Ectoparasites Infestation to Small Ruminants and Practical Attitudes among Farmers toward Acaricides Treatment in Central Region of Java, Indonesia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
2.2. Animals
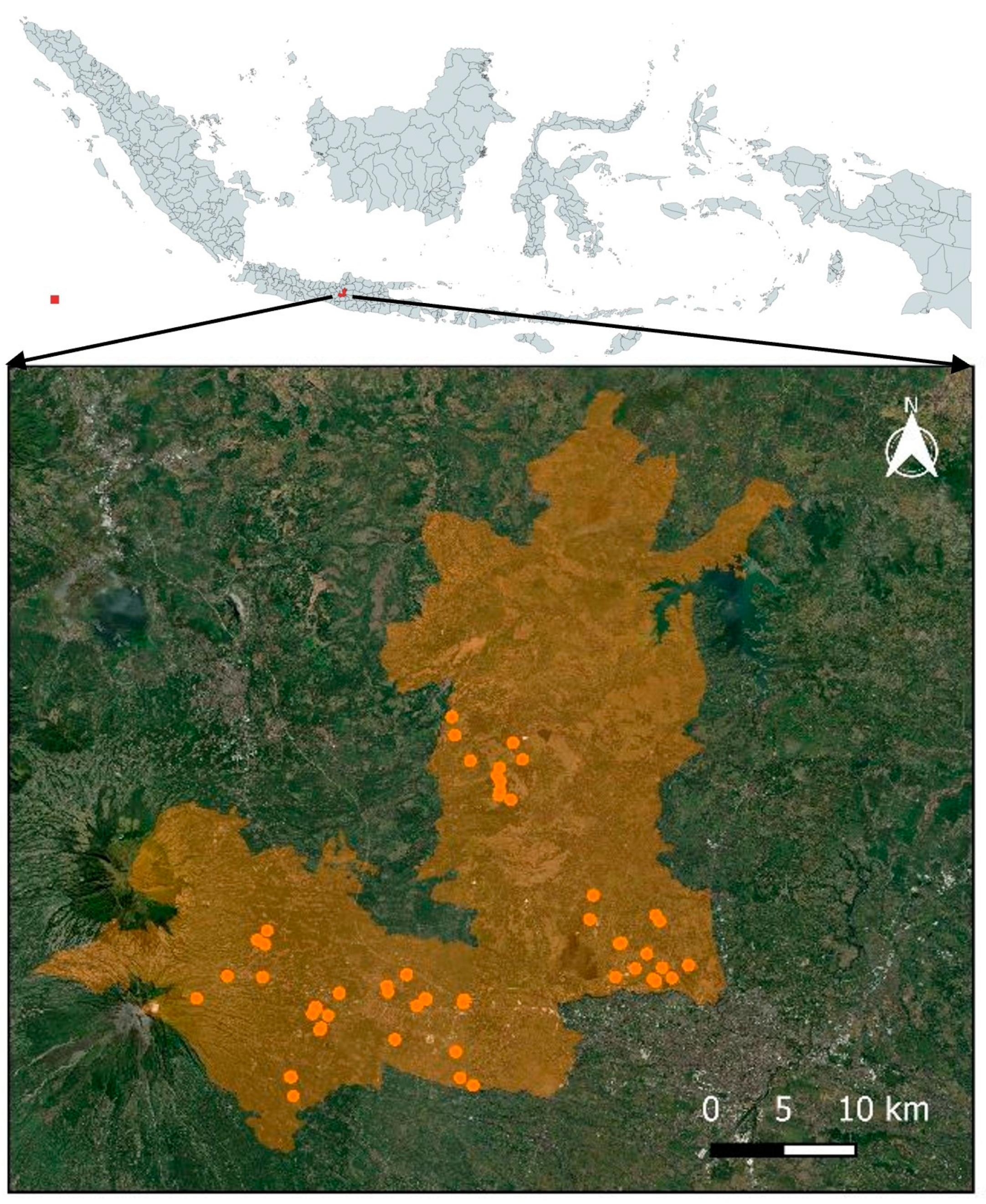
2.3. Locations
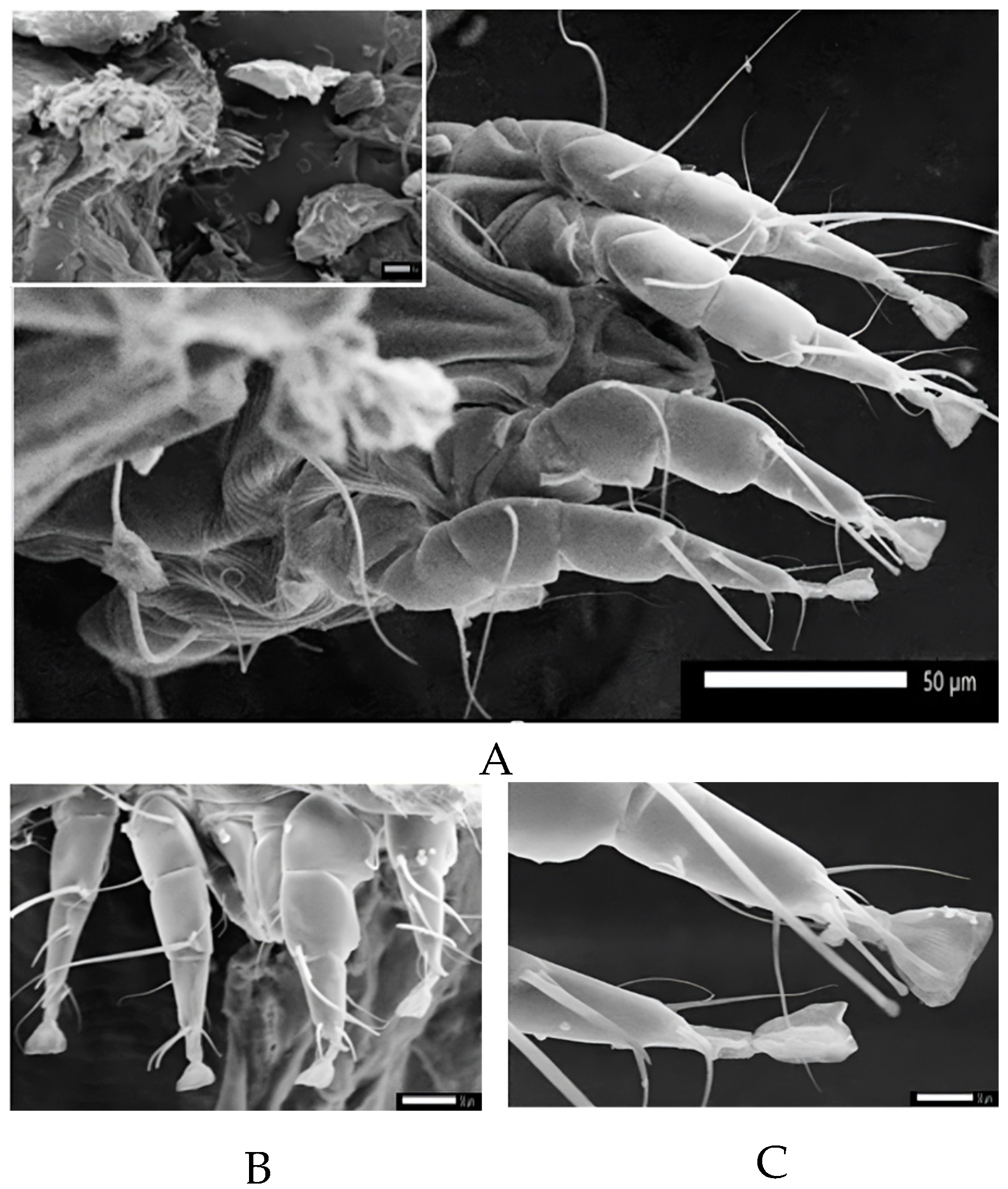
2.4. Parasites Collection and Identifications
2.5. Farmers Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Results
3.2. Lice Infestation
3.3. Mange Infestation
3.4. Tick Infestation
3.5. Flea Infestation
3.6. Farmers’ Knowledge and Practices to Ectoparasites Cases in Boyolali
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistik, B.P. Populasi Ternak Menurut Kecamatan dan Jenis Ternak di Kabupaten Boyolali 2018–2020; Badan Pusat Statistik Kabupaten Boyolali: Jawa Tengah, Indonesia, 2021. [Google Scholar]
- Udo, H.M.J.; Budisatria, I.G.S. Fat-tailed sheep in Indonesia; an essential resource for smallholders. Trop. Anim. Health Prod. 2011, 43, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Budisatria, I.G.S.; Udo, H.M.; van der Zijpp, A.J.; Baliarti, E.; Murti, T.W. Religious Festivities and Marketing of Small Ruminants in Central Java–Indonesia. Asian J. Agric. Dev. 2008, 5, 57–73. [Google Scholar] [CrossRef]
- Tiesnamurti, B.; Sinulingga, S.; Gatenby, R.J.W. Small Ruminant Community Breeding Program in Indonesia. Wartazoa 2020, 30, 163–175. [Google Scholar] [CrossRef]
- Akinmoladun, O.F.; Muchenje, V.; Fon, F.N.; Mpendulo, C.T. Small ruminants: Farmers’ hope in a world threatened by water scarcity. Animals 2019, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Budisatria, I.; Udo, H.; Eilers, C.; Van Der Zijpp, A. Dynamics of small ruminant production: A case study of Central Java, Indonesia. Outlook Agric. 2007, 36, 145–152. [Google Scholar] [CrossRef]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. A Textbook of the Diseases of Cattle, Sheep, Goats, Pigs and Horses; Canadian Veterinary Medical Association: Booth St Ottawa, ON, Canada, 2007; pp. 1576–1580. [Google Scholar]
- Iqbal, A.; Sajid, M.S.; Khan, M.N.; Muhammad, G. Epizootiology of Ectoparasitic Fauna Infesting Selected Domestic Cattle Population of Punjab, Pakistan. Int. J. Agric. Biol. 2014, 16, 443–446. [Google Scholar]
- Sahito, H.A.; Kousar, T.; Mughal, M.A.; Mangrio, W.M.; Shah, Z.; Ghumro, B. Prevalence of cattle lice; Haematopinus tuberculastus and Ticks; Haemaphysalis bispinosa on cattle at region Sukkur, Sindh-Pakistan. Int. J. Res. Stud. Biosci. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Tirosh-Levy, S.; Gottlieb, Y.; Mumcuoglu, K.Y.; Steinman, A.J.P. Species distribution and seasonal dynamics of equine tick infestation in two Mediterranean climate niches in Israel. Parasites Vectors 2018, 11, 546. [Google Scholar] [CrossRef]
- Pavela, R.; Canale, A.; Mehlhorn, H.; Benelli, G.J.R.i.v.s. Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: A review. Res. Vet. Sci. 2016, 109, 1–9. [Google Scholar] [CrossRef]
- Ahmed, J.S.; Luo, J.; Schnittger, L.; Seitzer, U.; Jongejan, F.; Yin, H. Phylogenetic position of small-ruminant infecting piroplasms. Ann. N. Y. Acad. Sci. 2006, 1081, 498–504. [Google Scholar] [CrossRef]
- Khalil, S.; Abbas, O.; Kibbi, A.G.; Kurban, M. Scabies in the age of increasing drug resistance. PLoS Neglected Trop. Dis. 2017, 11, e0005920. [Google Scholar] [CrossRef]
- Mencke, N.; Larsen, K.S.; Eydal, M.; Sigurðsson, H. Natural infestation of the chewing lice (Werneckiella equi) on horses and treatment with imidacloprid and phoxim. Parasitol. Res. 2004, 94, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Wright, R. Lice on horses. Can. Vet. J. 1999, 40, 590. [Google Scholar] [PubMed]
- Littlewood, J.D.; Rose, J.F.; Paterson, S. Oral ivermectin paste for the treatment of chorioptic mange in horses. Vet. Rec. 1995, 137, 661–663. [Google Scholar] [PubMed]
- Osman, S.; Hanafy, A.; Amer, S. Clinical and therapeutic studies on mange in horses. Vet. Parasitol. 2006, 141, 191–195. [Google Scholar] [CrossRef]
- Mencke, N.; Larsen, K.S.; Eydal, M.; Sigurðsson, H. Dermatological and parasitological evaluation of infestations with chewing lice (Werneckiella equi) on horses and treatment using imidacloprid. Parasitol. Res. 2005, 97, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.d.S.; Daemon, E.; Monteiro, C.M.d.O.; Maturano, R.; Brito, F.C.; Massoni, T. Acaricidal activity of thymol on larvae and nymphs of Amblyomma cajennense (Acari: Ixodidae). Vet. Parasitol. 2011, 183, 136–139. [Google Scholar] [CrossRef]
- French, D.; Craig, T.; Hogsette, J.; Pelzel-McCluskey, A.; Mittel, L.; Morgan, K.; Pugh, D.; Vaala, W. External Parasites and Vector Control Guidelines; American association of Equine Practitioners: Lexington, KY, USA, 2016. [Google Scholar]
- Martin, S.J. Acaricide (pyrethroid) resistance in Varroa destructor. Bee World 2004, 85, 67–69. [Google Scholar] [CrossRef]
- Chosidow, O. Scabies. N. Engl. J. Med. 2006, 354, 1718–1727. [Google Scholar] [CrossRef]
- Thullner, F.; Willadsen, P.; Kemp, D. Acaricide rotation strategy for managing resistance in the tick Rhipicephalus (Boophilus) microplus (Acarina: Ixodidae): Laboratory experiment with a field strain from Costa Rica. J. Med. Entomol. 2007, 44, 817–821. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Syamsul, V.S.; Okene, I.A.-A.; Yahya, S.N.C.; Hamdan, R.H.; Lee, S.H.; Tan, L.P. Prevalence of ectoparasitism on small ruminants in Kelantan, Malaysia. Trop. Life Sci. Res. 2020, 31, 45. [Google Scholar] [CrossRef]
- Laksono, T.T.; Yuliani, G.A.; Sunarso, A.; Lastuti, N.D.R.; Suwanti, L.T. Prevalence and Saverity Level of Scabies (Sarcoptes scabiei) on Rabbits in Sajen Village, Pacet SUB-District, Mojokerto Regency. J. Parasite Sci. (JoPS) 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Ahmad, M.; Zafar, M.; Sultana, S.; Ahmad, M.; Abbas, Q.; Ayoub, M.; Bahadur, S.; Ullah, F. Identification of green energy ranunculaceous flora of district Chitral, Northern Pakistan using pollen features through scanning electron microscopy. Microsc. Res. Tech. 2018, 81, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Perveen, F.; Jabeen, T.; Ali, N.; Yasmin, N.; Navqi, S.N.H. Distribution of fleas (Siphonaptera) on livestock and stockmen in Mansehra, Pakistan. Pak. J. Entomol. Karachi 2011, 25, 5. [Google Scholar]
- Jaiswal, K.; Mishra, S.; Bee, A. Prevalence of Gastrointestinal Helminth Parasites in Gallus gallus domesticus in Lucknow, U. P, India. Adv. Zool. Bot. 2020, 8, 422–430. [Google Scholar] [CrossRef]
- Seyoum, Z.; Tadesse, T.; Addisu, A. Ectoparasites prevalence in small ruminants in and around Sekela, Amhara Regional State, Northwest Ethiopia. J. Vet. Med. 2015, 2015, 216085. [Google Scholar] [CrossRef]
- Guswanto, A.; Allamanda, P.; Mariamah, E.S.; Sodirun, S.; Wibowo, P.E.; Indrayani, L.; Nugroho, R.H.; Wirata, I.K.; Jannah, N.; Dias, L.P. Molecular and serological detection of bovine babesiosis in Indonesia. Parasites Vectors 2017, 10, 550. [Google Scholar] [CrossRef]
- Hamid, P.H.; Cahyadi, M.; Whardana, A.H.; Savitri, D.H.; Arvita, N.; Insyariati, T.; Kurnianto, H.; Hermosilla, C.R. First Autochthonous Report on Cattle Babesia naoakii in Central Java, Indonesia, and Identification of Haemaphysalis bispinosa Ticks in the Investigated Area. Pathogens 2023, 12, 59. [Google Scholar] [CrossRef]
- Gubbels, M.-J.; Yin, H.; Bai, Q.; Liu, G.; Nijman, I.J.; Jongejan, F. The phylogenetic position of the Theileria buffeli group in relation to other Theileria species. Parasitol. Res. 2002, 88, S28–S32. [Google Scholar] [CrossRef]
- Blieck, L.; Kaligis, J. Pseudokustkoorts en anaplasmosis bij buffels op Java. Veearts Bl. 1912, 24, 253–260. [Google Scholar]
- Mahatmi, H.; Setiyono, A.; Soejoedono, R.; Pasaribu, F. Deteksi Coxiella burnetii penyebab Q fever pada sapi, domba dan kambing di Bogor dan Bali. J. Vet. 2007, 8, 180–187. [Google Scholar]
- Aulyani, T.L.; Fatullah, A.L.; Nuraeni, N.; Andy, A. Prevalensi Scabies pada Kambing di Kecamatan Bontomatene Kabupaten Kepulauan Selayar: Prevalence of Scabies on Goats in Bontomatene District, Kepulauan Selayar. J. Agrisistem 2022, 18, 71–75. [Google Scholar] [CrossRef]
- Nugroho, Y.D.; Humaidah, N.; Suryanto, D. Studi Kajian Prevalensi Scabies pada Kambing di Kecamatan Paloh Kabupaten Sambas. Din. Rekasatwa J. Ilm. 2022, 5, 266–274. [Google Scholar]
- Rotan, H.; Ginting, Y.; Loesnihari, R.; Kembaren, T.; Marpaung, B. Correlation between chronic arthritis patients confirmed with questionnaire and serologic test of Lyme disease. In IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 15–18 November 2017; IOP Publishing: Bristol, UK, 2018; Volume 125, p. 012043. [Google Scholar]
- Nururrozi, A.; Indarjulianto, S.; Yanuartono, Y.; Purnamaningsih, H.; Raharjo, S.; Rusmihayati, R. Bovine Ephemeral Fever (BEF): Penyebab, Epidemiologi, Diagnosa, dan Terapi. J. Sain Vet. 2020, 38, 77–91. [Google Scholar] [CrossRef]
- Lubis, C.N.B.; Suwandono, A.; Sakundarno, M. Gambaran Perilaku Masyarakat Terhadap Risiko Penyakit Pes Pada Dusun Fokus Dan Dusun Terancam Pes. J. Kesehat. Masy. 2016, 4, 334–340. [Google Scholar] [CrossRef]
- Wardhana, A.H.; Muharsini, S. Kasus Myasis yang disebabkan oleh Chrysomya bezziana di Pulau Jawa. In Proceedings of the Prosiding Seminar Nasional Teknologi Peternakan dan Veteriner, Bogor, Indonesia, 12–13 September2005; pp. 12–13. [Google Scholar]
- Suartha, I.N.; Septyawati, R.; Gunata, I.K. Bentuk dan sebaran lesi demodekosis pada sapi bali. J. Vet. 2014, 15, 395–400. [Google Scholar]
- Sari, N.V.V.; Sunarso, A.; Harijani, N.; Suprihati, E.; Hastutiek, P. Prevalence of Ectoparasites in Bean Goats on the Sub-District of Prambon, District of Nganjuk. J. Parasite Sci. 2020, 4, 7–10. [Google Scholar]
- Rahmadani, I. Prevalensi Ektoparasit Pada Kambing Saanen (Capra aegagrus hircus.) Yang Diberi Ransum Daun Pepaya (Carica papaya L.) dan Konsentrat di Kecamatan Sunggal, Kabupaten Deli Serdang, Sumatera Utara; Universitas Sumatera Utara: Medan, Indonesia, 2022. [Google Scholar]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.-U.; Cabezas-Cruz, A.; Jabbar, A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef]
- Kumar, A.; Rawat, B.; Saxena, A.; Agarwal, G. Prevalence of ectoparasites on goats in Dehradun (India). Appl. Parasitol. 1994, 35, 227–236. [Google Scholar]
- Yakhchali, M.; Hosseine, A. Prevalence and ectoparasites fauna of sheep and goats flocks in Urmia suburb, Iran. Vet. Arh. 2006, 76, 431–442. [Google Scholar]
- Ugochukwu, E.; Apeh, A. Prevalence of ectoparasites of small ruminants in Nsukka, Nigeria. Int. J. Zoonoses 1985, 12, 313–317. [Google Scholar] [PubMed]
- Yasine, A.; Kumsa, B.; Hailu, Y.; Ayana, D. Mites of sheep and goats in Oromia Zone of Amhara Region, North Eastern Ethiopia: Species, prevalence and farmers awareness. BMC Vet. Res. 2015, 11, 122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gebreselama, M.; Zeru, F.; Romha, G. Identification and prevalence of ectoparasites in cattle and sheep in and around Bishoftu town, central Ethiopia. Anim. Vet. Sci. 2014, 2, 124–129. [Google Scholar] [CrossRef]
- Kusiluka, L.; Kambarage, D.; Matthewman, R.; Daborn, C.; Harrison, L. Prevalence of ectoparasites of goats in Tanzania. J. Appl. Anim. Res. 1995, 7, 69–74. [Google Scholar] [CrossRef]
- Cornall, K.; Wall, R. Ectoparasites of goats in the UK. Vet. Parasitol. 2015, 207, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.; Santos, A.; Guerra, R.M. Ectoparasites in goat and sheep folks from Alto Mearim and Grajaú Microregion, state of Maranhão. Rev. Bras. Parasitol. Vet. 2005, 14, 59–63. [Google Scholar] [PubMed]
- Ahmed Ali, B.; Abdulqader Naqid, I.; Kadir Zangana, I. Distribution of Ectoparasites Infested Sheep and Goats in Duhok Province, North Iraq. Basrah J. Vet. Res. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Taylor, M.; Coop, R.; Wall, R. Veterinary Parasitology, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar]
- Ajith, Y.; Dimri, U.; Gopalakrishnan, A.; Devi, G. A study on prevalence and factors associated with ectoparasitism in goats of two agro-climatic regions in India. J. Parasit. Dis. 2017, 41, 739–746. [Google Scholar] [CrossRef]
- El-Seify, M.; Ga, Z.; Aggour, M. Some Studies on Lice Infesting Goats in Beni-Suef, Middle Egypt. Assiut Vet. Med. J. 1990, 23, 64–74. [Google Scholar] [CrossRef]
- Gabaj, M.; Beesley, W.; Awan, M. Lice of farm animals in Libya. Med. Vet. Entomol. 1993, 7, 138–140. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.B.; Cançado, P.H.D.; Piranda, E.M.; Faccini, J.L.H. Infestações Por Linognathus africanus (Kellogg e Paine, 1911)(Linognathidae) e Bovicola caprae (Ewing, 1936)(Trichodectidae) em Rebanho caprino no estado do rio de Janeiro, Brasil. Rev. Bras. Parasitol. Veterinária 2006, 15, 41–43. [Google Scholar]
- Asp, J.; Tauni, M. Skin diseases on Ethiopian sheep and their effect on the pickled skin. A minor field study. Swed. Univ. Agric. Sci. 1988, 89, 29. [Google Scholar]
- Heath, A.; Bishop, D.; Cole, D.; Pfeffer, A. The development of cockle, a sheep pelt defect, in relation to size of infestation and time of exposure to Bovicola ovis, the sheep-biting louse. Vet. Parasitol. 1996, 67, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Armstrong, R. Inspection of wool lots at sales as a diagnostic test for louse infestation. Vet. Parasitol. 2000, 90, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.L.; Shearer, D. Veterinary Ectoparasites: Biology, Pathology and Control; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Arlian, L.G.; Morgan, M.S. A review of Sarcoptes scabiei: Past, present and future. Parasites Vectors 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Lavin, S.; Ruiz-Bascaran, M.; Marco, I.; Fondevila, M.; Ramis, A.J. Experimental infection of chamois (Rupicapra pyrenaica parva) with Sarcoptes scabiei derived from naturally infected goats. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; Al-Quraishy, S.; Mares, M.M. Survey of mange mite infesting sheep in Riyadh region, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 595–600. [Google Scholar] [CrossRef]
- Lusat, J.; Morgan, E.; Wall, R. Mange in alpacas, llamas and goats in the UK: Incidence and risk. Vet. Parasitol. 2009, 163, 179–184. [Google Scholar] [CrossRef]
- Kollbrunner, M.; Luginbühl, A.; Pfister, K. Epidemiologische Aspekte zur Chorioptes-Räude bei Milchkühen in der Schweiz: Eine Felduntersuchung. Im. Druck. 2010, 152, 231–236. [Google Scholar] [CrossRef]
- Kho, K.-L.; Koh, F.-X.; Jaafar, T.; Hassan Nizam, Q.N.; Tay, S.-T. Prevalence and molecular heterogeneity of Bartonella bovis in cattle and Haemaphysalis bispinosa ticks in Peninsular Malaysia. BMC Vet. Res. 2015, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.L.; Ng, A. The detection of three new Haemaphysalis ticks (Acari: Ixodidae) in Singapore and their potential threat for public health, companion animals, and wildlife. Acarologia 2022, 62, 927–940. [Google Scholar] [CrossRef]
- Naren Babu, N.; Jayaram, A.; Hemanth Kumar, H.; Pareet, P.; Pattanaik, S.; Auti, A.M.; Abdulmajeed, J.; Maity, H.; Devadiga, S.; Bhandari, Y. Spatial distribution of Haemaphysalis species ticks and human Kyasanur Forest Disease cases along the Western Ghats of India, 2017–2018. Exp. Appl. Acarol. 2019, 77, 435–447. [Google Scholar] [CrossRef]
- Ghosh, S.; Bansal, G.C.; Gupta, S.C.; Ray, D.; Khan, M.Q.; Irshad, H.; Shahiduzzaman, M.; Seitzer, U.; Ahmed, J.S. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol. Res. 2007, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Tuvshintulga, B.; Zhyldyz, A.; Kothalawala, H.; Yapa, P.R.; Kanagaratnam, R.; Vimalakumar, S.C.; Abeysekera, T.S.; Weerasingha, A.S.; Yamagishi, J. Genetic analysis of Babesia isolates from cattle with clinical babesiosis in Sri Lanka. J. Clin. Microbiol. 2018, 56, e00895-18. [Google Scholar] [CrossRef] [PubMed]
- Vongphayloth, K.; Apanaskevich, D.A.; Lakeomany, K.; Phommavanh, N.; Sinh Nam, V.; Fiorenzano, J.M.; Hertz, J.C.; Sutherland, I.W.; Brey, P.T.; Robbins, R.G. The Genus Haemaphysalis (Acari: Ixodidae) in Laos: An Update of Species Records and a Review of Taxonomic Challenges. J. Med. Entomol. 2022, 59, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.; Fryxell, T.R.; Hickling, G.J.; Vail, K.; Ivey, J. Asian Longhorned Tick; Virginia Tech: Blacksburg, VA, USA, 2019. [Google Scholar]
- Sertse, T.; Wossene, A.J.S.R.R. A study on ectoparasites of sheep and goats in eastern part of Amhara region, northeast Ethiopia. Small Rumin. Res. 2007, 69, 62–67. [Google Scholar] [CrossRef]
- Obasaju, M.; Otesile, E. Ctenocephalides canis infestation of sheep and goats. Trop. Anim. Health Prod. 1980, 12, 116–118. [Google Scholar] [CrossRef]
- Dahm, J.R.; Bailey, J.B.; Kelly, R.F.; Chikungwa, P.; Chulu, J.; Junior, L.C.; Freeman, E.J.; Mayer, D.; Mazeri, S.; Sargison, N.D. Risk factors associated with Ctenocephalides felis flea infestation of peri-urban goats: A neglected parasite in an under-appreciated host. Trop. Anim. Health Prod. 2021, 53, 181. [Google Scholar] [CrossRef]
- Güvendi, M.; Can, H.; Köseoğlu, A.E.; Alak, S.E.; Kandemir, Ç.; Taşkın, T.; Sürgeç, E.; Demir, S.; Döşkaya, A.D.; Karakavuk, M. Investigation of the genetic diversity and flea-borne pathogens in Ctenocephalides felis samples collected from goats in İzmir and Şanlıurfa provinces of Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2022, 90, 101896. [Google Scholar] [CrossRef]
- Kesuma, A.P. Pinjal (Fleas). BALABA J. Litbang Pengendali. Penyakit Bersumber Binatang Banjarnegara 2007, ed. 004, 20. [Google Scholar]
- Eisen, R.J.; Borchert, J.N.; Holmes, J.L.; Amatre, G.; Van Wyk, K.; Enscore, R.E.; Babi, N.; Atiku, L.A.; Wilder, A.P.; Vetter, S.M. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am. J. Trop. Med. Hyg. 2008, 78, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Civen, R.; Ngo, V. Murine typhus: An unrecognized suburban vectorborne disease. Clin. Infect. Dis. 2008, 46, 913–918. [Google Scholar] [CrossRef] [PubMed]


| Infestations | Goat (n = 537) | Sheep (n = 114) | ||
|---|---|---|---|---|
| Prevalence (%) | 95% CI | Prevalence (%) | 95% CI | |
| Mite | 6.30 | 4.27–8.40 | - | - |
| Tick | 4.30 | 2.57–6.00 | - | - |
| Lice | 97.40 | 96.04–98.74 | 100.0 | 100.00 |
| Flea | 0.20 | 0.00–1.03−018–055 | - | - |
| Overall | 97.4 | 96.04–98.74 | 100.0 | 100.00 |
| Breed | n | Ectoparasite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mite | Tick | Lice | Flea | Total | |||||||
| Positive (n) | % | Positive (n) | % | Positive (n) | % | Positive (n) | % | Positive (n) | % | ||
| Goat | |||||||||||
| Boer | 2 | 1 | 50.0 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | 2 | 100.0 |
| Jawarandu | 375 | 29 | 7.7 | 23 | 6.1 | 361 | 96.3 | 1 | 0.3 | 361 | 96.3 |
| Boer × Jawarandu | 81 | 2 | 2.5 | 0 | 0.0 | 81 | 100.0 | 0 | 0.0 | 81 | 100.0 |
| Ettawa crossbred | 37 | 0 | 0.0 | 0 | 0.0 | 37 | 100.0 | 0 | 0.0 | 37 | 100.0 |
| Saanen | 6 | 0 | 0.0 | 0 | 0.0 | 6 | 100.0 | 0 | 0.0 | 6 | 100.0 |
| Sapera | 36 | 2 | 5.6 | 0 | 0.0 | 36 | 100.0 | 0 | 0.0 | 36 | 100.0 |
| Total | 537 | 34 | 6.3 | 23 | 4.3 | 523 | 97.4 | 1 | 0.2 | 523 | 97.4 |
| Sheep | |||||||||||
| Merino | 12 | 0 | 0.0 | 0 | 0.0 | 12 | 100.0 | 0 | 0.0 | 12 | 100.0 |
| Thin-tailed | 102 | 0 | 0.0 | 0 | 0.0 | 102 | 100.0 | 0 | 0.0 | 102 | 100.0 |
| Total | 114 | 0 | 0.0 | 0 | 0.0 | 114 | 100.0 | 0 | 0.0 | 114 | 100.0 |
| Overall | 651 | 34 | 5.2 | 23 | 3.5 | 637 | 97.8 | 1 | 0.2 | 637 | 97.8 |
| Study Area (Subdistrict) | n | Ectoparasite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mite | Tick | Lice | Flea | Overall | |||||||
| Positive (n) | % | Positive (n) | % | Positive (n) | % | Positive (n) | % | Positive (n) | % | ||
| Boyolali | 155 | 2 | 1.3 | 0.0 | 0.0 | 155 | 100.0 | 1 | 0.6 | 155 | 100.0 |
| Cepogo | 30 | 2 | 6.7 | 0 | 0.0 | 30 | 100.0 | 0 | 0.0 | 30 | 100.0 |
| Ngemplak | 63 | 5 | 7.9 | 7 | 11.1 | 63 | 100.0 | 0 | 0.0 | 63 | 100.0 |
| Karanggede | 38 | 2 | 5.3 | 0 | 0.0 | 38 | 100.0 | 0 | 0.0 | 38 | 100.0 |
| Klego | 7 | 3 | 42.9 | 7 | 100.0 | 7 | 100.0 | 0 | 0.0 | 7 | 100.0 |
| Musuk | 31 | 6 | 19.4 | 9 | 29.0 | 17 | 54.8 | 0 | 0.0 | 17 | 54.8 |
| Mojosongo | 111 | 3 | 2.7 | 0 | 0.0 | 111 | 100.0 | 0 | 0.0 | 111 | 100.0 |
| Nogosari | 117 | 9 | 7.7 | 0 | 0.0 | 117 | 100.0 | 0 | 0.0 | 117 | 100.0 |
| Teras | 99 | 2 | 2.0 | 0 | 0.0 | 99 | 100.0 | 0 | 0.0 | 99 | 100.0 |
| Overall | 651 | 34 | 5.2 | 23 | 3.5 | 637 | 97.8 | 1 | 0.2 | 637 | 97.8 |
| Ectoparasites | Age | p Value | Sex | p Value | Housing Type | p Value | Management System | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <2 Years | >2 Years | Male | Female | Individu | Flock | Grazing | Non-Grazing | |||||
| (n = 372) | (n = 279) | (n = 64) | (n = 586) | (n = 102) | (n = 549) | (n = 38) | (n = 613) | |||||
| Mite | 20 (5.4%) | 14 (5.0%) | >0.05 | 6 (9.4%) | 28 (4.8%) | >0.05 | 8 (7.8%) | 26 (4.7%) | >0.05 | 2 (5.3%) | 32 (5.3%) | >0.05 |
| Tick | 10 (2.7%) | 13 (4.7%) | >0.05 | 5 (7.8%) | 18 (3.1) | >0.05 | 6 (5.9%) | 17 (3.1%) | >0.05 | 0 (0.0%) | 23 (3.8%) | >0.05 |
| Lice | 364 (97.8%) | 273 (97.8%) | >0.05 | 64 (100.0%) | 572 (97.6%) | >0.05 | 102 (100.0%) | 535 (97.4%) | >0.05 | 38 (100.0%) | 599 (97.7%) | >0.05 |
| Flea | 1 (0.3%) | 0 (0.0%) | >0.05 | 1 (1.6%) | 0 (0.0%) | >0.05 | 1 (1.0%) | 0 (0.0%) | >0.05 | 0 (0.0%) | 1 (0.2%) | >0.05 |
| Overall | 364 (97.8%) | 273 (97.8%) | >0.05 | 64 (100.0%) | 572 (97.6%) | >0.05 | 102 (100.0%) | 535 (97.4%) | >0.05 | 38 (100.0%) | 599 (97.7%) | >0.05 |
| Variable | Category | No. of Response (%) |
|---|---|---|
| Gender | Male | 89.7 |
| Female | 10.3 | |
| Ages | 18–35 | 12.8 |
| 36–45 | 23.1 | |
| >46 | 64.1 | |
| Education | No formal education | 0 |
| Elementary school (6 years) | 23.1 | |
| Junior high school (9 years) | 2.6 | |
| Senior high school (12 years) | 48.7 | |
| High education (university level) | 25.6 | |
| Total small ruminants | 1–10 | 64.1 |
| 11–50 | 23.1 | |
| 51–100 | 12.8 | |
| Ectoparasites knowledge | Flea | 2.6 |
| Lice | 66.7 | |
| Tick | 15.4 | |
| Mite | 43.6 | |
| Don’t know | 20.5 | |
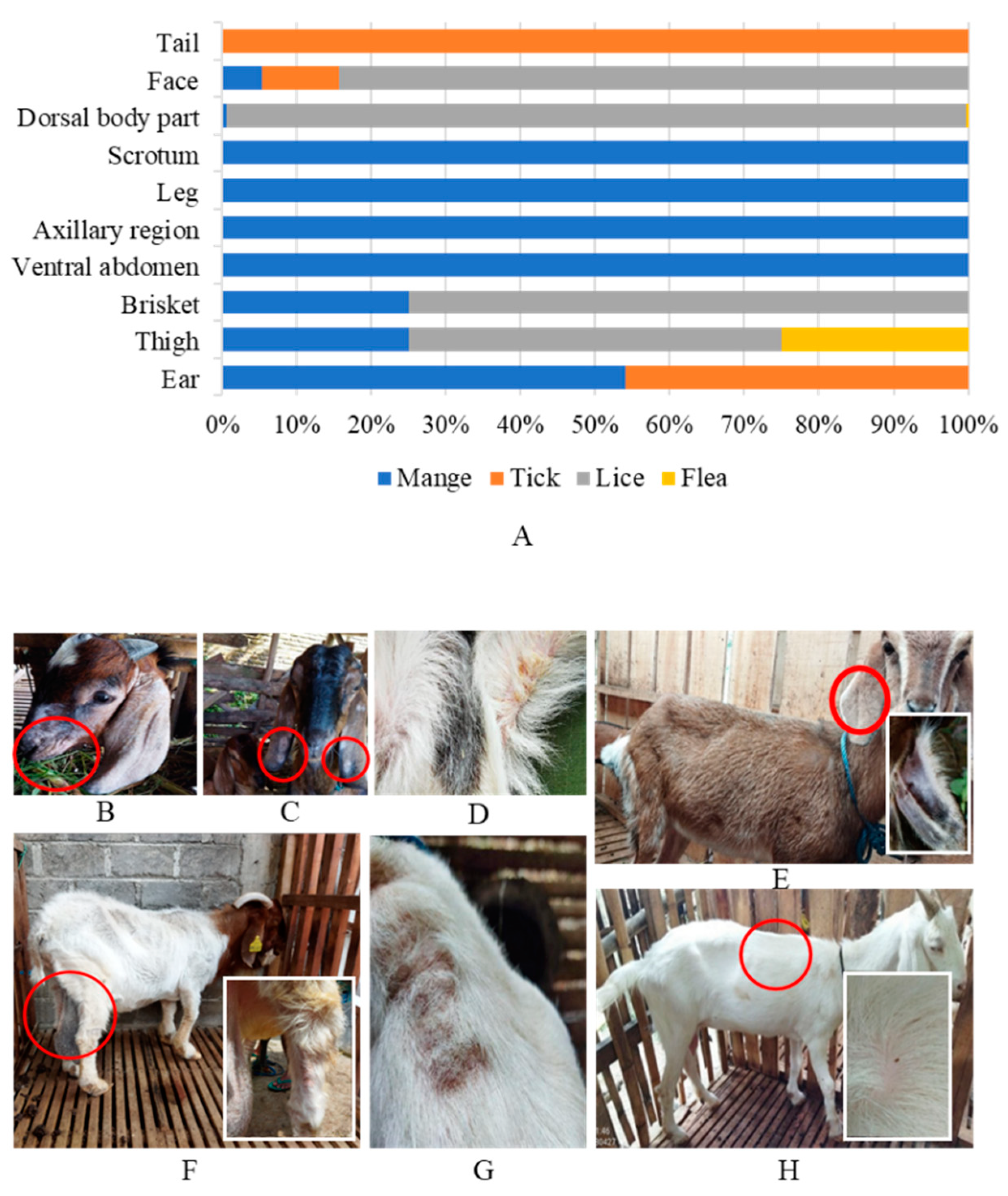
| Predilection site of ectoparasites mostly observed | Ear | 7.7 |
| Thigh | 0.5 | |
| Brisket | 0.6 | |
| Ventral abdomen | 0.3 | |
| Axilary region | 0.3 | |
| Leg | 0.6 | |
| Scrotum | 0.1 | |
| Dorsal body part | 76.2 | |
| Face | 25.5 | |
| Tail | 0.3 | |
| Knowledge of zoonotic potential | Know | 20.5 |
| Don’t know | 79.5 | |
| Acaricides treatment | Yes, pour on powder | 0 |
| Yes, topical ointment | 2.6 | |
| Yes, spray | 5.1 | |
| Yes, injection by local veterinarian | 41.1 | |
| Repetitive treatments by injection | <4 times scheduled injection by local veterinarian | 68.75 |
| >4 times scheduled injection by local veterinarian | 31.25 | |
| Repetitive treatments by pour on acaricides | <4 times self-treatment | 33.3 |
| >4 times self-treatment | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insyari’ati, T.; Hamid, P.H.; Rahayu, E.T.; Sugar, D.L.; Rahma, N.N.; Kusumarini, S.; Kurnianto, H.; Wardhana, A.H. Ectoparasites Infestation to Small Ruminants and Practical Attitudes among Farmers toward Acaricides Treatment in Central Region of Java, Indonesia. Vet. Sci. 2024, 11, 162. https://doi.org/10.3390/vetsci11040162
Insyari’ati T, Hamid PH, Rahayu ET, Sugar DL, Rahma NN, Kusumarini S, Kurnianto H, Wardhana AH. Ectoparasites Infestation to Small Ruminants and Practical Attitudes among Farmers toward Acaricides Treatment in Central Region of Java, Indonesia. Veterinary Sciences. 2024; 11(4):162. https://doi.org/10.3390/vetsci11040162
Chicago/Turabian StyleInsyari’ati, Titis, Penny Humaidah Hamid, Endang Tri Rahayu, Diah Lutfiah Sugar, Nadya Nurvita Rahma, Shelly Kusumarini, Heri Kurnianto, and April Hari Wardhana. 2024. "Ectoparasites Infestation to Small Ruminants and Practical Attitudes among Farmers toward Acaricides Treatment in Central Region of Java, Indonesia" Veterinary Sciences 11, no. 4: 162. https://doi.org/10.3390/vetsci11040162
APA StyleInsyari’ati, T., Hamid, P. H., Rahayu, E. T., Sugar, D. L., Rahma, N. N., Kusumarini, S., Kurnianto, H., & Wardhana, A. H. (2024). Ectoparasites Infestation to Small Ruminants and Practical Attitudes among Farmers toward Acaricides Treatment in Central Region of Java, Indonesia. Veterinary Sciences, 11(4), 162. https://doi.org/10.3390/vetsci11040162





