Simple Summary
Currently, animal skin infections are treated with antimicrobial drugs. However, due to developing resistance, alternatives are being sought. Such an alternative to these drugs could be ozonated oils, exercising antibacterial and antifungal properties. This article presents data evaluating the chemical parameters and antibacterial activity of four selected ozonated oils (linseed, hemp seed, sunflower, and olive). The ozonation of oils changed their chemical composition. This was related to antibacterial activity, as the results showed a tendency for the reference strains of S. aureus, E. faecalis, and E. coli to have broader zones of inhibition (p < 0.001) than clinical strains. Overall, ozonated linseed oil exhibited the highest antibacterial activity, while ozonated olive oil showed the lowest. Further characterisation of selected ozonated oils will be performed to determine antifungal properties and cytotoxicity together with evaluation of shelf life of biological activity, prior to preclinical animal studies.
Abstract
Infectious skin diseases are quite common in veterinary medicine. These diseases can be caused by both bacteria and pathogenic fungi. Antimicrobial drugs are usually used for treatment. An alternative to these drugs could be ozonated oils with antibacterial and antifungal properties. Four different ozonated oils (linseed, hemp seed, sunflower, and olive) were tested in order to develop an optimal pharmaceutical form for the treatment of skin infections in animals. Chemical parameters such as acid and acidity value, iodine and peroxide value, viscosity, and infrared spectres were analysed. The ozonation of oils resulted in changes in their chemical composition. The antimicrobial activity of the tested oils was evaluated by determining the minimum inhibitory concentrations and zones of inhibition in agar. After ozonation, the acid content increased in all the tested oils. The highest acidity was found in linseed oil (13.00 ± 0.11 mg KOH/g; 6.1%). Hemp oil, whose acidity was also significant (second only to linseed oil), was the least acidified by ozonation (11.45 ± 0.09 mg KOH/g; 5.75%). After ozonation, the iodine value in oils was significantly reduced (45–93%), and the highest amounts of iodine value remained in linseed (47.50 ± 11.94 g Iodine/100 g oil) and hemp (44.77 ± 1.41 Iodine/100 g oil) oils. The highest number of peroxides after the ozonation of oils was found in sunflower oil (382 ± 9.8 meqO2/kg). It was found that ozonated hemp and linseed oils do not solidify and remain in liquid form when the temperature drops. The results showed a tendency for the reference strains of S. aureus, E. faecalis, and E. coli to have broader zones of inhibition (p < 0.001) than clinical strains. Overall, ozonated linseed oil had the highest antibacterial activity, and ozonated olive oil had the lowest, as determined by both methods. It was found that ozonated linseed oil was the most effective on bacteria, while the most sensitive were S. aureus ATCC 25923, MRSA, and S. pseudointermedius (MIC 13.5 mg/mL, 4.6 mg/mL, and 13.5 mg/mL, respectively, and sterile zones 20.67 ± 0.98 mm, 20.25 ± 0.45 mm, and 18.25 ± 0.45 mm, respectively). The aim and new aspect of this work is the characterisation of selected ozonated vegetable oils, especially hemp oil, according to chemical and antibacterial parameters, in order to select suitable candidates for preclinical and clinical animal studies in the treatment of bacterial or fungal skin infections in terms of safety and efficacy.
1. Introduction
In clinical veterinary medicine, animal skin infections are one of the most common pathologies. Depending on the animal species, they include folliculitis; furunculosis; abscesses; superficial pyoderma; superficial, pustular, and crusting dermatitis; impetigo; otitis externa; infected wounds; mastitis; and interdigital necrobacillosis and if not controlled can lead to life-threatening septicaemia [1,2,3,4]. Dermal injuries can be colonised by aerobic and anaerobic bacteria; more detailed data related to the most frequent pathogens causing animal skin infections are listed in Table 1.
Bacterial skin infections can be treated with topical medications (creams, ointments, shampoos, lotions, mousses, sprays, and wipes containing antiseptics or antibiotics) or systemic antibiotic therapy [4]. Reducing the pressure of bacterial infections through therapeutic means not only improves animal health but also positively affects animal welfare and human safety. However, bacteria responsible for skin infections in animals have exhibited significant resistance to antibiotics, emphasising the need for heightened awareness, the rational utilisation of these medications, and research of alternatives [5,6,7,8,9,10]. The use of bacteriophages, bacteriocins, antimicrobial photodynamic therapy, phytochemicals, and ozone have recently been investigated as alternative treatment options [11]. Among the alternatives, research on ozonated oils has been increasing in recent years. It is worth noting that, compared to ozone gas and ozonated water, ozonated oils have better stability and ease of handling, have better storage, avoid rapid degradation, allow for treatment outside the hospital, and reduce the risk of using large and inappropriate doses [12].

Table 1.
The pathogenic bacteria related to animal skin infections [compiled from [1,2,3,4,8,10]].
Table 1.
The pathogenic bacteria related to animal skin infections [compiled from [1,2,3,4,8,10]].
| Animal Species | Bacterial Pathogens |
|---|---|
| Cattle | Actinomyces bovis, Bacteroide melaninogenicus, Staphylococcus aureus, Streptococcus dysgalactiae, Fusobacterium necroforum, Moraxella bovis, Trueperella pyogenes |
| Pigs | Dermatophylus congolensis, S. hyicus, S. intermedius, S. chromogenes, S. sciuri |
| Goats | Dermatophylus congolensis, S. aureus, S. hyicus, S. haemolyticus, S. warneri, S. epidermidis, S. chromogenes, S. caprae, S. simulans |
| Sheep | Dermatophylus congolensis, Corynebacterium pseudotuberculosis, Pithomyces fungus, S. aureus, S. xylosus, S. epidermidis, Str. Dysgalactiae |
| Poultry | S. aureus, S. hyicus |
| Dogs | Staphylococcus pseudintermedius, Pseudomonas aeruginosa, coagulase-negative Staphylococcus (CoNS) (S. xylosus, S. simulans, S. epidermidis, S. sciuri, S. chromogenes, S. hyicus, and S. cohnii), Streptococcus (S. canis, S. mitis, S. dysgalactiae, S. agalactiae), E. coli and Enterobacterales (Klebsiella pneumoniae, Proteus mirabilis, Raoultella ornithinolytica, Enterobacter cloacae, Serratia marcescens, and Citrobacter youngae), E. faecalis |
| Cats | coagulase-negative Staphylococcus, S. aureus, S. pseudintermedius, Streptococcus canis, Pseudomonas aeruginosa, E. coli and Klebsiella pneumoniae |
Several in vitro studies have evaluated the antimicrobial activity of ozonated oils. Bouzid et al. found that ozonated olive oil, depending on the concentration used (0.248 to 63.5 mg/mL), effectively inhibited the growth of Proteus mirabilis ATCC 35659, Escherichia coli ATCC 25922, and Staphylococcus aureus ATCC 6538; however, the highest ozone concentration was required to inhibit the growth of Listeria monocytogenes ATCC 15313, Pseudomonas aeruginosa ATCC 27853, and Klebsiella pneumoniae ATCC 4352 [13]. In addition, several studies have shown that many oils with varying levels of ozonation have antibacterial, fungicidal, and antiviral properties [14,15,16,17,18,19,20]. Consequently, ozonated oils have been successfully used in humans to treat various skin conditions and sensitivities such as atopic dermatitis; contact dermatitis; ichthyosis; psoriasis; acne; abrasions; pressure ulcers; insect bite blisters; first-degree burns; diabetic foot; and skin care after laser therapy, surgery, and sunburn [15]. The therapeutic activity of ozonated oil was attributed to its antimicrobial, antihypoxic, analgesic, and immunomodulatory effects [21,22]. The efficacy and safety of ozonated oils for veterinary purposes have also been tested. Ozonated olive oil was found to be effective in in vivo studies in rats against S. pyogenes and S. aureus [23]. The topical application of ozonated vegetable oil in MRSA-infected rat skin showed a slight reduction in bacteria load and improved wound healing [24]. Ozonated olive oil has been used effectively to treat cat ear mites (Otodectes cynotis) [25]. The ozonated oil ointment used in the study by Szponder T. et al. proved to be an effective and convenient means for the topical treatment of foot rot in sheep enabling precise administration and causing no undesirable effects [26]. Ozonated oils in liposomes plus hypromellose have been reported to have restorative and regenerative effects in external spontaneous ocular pathologies in animals and humans [27]. The successful use of ozonated oil in the treatment of pharmacodermia in female dogs has been reported [28].
In order to effectively use ozonated oils for the treatment of animal skin infections, it is necessary to study the parameters that could potentially influence their therapeutic effectiveness. Chemical tests of peroxide, iodine and acidity, viscosity, and spectroscopy (FT-IR, 1H NMR, and 13C NMR) [29], together with antimicrobial tests, have been performed to characterise the prepared ozonated oils [30,31]. It is observed that ozonated oil with a higher peroxide value has better antimicrobial activity [16,30,32]. The amount of peroxide in ozonated vegetable oils can be increased by adding water and increasing the ozonation time [16]. Two studies showed an increase in antimicrobial activity with ozonation times up to 5 h, after which antibacterial activity did not improve [32,33]. Different ozonation techniques, chemical parameters before and after ozonation, and their correlation with antimicrobial activity within single oil have been analysed in several studies with interesting observations [29,31,32,34,35].
However, Guerra-Blanco et al. confirmed that the ozonation kinetics of different oils (grapeseed, sunflower, avocado, and olive) differ [36]. Therefore, such an analysis of several different oils would help to gain a broader picture of the characteristics of ozonated oils and to find an optimal formulation suitable for the treatment of skin infection in animals that would be an alternative to the use of antibiotics and the prevention of antibiotic resistance. This study analyses the chemical parameters of linseed, hemp seed, sunflower, and olive oil before and after ozonation and the correlation between these parameters and the antimicrobial activity of the studied preparations.
As the first phase of the investigation, this study aimed to evaluate the chemical parameters (acid and acidity values, iodine values and peroxide values, viscosity, and infrared spectres) and antimicrobial activity of the selected ozonated oils (linseed, hemp seed, sunflower, and olive oil) in order to develop an optimal pharmaceutical form for the treatment of skin infections in animals.
2. Materials and Methods
2.1. Materials
For antimicrobial assays, Mueller–Hinton agar media (Sigma-Aldrich, Poznan, Poland) and LB broth (Miller) (Sigma-Aldrich, Poznan, Poland) were used. All reagents for chemical analysis were purchased from Sigma-Aldrich: sodium thiosulfate anhydrous, hexane, glacial acetic acid, chloroform, methanol, potassium iodide, and potassium hydroxide. The ozonated oils used in this study (linseed, hemp seed, sunflower, and olive oil) were specifically prepared for research purposes and were generously provided by UAB Ozono Centras, Lithuania. Oils were ozonated for 4 h without water, and the ozonation time was chosen after analysing the results of other scientists’ research [30,36,37]. This was the minimum time during which good antimicrobial activity results are usually obtained. All used oils were extra virgin and cold-pressed.
2.2. Bacterial Strains
Four ozonated oils were tested against Gram-positive S. aureus (ATCC 25923 and a clinical strain), the MRSA clinical strain, S. pseudintermedius clinical strain, E. faecalis (ATCC 29212 and a clinical strain), Gram-negative P. aeruginosa (ATCC 27859 and a clinical strain) and E. coli (ATCC 25922 and a clinical strain). The clinical strains were obtained from the collection of the Institute of Microbiology and Virology (Faculty of Veterinary Medicine, Lithuanian University of Health Sciences).
2.3. FT-IR Analysis
FT-IR spectra of hemp seed, linseed, sunflower, and olive oil before and after ozonation were recorded by using a Shimadzu IRTracer-100 (Kyoto, Japan) FT-IR spectrophotometer. Transmission levels were measured for wave numbers of 4000–400 cm−1 with a resolution of 2 cm−1 for 45 scans and read as absorbance in triplicate before taking the averaged value. About 2 μL of the sample was deposited between two disks of KBr, avoiding air bubble formation.
2.4. Peroxide Value
The peroxide value (PV) is a measure of the amount of oxygen chemically bound to an oil or fat as peroxides, particularly hydroperoxides. It is one of the indicators of the strength of the ozonation process. PV is expressed in milliequivalents of active oxygen per kilogram of oil (meq/kg). PV was determined using the ISO standard 3960:2017 [38].
2.5. Acid Value and Acidity
The acid value is the number of milligrams of potassium hydroxide required to neutralise the free fatty acids present in 1 g of fat when determined in accordance with the procedure specified in LST EN ISO 660:2020 [39]. The acid value is expressed in milligrams per gram.
Acidity is the content of free fatty acids, also determined according to the procedure specified in LST EN ISO 660:2020 [39]. The acidity is expressed as a percentage by mass. If the result of the determination is reported as acidity without further explanation, this is, by convention, the acidity based on the oleic acid content.
2.6. Iodine Value
The iodine value (IV) is a mass of halogen, expressed as iodine, absorbed by the test portion following the specified procedure by ISO 3961:2018 [40], divided by the mass of the test portion. The IV is expressed as a mass fraction in grams per 100 grams of fat.
2.7. Viscosity
The dynamic viscosity of ozonated the oil samples was measured in an ANTON PAAR modular compact rheometer MCR-102 (Anton Paar GmbH, Graz, Austria), at different shear rates and three controlled temperatures (5, 22, and 38 ± 0.1 °C). The shear rate in the range from 0.1 to 100 s−1 was tested. The measurement was performed in a parallel plate system with a diameter of 50 mm at a gap of 0.2 mm. Data were processed using the Rheocompass software (ver. 1.14).
2.8. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) was determined using the broth microdilution method. The assay was carried out in 96-well microtitre plates filled with an LB medium. Ozonated oils were transferred to microplate wells in order to obtain two-fold serial dilution in concentrations ranging between 0.8 and 310 mg/mL. The final concentrations of bacteria in the wells were 1–3 × 105 CFU/mL. The plates were incubated at 37 °C for 24 h. There were three types of controls: negative control (only medium), positive control (medium and bacteria inoculum), and colour control (medium and ozonated oils in serial dilutions). Bacterial growth was evaluated with a microplate photometer Multiskan FC (Thermo Scientific, Foster city, CA, USA) in 570 nm wavelength. Based on the control samples, thresholds were defined to determine the criteria for classifying the obtained results as either bacterial growth or non-growth.
2.9. Agar Well Diffusion Method
The inoculum suspensions of the bacterial isolates were adjusted to 1.0 × 105 CFU/mL in sterile saline. Plates (90 mm diameter) with Mueller–Hinton agar were streaked evenly in three directions over the entire agar surface with a swab dipped into the bacterial inoculum suspension. The plates were allowed to dry before making 9 mm wells with a sterile metal cylinder. Wells were filled with control oils (not ozonated) and ozonated oils. The plates were incubated at 37 °C for 24 h. After incubation, the inhibition zones were measured.
2.10. Statistical Analysis
For agar well diffusion assay, the results are expressed as the mean ± SD of at least 12 independent measurements. The broth microdilution assay was repeated until consistent MIC results were obtained for each bacterial strain. Analysis of variance (ANOVA) was employed to compare the results between all four ozonated oils. Student’s t-test was used to compare the means between two groups (e.g., inhibition zones between clinical and reference strains). The correlations were determined using the Pearson test after verifying the normality using the Shapiro–Wilk test. For statistical calculations involving agar well diffusion data where the inhibition zone was not observed, a well diameter of 9 mm was employed in the analysis. Statistical analyses were applied to the data using the SPSS statistical package (IBM SPSS Statistics for Windows 29.0).
3. Results
3.1. Acid Value (AV) Results
An increase in the acid value (AV) after ozonation was observed in all four oils tested (Table 2). The highest increase was in linseed oil—18 times. It also had the highest acid value after ozonation, at 13 mg KOH/g. The lowest increase in the acid value after ozonation was observed in hemp seed oil (2.8 times), but it still had a relatively high acid value of 11.45 mg KOH/g. The lowest acid value after ozonation was in olive oil, at 3.65 mg KOH/g. As expected, acidity results were consistent with acid values of the same oils (Table 3).

Table 2.
Acid values before and after ozonation, mean ± SD.

Table 3.
Acidity before and after ozonation, in %.
3.2. Iodine Value (IV) Results
The iodine values before ozonation were compatible with ordinary values that were defined by other researchers [15,37], where linseed had the highest IV, and olive oil had the lowest. During the ozonation reaction, a decrease in iodine values was observed (Table 4). The iodine value difference before and after ozonation in all oils was relatively similar—on average 42.83 g/100 g oil (39.34–47.75). This could be due to the same ozonation time (4 h).

Table 4.
Iodine value before and after ozonation, mean ± SD.
3.3. Peroxide Value (PV) Results
The peroxide value increased in all tested oils after ozonation (Table 5). Sunflower oil had the highest peroxide value after ozonation. The other three oils had very similar peroxide values.

Table 5.
Peroxide values, meqO2/kg ± SD.
3.4. Viscosity
Viscosity results are shown in Table 6. A strong negative linear correlation was found: Lower temperatures showed higher consistency factor (K) measures of ozonated oils (r = −0.953–−0.881, p > 0.05). We additionally observed that ozonated sunflower oil (OSO) and ozonated olive oil (OOO) solidified in the refrigerator temperature (5 °C), but ozonated hemp seed oil (OHO) and ozonated linseed oil (OLO) remained liquid. OOO at 5 °C was the most viscous of all tested oils and the least viscous at 38 °C, with a 1712× difference between those two values. Such a change in the consistency factor is not characteristic of unprocessed olive oil. It is rather an impact of ozonation. The flow behaviour index characterised the ozonated oils as pseudoplastic fluids.

Table 6.
Consistency factor, mPa·s.
3.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
The transmittance % was measured in the mid-IR spectrum range (400–4000 cm−1) (see Figure 1). The main changes during ozonation occurred at 722–723 cm−1 (overlapping of the methylene rocking vibration and the out-of-plane vibration of cis-disubstituted olefins), 1105 cm−1 (–C–O stretching), 1155–1163 cm−1 (sv of –C–O), 1460–1465 cm−1 (the bending vibrations of CH2 and CH3 aliphatic groups), 1654 cm−1 (the C=C stretching vibration of cis-olefins), 3009 cm−1 (C=C–H stretching), and in the bands at 2922 and 2853 cm−1, which are attributed to the symmetric and asymmetric stretching vibration of the aliphatic CH2 group [41,42,43,44].
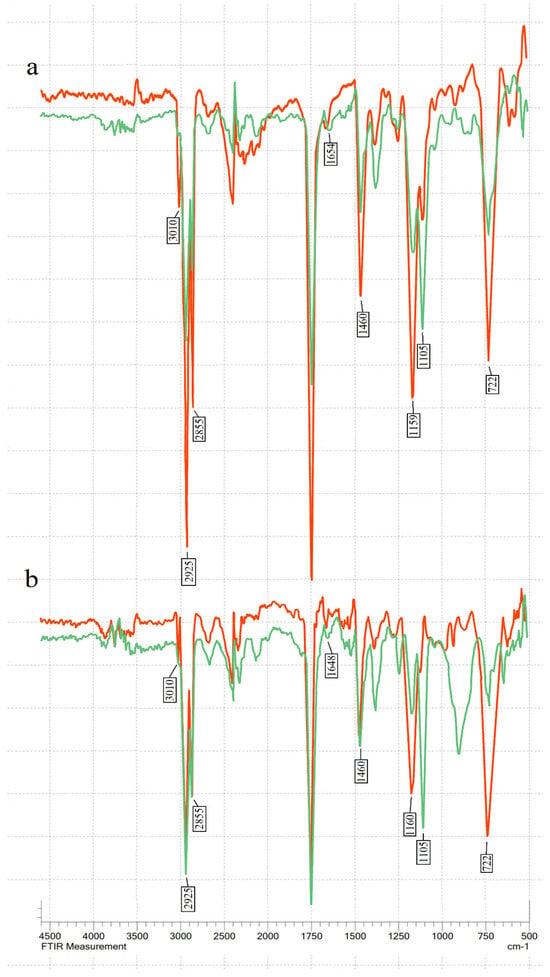
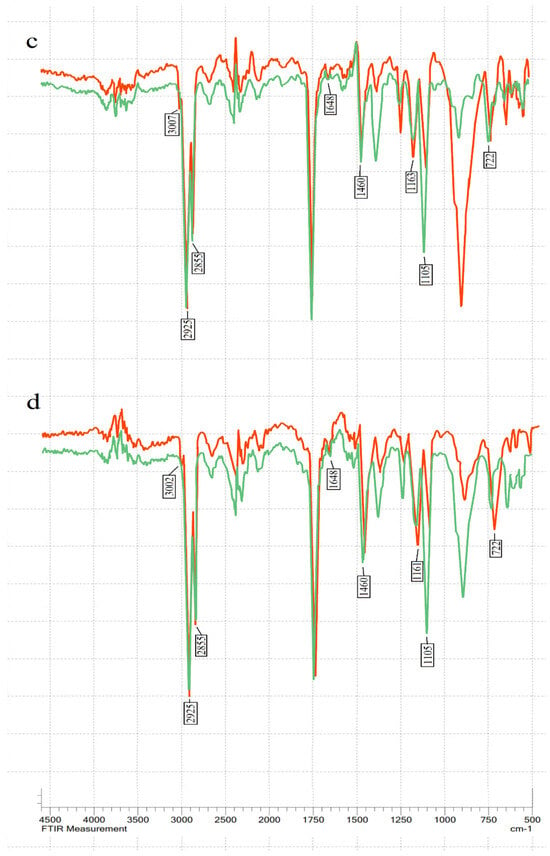
Figure 1.
FT-IR spectra of oils before (red line) and after (green line) ozonation: (a) linseed oil; (b) hemp seed oil; (c) sunflower oil; (d) olive oil.
The characteristic double-bond absorption bands at 722 cm−1, 1648–1654 cm−1, and 3009 cm−1 decreased after ozonation in all four oils, showing double-bond consumption. A decrease in absorption bands 1155–1163 cm−1 and 1460–1461 cm−1 was also visible. The peaks at 2925 and 2855 cm−1 decreased after ozonation in all tested oils. Meanwhile, the peak at C–O stretching in 1105 cm−1 increased after ozonation. This indicates ozonide formation.
There were no visible peaks between 3300 and 3600 cm−1, indicating the absence of hydroxyl groups in the tested ozonated oils [17]. In addition, no aldehydes were detected in FT-IR analysis after ozonation (no peaks were observed in aldehyde characteristic absorption bands between 2700 and 2800 cm−1 [34].
3.6. Minimal Inhibitory Concentrations
Gram-positive Staphylococcus aureus ATCC 25923 was the least resistant of the tested microorganisms (Table 7), which corresponds to other authors’ results [31,33]. OOO had the highest MIC between 190.5 and 300 mg/mL for all the microorganisms tested and was the least effective. The MIC values for OHO ranged from 18.5 to 74 mg/mL, and for the tested isolates from OSO, they ranged from 4.7 to 80 mg/mL. OLO showed the lowest MIC values against the tested microorganisms between 4.6 and 50 mg/mL, except for S. aureus ATCC 25923, where the MIC of OSO was lower than that of OLO. The MIC results had a strong negative correlation with the acid values of the same ozonated oils (R = −0.920, p < 0.001). The ozonated oils with higher acid values had lower MIC values (better antimicrobial activity). It was also observed that the higher the percentage of oil iodine consumption after ozonation, the higher the minimum inhibitory concentrations (R = 0.971, p = 0.029). OOO had the highest MICs for all the tested microorganisms in the range from 190.5 to 300 mg/mL and was the least effective.

Table 7.
MICs of ozonated oils against tested bacteria, mg/mL.
The control samples of oils before ozonation had no antibacterial activity. The MIC values for OHO against the tested isolates ranged from 18.5 to 74 mg/mL, and for OSO, they were in the range from 4.7 to 80 mg/mL. OLO showed the lowest MIC values against the tested microorganisms, ranging from 4.6 to 50 mg/mL, except for S. aureus ATCC 25923, where OSO had a lower MIC than OLO. MIC results had a strong negative correlation with acid values of the same ozonated oils (R = −0.920, p < 0.001). The ozonated oils with higher acid values had lower MIC values (better antimicrobial activity). It was also observed that the higher the percentage of iodine consumption of oil after ozonation, the higher the minimum inhibitory concentrations (R = 0.971, p = 0.029). MIC results had no correlation with peroxide values (R = −0.215, p > 0.05). The MIC assay with E. faecalis reference and clinical strains was not performed. In relation to the agar well diffusion method, this pathogen tends to require higher ozonated oil concentration, especially for the tested olive oil.
3.7. Agar Well Diffusion
The results showed that the reference strains of S. aureus, E. faecalis, and E. coli had wider zones of inhibition (p < 0.001) than clinical strains. There were some exceptions where no statistically significant difference between the two strains was observed for each bacterium: E. faecalis with OLO, S. aureus with OOO, and E. coli with OSO. The P. aeruginosa reference strain had smaller zones of inhibition than the clinical strain (p < 0.001 with OHO and OSO, p < 0.01 with OLO).
The antimicrobial efficacy of the ozonated oils in Gram-group bacteria was not clearly demonstrated. The difference between the diameters of the inhibition zones was more pronounced between two Gram-negative bacteria (E. coli and P. aeruginosa) than between different groups of Gram-negative bacteria. Control samples of oils before ozonation did not show zones of bacterial inhibition. The correlation between the two methods showed that the sensitivity of the bacteria to a given oil was consistent. Ozonated oils that produced lower MICs also produced wider zones of inhibition with the same bacteria (Table 8). Overall, OLO showed the highest antibacterial activity, and OOO showed the lowest, as observed by both methods. The strength and significance of this correlation varied slightly between the different bacteria. Both reference and clinical E. coli and P. aeruginosa strains had a statistically significant strong correlation (r = −0.981, p < 0.05, r = −0.970, p < 0.01, r = −0.978, respectively, p < 0.001). The results of MIC had a strong negative, but statistically non-significant, correlation with the results of agar well diffusion assay in S. pseudintermedius (r = −0.930, p = 0.070) and MRSA (r = −0.910, p = 0.090) for the same ozonated oils. The correlation between the two assays of S. aureus ATCC 25923, clinical S. aureus, and P. aeruginosa ATCC 27859 was weaker and more non-significant (r = −0.707, p = 0.293; r = −0.711, p = 0.011 and r = −0.652, p = 0.011).

Table 8.
Inhibition zone diameter in mm, mean ± SD.
4. Discussion
In order to standardise the ozonation process, many authors suggest monitoring the peroxide value and viscosity parameters during the process [18]. The values of peroxides obtained in our study are among the lower ones. This was due to the choice not to add water during the process and the selected ozonation time (4 h). The research of Moureu et al. clearly shows that the ozonation of oil with water leads to higher peroxide values (up to 2600 mEq of active oxygen/kg oil with water and 430 without) [33]. In addition, it should be emphasised that peroxide values can be highly dependent on the chosen peroxide determination method [29]. When tracking viscosity, it is important to pay attention to temperature. As our study shows, the viscosity of ozonated oils varies greatly at different temperatures. Unfortunately, some studies with ozonated oils do not specify the temperature for the viscosity parameter. Obtaining ozonated oil with precisely matched parameters by repeated tests remains a challenging task.
According to many authors, the peroxide content is the most important characteristic of the antimicrobial efficacy of ozonated oils [16,30]. In most cases, increasing levels of peroxide have better antimicrobial activity. In our research, the peroxide value had no correlation with antimicrobial activity. This may be due to relatively similar PV concentrations.
In this study, the highest PV (OSO) was only 72% higher than the lowest PV (OLO). It is also possible that there is a stronger correlation between the peroxide value and antimicrobial activity when comparing these parameters in the same oil than in different oils, as shown in our study. However, our antimicrobial results were correlated with the acid value and mole percentage of double bonds consumed. Moureu et al. observed that the acid value also correlated with antimicrobial activity [33]. In addition, Guerra-Blanco et al. observed that anti-inflammatory and wound-healing effects were achieved with ozonated grape seed and sunflower oil with a low degree of ozonation, implying that the superior therapeutic effect of ozonated oils is not necessarily due to a high peroxide value [36].
Diaz et al. observed that the oil with the lowest iodine index (highest oleic acid content) reacted faster with ozone, consuming 94 percent of the moles of double bonds, while other oils consumed 50–60% at the same time [31]. Our study supports this observation, with olive oil having the lowest iodine index before ozonation and the highest consumption of double bonds (93%) compared to other oils (45–60%). Diaz et al. (2021) analysed this phenomenon in more detail and discussed the possible reasons. It is worth noting that our oil with the highest consumption of double bonds (olive oil) and the one in the study by Diaz et al. (“Dende” oil) yielded opposite results regarding their PV and antimicrobial efficacy. Despite the similar IV pre-ozonation and double-bond rate, Dende oil had the strongest antimicrobial activity among the oils with the highest peroxide value, while our ozonated olive oil had the weakest antibacterial activity. These observations suggest that the ability of oils to absorb ozone is not the main determinant of their potential antimicrobial activity [18].
The main changes in the FT-IR spectrum after ozonation were consistent with earlier studies [24,35,37]. During FT-IR analysis, it was observed that different oils had slightly different frequency numbers for the same chemical groups, especially for the 3006 cm−1 band. Hemp seed and linseed oil had a peak at 3009.97 cm−1, sunflower at 3007 cm−1, and olive oil at 3002 cm−1.
The exact position of this band depends on the proportion of fatty acids [17]. Oils with higher unsaturation have a higher frequency [19]. After ozonation, the oils in our study had no deformations in the characteristic bands of aldehyde and hydroxyl. This was an expected result since ozonation did not use water. Ozonolysis can produce aldehydes and α-hydroxy hydroperoxides if a protic solvent (water) is present. However, FT-IR results do not necessarily mean that the sample is free of aldehydes. Kogawa et al. and Soriano et al. showed that ozonated oils contained a small amount of aldehyde when analysed using H NMR, even though FT-IR analysis showed no peaks in the characteristic aldehyde band [34,37].
Our study did not observe any evidence that Gram-positive or Gram-negative bacteria were more sensitive to ozonated oils compared to each other. Our results did not fully agree with other studies by different authors, which reported that Gram-positive bacteria are more sensitive to ozonated oils [31,45]. Although there are frequent references to the fact that Gram-positive bacteria are more sensitive to ozonated oils than Gram-negative bacteria, there is still a lack of comprehensive articles on this topic based on a larger number of representatives of both groups and providing stronger statistical support.
We examined the correlation between the two antimicrobial activity assay methods and found that the correlation was not significant for certain bacteria. It can be assumed that this non-significance is due to the identical MIC values observed with the different ozonated oils. However, in reality, the true MIC value can be anywhere between the last dilution that inhibits growth and the first dilution that does not inhibit growth [20]. Therefore, the exact values could have been different, leading to different levels of significance. In addition, some correlations were observed to be non-significant due to OSO having a lower minimum inhibitory concentration (MIC) than OLO against S. aureus ATCC 25923 or a larger zone of inhibition than OHO against S. pseudintermedius. The presence of such deviations can be attributed to the relatively higher peroxide value of sunflower oil compared to the other three oils. However, we cannot assume that certain bacteria are more sensitive to OSO, since the second method showed that OLO remained the most effective.
Promising results were obtained with the tested ozonated oils, allowing for the development of an effective and safe antimicrobial/antiseptic pharmaceutical formulation for external use against skin infections, which can be used as an alternative (or adjunctive) to veterinary antibiotics. A more extensive in vitro evaluation of the bioactivity of the selected ozonated oils is necessary before preclinical animal studies. Therefore, we plan to carry out an assessment of the antifungal properties and cytotoxicity of ozonated linseed, hemp, sunflower, and olive oils in cell lines and the shelf life of biological activity.
5. Conclusions
Ozone formulation and therapy are gaining popularity in research and clinical settings [14,46,47] as well as in veterinary medicine [48]. Four different ozonated oils (linseed, hemp, sunflower, and olive) were tested in order to develop an optimal pharmaceutical form for the treatment of skin infections in animals. The analysed chemical parameters were acids and acidity, iodine and peroxide content, viscosity, and infrared spectra. The ozonation of oils changed their chemical composition. The antimicrobial activity of the tested oils was evaluated by determining the minimum inhibitory concentrations and zones of inhibition in agar. The results showed that the reference strains of S. aureus, E. faecalis, and E. coli had wider zones of inhibition (p < 0.001) than clinical strains. Overall, ozonated linseed oil had the highest antibacterial activity, and ozonated olive oil had the least, as determined by both methods. Further characterisation of the selected ozonated linseed, hemp, sunflower, and olive oils is necessary to determine antifungal properties and cytotoxicity together with the evaluation of the shelf life of biological activity, prior to preclinical animal studies.
Author Contributions
Conceptualisation, G.S., A.G., L.I. and K.R.; methodology, M.I., I.S. and V.A.; formal analysis, G.S. and A.G.; investigation, G.S.; writing—original draft preparation, G.S. and G.D.; writing—review and editing, G.S., A.G., M.I., I.S., V.A., D.J., L.I., K.R. and G.D.; visualisation, G.S.; supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are included in the present article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Foster, A.P. Staphylococcal skin disease in livestock. Vet. Dermatol. 2012, 23, 342–351. [Google Scholar] [CrossRef]
- Costa, S.S.; Oliveira, V.; Serrano, M.; Pomba, C.; Couto, I. Phenotypic and Molecular Traits of Staphylococcus coagulans Associated with Canine Skin Infections in Portugal. Antibiotics 2021, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Mala, L.; Lalouckova, K.; Skrivanova, E. Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals 2021, 11, 2473. [Google Scholar] [CrossRef]
- Hoang, T.P.N.; Ghori, M.U.; Conway, B.R. Topical Antiseptic Formulations for Skin and Soft Tissue Infections. Pharmaceutics 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Ambrosio, M.; Fiorito, F.; Cortese, L.; De Martino, L. On Gram-Positive- and Gram-Negative-Bacteria-Associated Canine and Feline Skin Infections: A 4-Year Retrospective Study of the University Veterinary Microbiology Diagnostic Laboratory of Naples, Italy. Animals 2021, 11, 1603. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Couto, M.; Pavilonis, A.; Klimiene, I.; Siugzdiniene, R.; Virgailis, M.; Vaskeviciute, L.; Anskiene, L.; Pomba, C. Characterization of Staphylococcus pseudintermedius isolated from diseased dogs in Lithuania. Pol. J. Vet. Sci. 2016, 19, 7–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakaminami, H.; Okamura, Y.; Tanaka, S.; Wajima, T.; Murayama, N.; Noguchi, N. Prevalence of antimicrobial-resistant staphylococci in nares and affected sites of pet dogs with superficial pyoderma. J. Vet. Med. Sci. 2021, 83, 214–219. [Google Scholar] [CrossRef]
- Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.L.; Igrejas, G.; et al. Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms 2021, 9, 482. [Google Scholar] [CrossRef]
- Burke, M.; Santoro, D. Prevalence of multidrug-resistant coagulase-positive staphylococci in canine and feline dermatological patients over a 10-year period: A retrospective study. Microbiology 2023, 169, 001300. [Google Scholar] [CrossRef]
- Feßler, A.T.; Scholtzek, A.D.; Schug, A.R.; Kohn, B.; Weingart, C.; Hanke, D.; Schink, A.-K.; Bethe, A.; Lübke-Becker, A.; Schwarz, S. Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions. Antibiotics 2022, 11, 152. [Google Scholar] [CrossRef]
- Rizzo, A.; Piccinno, M.; Lillo, E.; Carbonari, A.; Jirillo, F.; Sciorsci, R.L. Antimicrobial resistance and current alternatives in veterinary practice: A review. Curr. Pharm. Des. 2023, 29, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Anzolin, A.P.; Silveira-Kaross, N.L.; Bertol, C.D. Ozonated oil in wound healing: What has already been proven? Med. Gas Res. 2020, 10, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Merzouki, S.; Boukhebti, H.; Zerroug, M.M. Various Antimicrobial Agent of Ozonized Olive Oil. Ozone Sci. Eng. 2021, 43, 606–612. [Google Scholar] [CrossRef]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Radzimierska-Kaźmierczak, M.; Śmigielski, K.; Sikora, M.; Nowak, A.; Plucińska, A.; Kunicka-Styczyńska, A.; Czarnecka-Chrebelska, K.H. Olive Oil with Ozone-Modified Properties and Its Application. Molecules 2021, 26, 3074. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef]
- Ledea-Lozano, O.E.; Fernández-García, L.A.; Gil-Ibarra, D.; Bootello, M.Á.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Characterization of different ozonized sunflower oils II. Triacylglycerol condensation and physical properties. Grasas y Aceites 2019, 70, e330. [Google Scholar] [CrossRef]
- Díaz, M.F.; Veloso, M.C.C.; Pereira, P.A.P.; Sanchez, Y.; Fernandez, I.; Andrade, J.B. Assessment of the Physicochemical Quality Indicators and Microbiological Effects of Brazilian Ozonized Vegetable Oils. J. Braz. Chem. Soc. 2021, 32, 216–224. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Relationships between the composition of edible oils and lard and the ratio of the absorbance of specific bands of their Fourier transform infrared spectra. Role of some bands of the fingerprint region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- van de Kassteele, J.; van Santen-Verheuvel, M.G.; Koedijk, F.D.H.; van Dam, A.P.; van der Sande, M.A.B.; de Neeling, A.J. New Statistical Technique for Analyzing MIC-Based Susceptibility Data. Antimicrob. Agents Chemother. 2012, 56, 1557–1563. [Google Scholar] [CrossRef]
- Currò, M.; Russo, T.; Ferlazzo, N.; Caccamo, D.; Antonuccio, P.; Arena, S.; Parisi, S.; Perrone, P.; Ientile, R.; Romeo, C.; et al. Anti-inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans. Molecules 2018, 23, 645. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Lim, Y.; Belmonte, G.; Miracco, C.; Zanardi, I.; Bocci, V.; Travagli, V. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Rep. Reg. 2011, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Varol, K.; Birdane, F.M.; Keles, I. Effect of ozonated olive oil on experimentally induced skin infection by Streptococcus pyogenes and Staphylococcus aureus in rats. Ind. J. Exp. Biol. 2018, 56, 665–673. [Google Scholar]
- Silva, V.; Peirone, C.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Pires, I.; Maltez, L.; Pereira, J.E.; Igrejas, G.; Poeta, P. Topical Application of Ozonated Oils for the Treatment of MRSA Skin Infection in an Animal Model of Infected Ulcer. Biology 2021, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Altinok, Y.F.; Abuzer, A.; Mustafa, Y. Effect of some essential oils (Allium sativum L., Origanum majorana L.) and ozonated olive oil on the treatment of ear mites (Otodectes cynotis) in cats. Turk. J. Vet. Anim. Sci. 2016, 40, 782–787. [Google Scholar] [CrossRef]
- Szponder, T.; Zdziennicka, J.; Nowakiewicz, A.; Świeca, M.; Sobczyńska-Rak, A.; Żylińska, B.; Patkowski, K.; Junkuszew, A.; Wessely-Szponder, J. Effects of topical treatment of foot rot in sheep using ozonated olive ointment. J. Vet. Res. 2021, 65, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Tonti, E.; Spaterna, A.; Marchegiani, A. Use of Ozone-Based Eye Drops: A Series of Cases in Veterinary and Human Spontaneous Ocular Pathologies. Case Rep. Ophthalmol. 2018, 9, 287–298. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Silva, J.I., Jr.; Florêncio dos Santos, C.S.; da Silva, B.M.; Charas dos Santos, I.F.; Ferro, B.S.; Silva Barros, T.I.; Tomacheuski, R.M.; Simões-Mattos, L. Topical Ozone Therapy in the Treatment of Pharmacodermia in a Dog (Canis lupus familiaris). Acta Sci. Vet. 2019, 47, 425. [Google Scholar] [CrossRef]
- Sega, A.; Zanardi, I.; Chiasserini, L.; Gabbrielli, A.; Bocci, V.; Travagli, V. Properties of sesame oil by detailed 1H and 13C NMR assignments before and after ozonation and their correlation with iodine value, peroxide value, and viscosity measurements. Chem. Phys. Lipids 2010, 163, 148–156. [Google Scholar] [CrossRef]
- Skalska, K.; Ledakowicz, S.; Perkowski, J.; Sencio, B. Germicidal Properties of Ozonated Sunflower Oil, Ozone. Sci. Eng. 2009, 31, 232–237. [Google Scholar] [CrossRef]
- Amri, Z.; Hamida, S.B.; Dbeibia, A.; Ghorbel, A.; Mahdhi, A.; Mansour Znati, M.; Ambat, I.; Haapaniemi, E.; Sillanpää, M.; Hammami, M. Physico-chemical Characterization and Antibacterial Activity of Ozonated Pomegranate Seeds Oil, Ozone. Sci. Eng. 2020, 42, 531–538. [Google Scholar] [CrossRef]
- Díaz, M.F.; Sánchez, Y.; Gómez, M.; Hernández, F.; Da, C.; Veloso, M.C.; De, P.; Pereira, P.A.; De Andrade, J.B. Physicochemical characteristics of ozonated sunflower oils obtained by different procedures. Grasas y Aceites 2012, 63, 466–474. [Google Scholar] [CrossRef]
- Moureu, S.; Violleau, F.; Ali Haimoud-Lekhal, D.; Calmon, A. Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem. Phys. Lipids 2015, 186, 79–85. [Google Scholar] [CrossRef]
- Soriano, N.U.; Migo, V.P.; Matsumura, M. Ozonation of sunflower oil: Spectroscopic monitoring of the degree of unsaturation. J. Am. Oil Chem. Soc. 2003, 80, 997–1001. [Google Scholar] [CrossRef]
- Balea, A.; Ciotlăuș, I.; Pojar-Feneșan, M.; Carpa, R. Comparative chemical and antimicrobial characterization of non-ozonated and ozonated vegetable oils. Stud. Univ. Babes-Bolyai Chem. 2023, 285–301. [Google Scholar] [CrossRef]
- Guerra-Blanco, P.; Chairez, I.; Poznyak, T.; Brito-Arias, M. Kinetic Analysis of Ozonation Degree Effect on the Physicochemical Properties of Ozonated Vegetable Oils, Ozone. Sci. Eng. 2021, 43, 546–561. [Google Scholar] [CrossRef]
- Rodrigues De Almeida Kogawa, N.; José De Arruda, E.; Micheletti, A.C.; De Fatima Cepa Matos, M.; Silva De Oliveira, L.C.; Pires De Lima, D.; Pereira Carvalho, N.C.; Dias De Oliveira, P.; De Castro Cunha, M.; Ojeda, M.; et al. Synthesis, characterization, thermal behaviour, and biological activity of ozonides from vegetable oils. RSC Adv. 2015, 5, 65427–65436. [Google Scholar] [CrossRef]
- EN ISO 3960:2017; Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination. 5th ed. International Organization for Standardization: Geneva, Switzerland, 2017; pp. 1–10.
- LST EN ISO 660:2020; Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity. 4th ed. International Organization for Standardization: Geneva, Switzerland, 2020; pp. 1–12.
- EN ISO 3961:2018; Animal and Vegetable Fats and Oils-Determination of Iodine Value. 6th ed. International Organization for Standardization: Geneva, Switzerland, 2018; pp. 1–12.
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: New York, NY, USA, 2014; pp. 73–119. [Google Scholar]
- Guillén, M.D.; Cabo, N. Some of the most significant changes in the Fourier transform infrared spectra of edible oils under oxidative conditions. J. Agric. Food Chem. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Kaynak, E.G.; Ibanoglu, E.; Ibanoglu, S. Chemical and structural variations in hazelnut and soybean oils after ozone treatments. Grasas y Aceites 2018, 69, e253. [Google Scholar] [CrossRef]
- Chung, K.T.; Kim, B.W. Anti-microbial Activity Effects of Ozonized Olive Oil Against Bacteria and Candida albicans. J. Life Sci. 2019, 29, 223–230. [Google Scholar] [CrossRef]
- Ouf, S.A.; Moussa, T.A.; Abd-Elmegeed, A.M.; Eltahlawy, S.R. Anti-fungal potential of ozone against some dermatophytes. Braz. J. Microbiol. 2016, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, L.; Gao, L.; Zeng, J.; Lu, J. Ozone therapy for skin diseases: Cellular and molecular mechanisms. Int. Wound J. 2022, 20, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Sciorsci, R.L.; Lillo, E.; Occhiogrosso, L.; Rizzo, A. Ozone therapy in veterinary medicine: A review. Res. Vet. Sci. 2020, 130, 240–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).