In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Viruses and Cell Lines
2.3. Cytotoxicity
2.4. Antiviral Assay
2.5. Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
2.6. Confocal Fluorescence Imaging
2.7. Statistical Analysis
3. Results
3.1. Cytotoxicity of the Extracts and Bavachin from P. corylifolia in MA-104 Cells
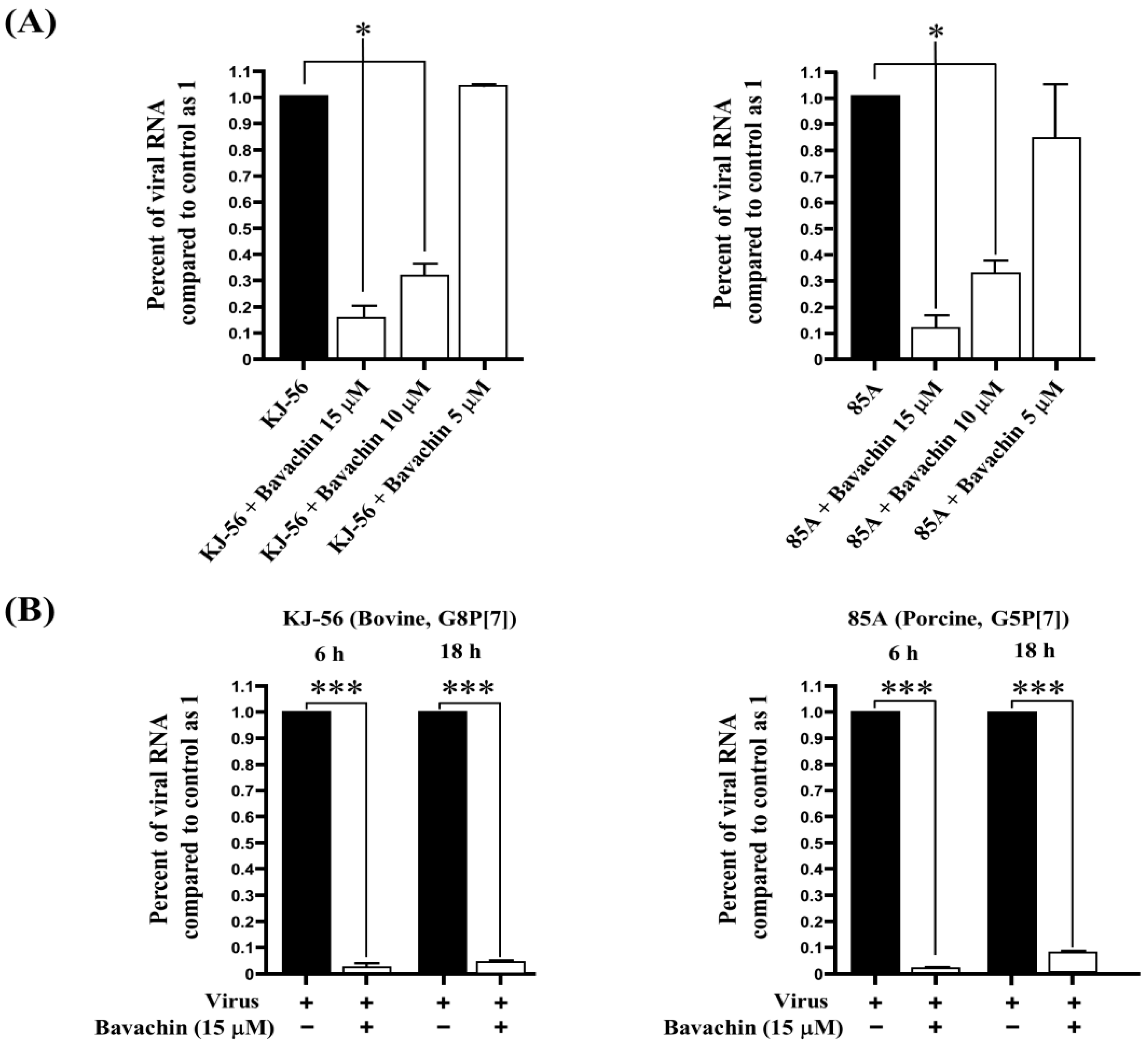
3.2. Antiviral Activity of Bavachin Isolated from P. corylifolia against Rotavirus
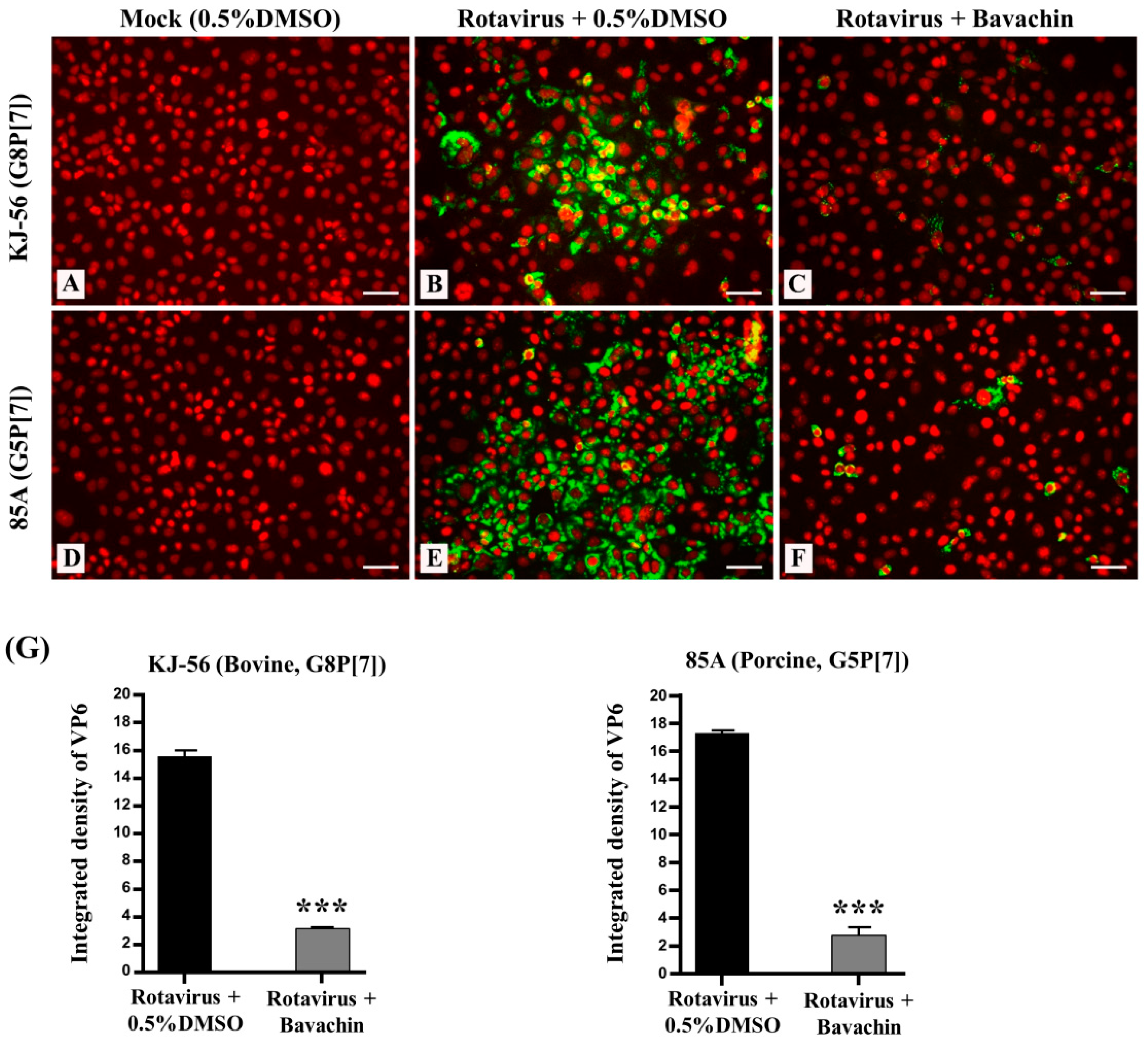
3.3. Inhibition of Viral RNA and Viral Protein Synthesis during Rotavirus Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estes, M.K.; Greenberg, H.B. Rotaviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1917–1974. [Google Scholar]
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Kellner, J.D.; Davies, H.D. Rotavirus gastroenteritis. Ade Ther. 2005, 22, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Téllez, M.A.; Téllez, A.N.; Vélez, F.; Ulloa, J.C. In vitro antiviral activity against rotavirus and astrovirus infection exerted by substances obtained from Achyrocline bogotensis (Kunth) DC. (Compositae). BMC Complement. Altern. Med. 2015, 15, 428. [Google Scholar] [CrossRef] [PubMed]
- Cecílio, A.B.; de Faria, D.B.; de Oliveira, P.C.; Caldas, S.; de Oliveira, D.A.; Sobral, M.E.; Duarte, M.G.; Moreira, C.P.; Silva, C.G.; de Almeida, V.L. Screening of Brazilian medicinal plants for antiviral activity against rotavirus. J. Ethnopharmacol. 2012, 141, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.I.; Roner, M.R. Characterization of in vivo anti-rotavirus activities of saponin extracts from Quillaja saponaria Molina. Antivir. Res. 2011, 90, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Zhou, G.; Zhu, Z.; Cui, Y.; Pu, R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine 2020, 77, 153230. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Bae, J.; Jung, J.; Kim, J.S.; Park, S.J. In vitro antiviral activity of abietane diterpenoids isolated from Torreya nucifera against rotavirus infection. Acta Virol. 2023, 67, 11630. [Google Scholar] [CrossRef]
- Zhu, Y.P. Chinese Material Medica: Chemistry, Pharmacology and Appli Cations; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998; pp. 609–612. [Google Scholar]
- Lim, S.H.; Ha, T.Y.; Ahn, J.; Kim, S. Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents. Phytomedicine 2011, 18, 425–430. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P.; Kumar, A.; Kumar, A.; Sanyal, I. Genus Psoralea: A review of the traditional and modern uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2019, 232, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.J.; Hsin, W.C.; Chen, C.C. Antiplatelet flavonoids from seeds of Psoralea corylifolia. J. Nat Prod. 1996, 59, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Tomonari, Y.; Kawashima, K.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Bavachin induces the apoptosis of multiple myeloma cell lines by inhibiting the activation of nuclear factor kappa B and signal transducer and activator of transcription 3. Biomed. Pharmacother. 2018, 100, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Kim, D.E.; Jang, M.S.; Min, J.S.; Kwon, S. Bavachin produces immunoadjuvant activity by targeting the NFAT signaling pathway. Phytomedicine 2021, 93, 153796. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yu-Feng, S.; Yang, H.; Lei, L.; Wei-Chao, C.; Gao-Xue, W.; Bin, Z. Highly efficient inhibition of spring viraemia of carp virus replication in vitro mediated by bavachin, a major constituent of psoralea corlifonia Lynn. Virus Res. 2018, 255, 24–35. [Google Scholar] [CrossRef]
- Lee, S.W.; Yun, B.R.; Kim, M.H.; Park, C.S.; Lee, W.S.; Oh, H.M.; Rho, M.C. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med. 2012, 78, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kwon, H.J.; Ryu, Y.B.; Chang, J.S.; Cho, K.O.; Hosmillo, M.D.; Rho, M.C.; Park, S.J.; Lee, W.S. Antiviral activity of Alpinia katsumadai extracts against rotaviruses. Res. Vet. Sci. 2012, 92, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kwon, H.J.; Park, J.S.; Jung, J.; Ryu, Y.B.; Kim, W.S.; Lee, J.H.; Jeong, J.H.; Lim, J.S.; Lee, W.S.; et al. Abietane Diterpenoids Isolated from Torreya nucifera Disrupt Replication of Influenza Virus by Blocking the Phosphatidylinositol-3-Kinase (PI3K)-Akt and ERK Signaling Pathway. Curr. Issues Mol. Biol. 2023, 45, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Matsuda, M.; Ohashi, K.; Taniguchi, K.; Nakagomi, O.; Abe, Y.; Mori, S.; Sato, N.; Okutani, K.; Shigeta, S. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antivir. Res. 2001, 49, 15–24. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Liang, L.; Song, L.; Zhao, W. Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected Caco-2 cells. Arch. Virol. 2015, 160, 1421–1433. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, B.; Yang, H.; Ma, T. Shikonin Inhibits Intestinal Calcium-Activated Chloride Channels and Prevents Rotaviral Diarrhea. Front. Pharmacol. 2016, 7, 270. [Google Scholar] [CrossRef]
- Kim, D.W.; Seo, K.H.; Curtis-Long, M.J.; Oh, K.Y.; Oh, J.W.; Cho, J.K.; Lee, K.H.; Park, K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014, 29, 59–63. [Google Scholar] [CrossRef]
- Shoji, M.; Arakaki, Y.; Esumi, T.; Kohnomi, S.; Yamamoto, C.; Suzuki, Y.; Takahashi, E.; Konishi, S.; Kido, H.; Kuzuhara, T. Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation. J. Biol. Chem. 2015, 290, 28001–28017. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, T.X.; Wang, T.Y.; Wang, G.; Liu, Y.G.; Liu, S.G.; Tang, Y.D.; Cai, X.H. Isobavachalcone inhibits post-entry stages of the porcine reproductive and respiratory syndrome virus life cycle. Arch. Virol. 2018, 163, 1263–1270. [Google Scholar] [CrossRef]
- Trejo-Cerro, Ó.; Eichwald, C.; Schraner, E.M.; Silva-Ayala, D.; López, S.; Arias, C.F. Actin-Dependent Nonlytic Rotavirus Exit and Infectious Virus Morphogenetic Pathway in Nonpolarized Cells. J. Virol. 2018, 92, e02076-17. [Google Scholar] [CrossRef]
- Urasawa, T.; Urasawa, S.; Taniguchi, K. Sequential Passages of Human Rotavirus in MA-104 Cells. Microb. Immunol. 1981, 25, 1025–1035. [Google Scholar] [CrossRef]
- Halasz, P.; Holloway, G.; Turner, S.J.; Coulson, B.S. Rotavirus replication in intestinal cells differentially regulates integrin expression by a phosphatidylinositol 3-kinase-dependent pathway, resulting in increased cell adhesion and virus yield. J. Virol. 2008, 82, 148–160. [Google Scholar] [CrossRef]
- Dutta, D.; Bagchi, P.; Chatterjee, A.; Nayak, M.K.; Mukherjee, A.; Chattopadhyay, S.; Nagashima, S.; Kobayashi, N.; Komoto, S.; Taniguchi, K.; et al. The molecular chaperone heat shock protein-90 positively regulates rotavirus infection. Virology 2009, 391, 325–333. [Google Scholar] [CrossRef]
- Wortzel, I.; Seger, R. The ERK cascade: Distinct functions within various subcellular organelles. Genes. Cancer. 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Diehl, N.; Schaal, H. Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Holloway, G.; Coulson, B.S. Rotavirus activates JNK and p38 signaling pathways in intestinal cells, leading to AP-1-driven transcriptional responses and enhanced virus replication. J. Virol. 2006, 80, 10624–10633. [Google Scholar] [CrossRef]
- Jafri, M.; Donnelly, B.; McNeal, M.; Ward, R.; Tiao, G. MAPK signaling contributes to rotaviral-induced cholangiocyte injury and viral replication. Surgery 2007, 142, 192–201. [Google Scholar] [CrossRef]
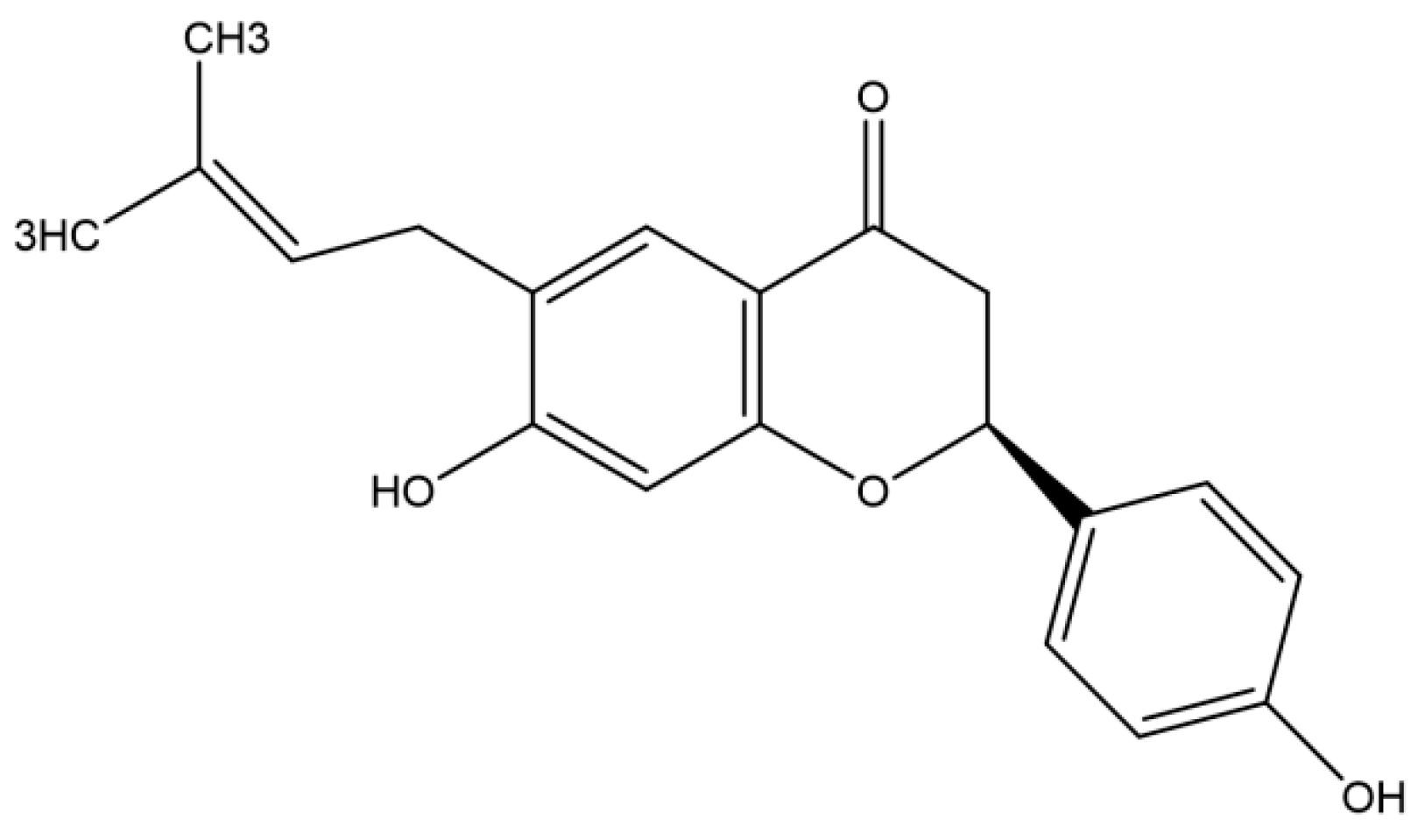
| Extracts or Compounds | CPE Reduction Assay | ||||
|---|---|---|---|---|---|
| CC50 a | KJ-56 (G8P[7]) | 85A (G5P[7]) | |||
| EC50 b | SI c | EC50 b | SI c | ||
| MeOH extract | 25.4 ± 7.0 μg/mL | - | - | 12.4 ± 1.9 μg/mL | 2.05 |
| EtOAc extract | 28.9 ± 5.7 μg/mL | 13.5 ± 4.0 μg/mL | 2.14 | 14.0 ± 0.4 μg/mL | 2.06 |
| Bavachin | 25.2 ± 1.2 μM | 10.6 ± 0.3 μM | 2.38 | 13.0 ± 4.9 μM | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Bae, J.; Park, J.S.; Lee, S.W.; Jeong, J.-H.; Park, S.-J. In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae). Vet. Sci. 2024, 11, 188. https://doi.org/10.3390/vetsci11050188
Jung J, Bae J, Park JS, Lee SW, Jeong J-H, Park S-J. In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae). Veterinary Sciences. 2024; 11(5):188. https://doi.org/10.3390/vetsci11050188
Chicago/Turabian StyleJung, Jinseok, Jaehoon Bae, Ji Sun Park, Seung Woong Lee, Jae-Ho Jeong, and Su-Jin Park. 2024. "In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae)" Veterinary Sciences 11, no. 5: 188. https://doi.org/10.3390/vetsci11050188
APA StyleJung, J., Bae, J., Park, J. S., Lee, S. W., Jeong, J.-H., & Park, S.-J. (2024). In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae). Veterinary Sciences, 11(5), 188. https://doi.org/10.3390/vetsci11050188







