatic Profile
2.3. Statistical Analysis
3. Results
3.1. Patient Signalment
3.2. Clinicopathological Abnormalities in Dogs with Vascular Neoplasms
3.3. Logistic Regression Analysis of Identifiable Canine Vascular Neoplasms Using Clinicopathological Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Canine and feline haemangiosarcoma. Vet. Rec. 2021, 189, e585. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.; Clifford, C.A. Miscellaneous Tumors: Hemangiosarcoma. In Withrow & MacEwen’s Small animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier, Inc.: St. Louis, MO, USA, 2020; pp. 773–774. [Google Scholar]
- Muller, J.; Henrich, M.; Hoogen-Merkel, J.; Hartung, S. Granulation tissue-type hemangioma in a 6-week-old puppy—A case report. BMC Vet. Res. 2022, 18, 431. [Google Scholar] [CrossRef] [PubMed]
- Lamerato-Kozicki, A.R.; Helm, K.M.; Jubala, C.M.; Cutter, G.C.; Modiano, J.F. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp. Hematol. 2006, 34, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Graef, A.J.; Dickerson, E.B.; Modiano, J.F. Pathobiology of Hemangiosarcoma in Dogs: Research Advances and Future Perspectives. Vet. Sci. 2015, 2, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W. Deep dermal and subcutaneous canine hemangiosarcoma in the perianal area: Diagnosis of perianal mass in a dog. BMC Vet. Res. 2019, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Hargis, A.M.; Feldman, B.F. Evaluation of hemostatic defects secondary to vascular tumors in dogs: 11 cases (1983–1988). J. Am. Vet. Med. Assoc. 1991, 198, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Couto, C.G.; Swardson, C.; Getzy, D. Hemostatic abnormalities in dogs with hemangiosarcoma. J. Vet. Intern. Med. 1991, 5, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Miura, T.; Sakai, M.; Koie, H.; Yamaya, Y.; Shibuya, H.; Sato, T.; Watari, T.; Takeuchi, A.; Tokuriki, M.; et al. The incidence of disseminated intravascular coagulation in dogs with malignant tumor. J. Vet. Med. Sci. 2004, 66, 573–575. [Google Scholar] [CrossRef]
- Hammond, T.N.; Pesillo-Crosby, S.A. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003–2005). J. Am. Vet. Med. Assoc. 2008, 232, 553–558. [Google Scholar] [CrossRef]
- Hendrick, M.J. Mesenchymal Tumors of the Skin and Soft Tissues. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley-Blackwell: Ames, IA, USA, 2017; pp. 142–175. [Google Scholar]
- Bertazzolo, W.; Dell’Orco, M.; Bonfanti, U.; Ghisleni, G.; Caniatti, M.; Masserdotti, C.; Antoniazzi, E.; Crippa, L.; Roccabianca, P. Canine angiosarcoma: Cytologic, histologic, and immunohistochemical correlations. Vet. Clin. Path. 2008, 34, 28–34. [Google Scholar] [CrossRef]
- McCourt, M.R.; Rizzi, T.E. Hematolgy of Dogs. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., Weiss, D.J., Eds.; Wiley-Blackwel: Hoboken, NJ, USA, 2022; pp. 969–982. [Google Scholar]
- Meuten, D.; Sample, S.; Bohn, A.A.; Weiser, G.; Allison, R.W. Clinical Chemistry of Common Domestic Species. In Veterinary Hematology, Clinical Chemistry, and Cytology, 3rd ed.; Thrall, M.A., Weiser, G., Allison, R.W., Campbell, T.W., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2022; pp. 341–586. [Google Scholar]
- Moe, L.; Gamlem, H.; Dahl, K.; Glattre, E. Canine neoplasia--population-based incidence of vascular tumours. APMIS Suppl. 2008, 125, 63–68. [Google Scholar] [CrossRef]
- Davies, O.; Taylor, A.J. Refining the “double two-thirds” rule: Genotype-based breed grouping and clinical presentation help predict the diagnosis of canine splenic mass lesions in 288 dogs. Vet. Comp. Oncol. 2020, 18, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Hargis, A.M.; Ihrke, P.J.; Spangler, W.L.; Stannard, A.A. A retrospective clinicopathologic study of 212 dogs with cutaneous hemangiomas and hemangiosarcomas. Vet. Pathol. 1992, 29, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Gamlem, H.; Nordstoga, K.; Arnesen, K. Canine vascular neoplasia--a population-based clinicopathologic study of 439 tumours and tumour-like lesions in 420 dogs. APMIS Suppl. 2008, 125, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wing, L.Y.; Lin, M.T. Pathogenetic roles of angiogenic factors in pyogenic granulomas in pregnancy are modulated by female sex hormones. J. Periodontol. 2002, 73, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.T.; Friedman, A.B. Hemangiomas and vascular malformations: Current theory and management. Int. J. Pediatr. 2012, 2012, 645678. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Vascular tumors. In Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis, 2nd ed.; Gross, T.L., Ihrke, P.J., Walder, E.J., Affolter, V.K., Eds.; Blackwell Science Ltd.: Ames, IA, USA, 2005; pp. 735–758. [Google Scholar]
- Schultheiss, P.C. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. Investig. 2004, 16, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.; Clifford, C.A. Histiocytic Sarcoma and Hemangiosarcoma Update. Vet. Clin. North. Am. Small Anim. Pract. 2019, 49, 855–879. [Google Scholar] [CrossRef]
- Aronsohn, M.G.; Dubiel, B.; Roberts, B.; Powers, B.E. Prognosis for acute nontraumatic hemoperitoneum in the dog: A retrospective analysis of 60 cases (2003–2006). J. Am. Anim. Hosp. Assoc. 2009, 45, 72–77. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hoshi, K.; Hirakawa, A.; Chimura, S.; Kobayashi, M.; Machida, N. Epidemiological, clinical and pathological features of primary cardiac hemangiosarcoma in dogs: A review of 51 cases. J. Vet. Med. Sci. 2013, 75, 1433–1441. [Google Scholar] [CrossRef]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A descriptive review of cardiac tumours in dogs and cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef]
- Boston, S.E.; Higginson, G.; Monteith, G. Concurrent splenic and right atrial mass at presentation in dogs with HSA: A retrospective study. J. Am. Anim. Hosp. Assoc. 2011, 47, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Gonsalves, M.N.; Huber, M.L.; Rich, L.; Strom, A. Erythrocyte and Biochemical Abnormalities as Diagnostic Markers in Dogs With Hemangiosarcoma Related Hemoabdomen. Vet. Surg. 2015, 44, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.O. Hematologic abnormalities in the small animal cancer patient. Vet. Clin. North. Am. Small Anim. Pract. 2012, 42, 123–155. [Google Scholar] [CrossRef]
- Sharma, D. Hemangiosarcoma in a geriatric Labrador retriever. Can. Vet. J. 2012, 53, 889–892. [Google Scholar] [PubMed]
- Masyr, A.R.; Rendahl, A.K.; Winter, A.L.; Borgatti, A.; Modiano, J.F. Retrospective evaluation of thrombocytopenia and tumor stage as prognostic indicators in dogs with splenic hemangiosarcoma. J. Am. Vet. Med. Assoc. 2021, 258, 630–637. [Google Scholar] [CrossRef]
- Pazzi, P.; Fosgate, G.T.; Rixon, A.; Hanekom, J.; Kristensen, A.T.; Goddard, A. A prospective evaluation of the prevalence of thromboemboli and associated hemostatic dysfunction in dogs with carcinoma or sarcoma. J. Vet. Intern. Med. 2023, 37, 1848–1863. [Google Scholar] [CrossRef]
- Andreasen, E.B.; Tranholm, M.; Wiinberg, B.; Markussen, B.; Kristensen, A.T. Haemostatic alterations in a group of canine cancer patients are associated with cancer type and disease progression. Acta Vet. Scand. 2012, 54, 3. [Google Scholar] [CrossRef]
| Signalments | Variables | No. (%) of Dogs |
|---|---|---|
| Sex | HMA | |
| Male | 56 (54.4) | |
| Female | 47 (45.6) | |
| HSA | ||
| Male | 40 (53.3) | |
| Female | 35 (46.7) | |
| Breeds | HMA | |
| Mixed breed | 49 (47.6) | |
| Golden retriever | 12 (11.6) | |
| Poodle | 10 (9.7) | |
| Labrador retriever | 7 (6.8) | |
| Bangkaew | 5 (4.8) | |
| Beagle | 5 (4.8) | |
| Pomeranian | 3 (2.9) | |
| Other 1 | 12 (11.6) | |
| HSA | ||
| Mixed breed | 30 (40.0) | |
| Golden retriever | 9 (12.0) | |
| Beagle | 6 (8.0) | |
| Siberian husky | 4 (5.3) | |
| Miniature schnauzer | 4 (5.3) | |
| Poodle | 4 (5.3) | |
| Pit bull | 3 (4.0) | |
| Shih tzu | 3 (4.0) | |
| Other 2 | 12 (16.0) | |
| Anatomic locations | HMA | |
| Non-visceral 3 | 96 (93.2) | |
| Spleen | 7 (6.8) | |
| HSA | ||
| Spleen | 37 (49.4) | |
| Non-visceral 3 | 33 (44.3) | |
| Omentum | 3 (3.8) | |
| Liver | 2 (2.5) |
| Variables | Initial Model 1 | Multivariate Model 2 | ||
|---|---|---|---|---|
| p-Value | OR 3 (95% CI) | p-Value | OR 3 (95% CI) | |
| Anemia (by RBC) | 0.002 | 7.00 (1.99–24.51) | 0.012 | 5.49 (1.46–20.65) |
| Anemia (by HGB) | <0.0001 | 4.82 (2.42–9.61) | — | — |
| Anemia (by HCT) | <0.0001 | 4.12 (2.29–7.44) | — | — |
| Hypochromia | 0.063 | 0.13 (0.01–1.11) | — | — |
| Leukocytosis | <0.0001 | 0.29 (0.16–0.56) | — | — |
| Neutrophilia | 0.058 | 0.55 (0.30–1.02) | — | — |
| Lymphopenia | <0.0001 | 97.78 (13.09–730.57) | 0.001 | 32.99 (4.28–254.40) |
| Thrombocytopenia | 0.047 | 0.56 (0.31–0.99) | — | — |
| Prolonged PT | n/a | 1 | — | — |
| Prolonged APTT | 0.072 | 5.26 (0.86–31.61) | — | — |
| Hyperfibrinogenemia | <0.0001 | 40.00 (6.98–229.30) | — | — |
| Increased BUN | 0.034 | 4.76 (1.12–15.31) | — | — |
| Increased creatinine | 0.980 | 1.02 (0.20–5.18) | — | — |
| Increased ALP | n/a | 1 | — | — |
| Hypoalbuminemia | 0.811 | 1.12 (0.44–2.82) | — | — |
| Variables | Initial Model 1 | Multivariate Model 2 | ||
|---|---|---|---|---|
| p-Value | OR 3 (95%CI) | p-Value | OR 3 (95%CI) | |
| Anemia (by RBC) | <0.0001 | 18.17 (5.03–65.61) | — | — |
| Anemia (by HGB) | <0.0001 | 15.97 (7.43–34.31) | 0.011 | 42.50 (2.39–754.14) |
| Anemia (by HCT) | <0.0001 | 15.5 (6.68–36.09) | — | — |
| Hypochromia | 0.463 | 0.59 (0.15–2.39) | — | — |
| Leukocytosis | 0.215 | 0.68 (0.36–1.26) | — | — |
| Neutrophilia | 0.834 | 1.07 (0.65–2.04) | — | — |
| Lymphopenia | <0.0001 | 205.4 (26.74–1577.5) | 0.001 | 345.66 (12.39–9653.14) |
| Thrombocytopenia | 0.515 | 1.22 (0.67–2.24) | — | — |
| Prolonged PT | <0.0001 | 2.74 (6.76–111.23) | — | — |
| Prolonged APTT | <0.0001 | 9.43 (2.92–30.30) | — | — |
| Hyperfibrinogenemia | <0.0001 | 24.39 (6.67–86.43) | 0.004 | 92.73 (4.41–1951.60) |
| Increased BUN | <0.0001 | 12.38 (3.47–44.13) | — | — |
| Increased creatinine | 0.009 | 5.88 (1.55–22.27) | — | — |
| Increased ALP | n/a | 1 | — | — |
| Hypoalbuminemia | 0.144 | 2.03 (0.79–5.23) | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suphonkhan, J.; Klaymongkol, C.; Khomsiri, W.; Wanprom, J.; Jeamsripong, S.; Chimnakboon, N.; Rungsipipat, A.; Radtanakatikanon, A. Retrospective Study of Clinicopathological Changes and Prediction Model for Canine Vascular Neoplasms. Vet. Sci. 2024, 11, 189. https://doi.org/10.3390/vetsci11050189
Suphonkhan J, Klaymongkol C, Khomsiri W, Wanprom J, Jeamsripong S, Chimnakboon N, Rungsipipat A, Radtanakatikanon A. Retrospective Study of Clinicopathological Changes and Prediction Model for Canine Vascular Neoplasms. Veterinary Sciences. 2024; 11(5):189. https://doi.org/10.3390/vetsci11050189
Chicago/Turabian StyleSuphonkhan, Jidapa, Chananchida Klaymongkol, Wijittra Khomsiri, Jedsada Wanprom, Saharuetai Jeamsripong, Narisara Chimnakboon, Anudep Rungsipipat, and Araya Radtanakatikanon. 2024. "Retrospective Study of Clinicopathological Changes and Prediction Model for Canine Vascular Neoplasms" Veterinary Sciences 11, no. 5: 189. https://doi.org/10.3390/vetsci11050189
APA StyleSuphonkhan, J., Klaymongkol, C., Khomsiri, W., Wanprom, J., Jeamsripong, S., Chimnakboon, N., Rungsipipat, A., & Radtanakatikanon, A. (2024). Retrospective Study of Clinicopathological Changes and Prediction Model for Canine Vascular Neoplasms. Veterinary Sciences, 11(5), 189. https://doi.org/10.3390/vetsci11050189





